Abstract
Purpose: To evaluate the feasibility of precoagulation with microwave ablation (MWA) for hepatic parenchymal transection during liver partial resection.
Methods: A total of 66 eligible patients were enrolled in this double-blind, randomized, controlled study. Patients were randomized to receive either the traditional clamp-crushing method (Control group) or the MWA precoagulation method (MWA group) for hepatic parenchymal transection during liver partial resection. The operative time, hepatic portal occlusion time, intraoperative blood loss and transfusion, postoperative complications and recovery outcomes were compared.
Results: Compared to the Control group, the MWA group had significantly less intraoperative blood loss. Fewer red blood cell transfusions were observed in the MWA group but without statistical significance. The MWA group showed significantly higher serum alanine aminotransferase and aspartate aminotransferase levels at day 1 postoperatively, but no differences between the MWA and Control groups were found at days 3 and 7. There were no significant differences in terms of operative time, hepatic portal occlusion time, postoperative total bilirubin levels, human albumin solution consumption or length of hospital stay. Postoperative complications such as impaired renal function, pyrexia, admission to ICU, abscess, biliary leakage, intrahepatic and distant tumor recurrence and in-hospital mortality were comparable between the two groups.
Conclusion: Precoagulation with MWA reduced intraoperative blood loss with similar postoperative complications, providing a safe, effective, novel alternative for hepatic parenchymal transection during liver partial resection. Additional results from larger series are recommended to confirm these findings.
Introduction
Surgical resection, especially partial liver resection, is the gold standard for treating patients with early-stage hepatocellular carcinoma and metastatic lesions. However, partial hepatectomy is often accompanied by severe complications such as intraoperative and postoperative blood loss, biliary leakage and liver dysfunction or failure, significantly impairing patient recovery and even causing increased in-hospital mortality. Liver transection is one of the most important steps during hepatectomy. The first method described was the finger-fracture technique and, alternatively, the clamp-crushing method using small forceps. However, this potentially exacerbates liver dysfunction due to additional liver parenchyma loss caused by suture and ligation at the margin site. Thus, several methods have been used for liver parenchyma transection [Citation1].
Microwave ablation (MWA) is an emerging treatment for primary and metastatic solid tumors [Citation2]. MWA delivers high-frequency electromagnetic fields to the target tumor, rapidly and efficiently giving rise to high temperatures and causing tissues to desiccate and char. MWA showed similar tumor recurrence and survival rates as surgical resection for patients with small-sized HCC. Coincident with gradual technological improvements and understanding of MWA, studies have shown its application in treating larger tumors. Furthermore, MWA has several advantages compared with other local ablative techniques such as radiofrequency ablation (RFA), cryoablation and high-intensity focused ultrasound (HIFU).
Novel applications of MWA in other clinical settings have been reported. MWA successfully stopped post-liver biopsy hemorrhage, hemobilia and uterine bleeding [Citation3–5]. In a previous study, we reported successful control of emergent intraoperative hepatic tumor bleeding by MWA [Citation6]. Recently, microwave technology has been used for cutting by coagulating the liver parenchyma prior to transection [Citation7–9]. Nevertheless, further studies are needed to confirm the efficiency and safety of precoagulation with MWA for hepatic parenchymal transection during liver partial resection.
Here, we conducted a randomized, controlled clinical trial. Compared to the control group using the clamp-crushing technique, the precoagulation with MWA group had less intraoperative blood loss, with no significant differences in terms of operation time, postoperative liver function or length of hospital stay. Similar complication rates were observed between the groups as well. Thus, precoagulation with MWA may be a novel promising technique for hepatic parenchymal transection in patients undergoing partial hepatectomy.
Materials and methods
Patient enrollment
This study was carried out at the First Affiliated Hospital of Nanjing Medical University after approval from the institute’s ethics committee (2017-SR-336). Written informed consent, including details of the treatment procedure, was obtained from each patient.
Patients undergoing selective partial hepatectomy for liver tumor were eligible for inclusion. The exclusion criteria included (1) unresectable tumor after laparotomy, (2) benign tumor as identified by postoperative histopathology examination, (3) history of previous hepatic surgery and (4) severe cardio-pulmonary or renal dysfunction. A total of 66 eligible patients were enrolled and randomized using the sealed envelope method to undergo either the traditional clamp-crushing method using small forceps (Control group) or the WMA precoagulation method (MWA group) for hepatic parenchymal transection during liver partial resection.
Surgical procedure and hepatic treatment
All enrolled patients underwent open laparotomy under general anesthesia with a reverse ‘L’-shaped or middle line incision according to the tumor location as evaluated by preoperative computed tomography or magnetic resonance imaging. The hepatic portal triad was occluded using the Pringle maneuver depending on the severity of intraoperative hepatic bleeding. For the Control group, the conventional clamp crushing method was used for hepatic parenchymal transection. For the MWA group, patients were treated by applying precoagulation of the liver transection lines using a microwave probe positioned in parallel to the line of resection by an open approach after intra-operative assessment for localization of the tumor and line of resection. The microwave needle was inserted into the liver parenchyma at various sites along the transection line, followed by MWA with 50 W for 10–15 s at each site using the ECO system (ECO-100E, Nanjing, China).
Data collection
The operative time, hepatic portal occlusion time, intraoperative blood loss and transfusion and postoperative recovery outcomes were evaluated and recorded in a predesigned data collection form that was double-checked by another doctor. The length of hospital stay, incidence of ICU admission, postoperative human albumin solution consumption and hospital costs were all recorded. Postoperative liver and renal function measured at 1, 3 and 7 days after surgery were compared between groups. Potential side-effects such as pyrexia, abscess, biliary leakage, intrahepatic and distant tumor recurrence and in-hospital mortality were also evaluated.
Statistical analysis
If the continuous variables were normally distributed, they were expressed as means ± standard deviations; if not, they were expressed as median (range). We used the unpaired, two-sided t-test to compare the mean difference of continuous variables between two groups. Meanwhile, Welch’s t-test was used if the variances were unequal. Categorical variables were assessed by Pearson’s χ2 test or Fisher’s exact test. p values less than .05 were considered as significant. Statistical analyses were performed using statistics software (State version 11.0, StataCorp, College Station, TX).
Results
Preoperative patient/tumor characteristics
A total of 66 eligible patients were enrolled and randomly assigned into the two groups (33 patients in each group). One patient in the control group did not complete the study due to unresectable tumor because of multiple intrahepatic and intraperitoneal metastasis. shows that the biometric data were not significantly different between groups in terms of age, gender, body mass index, history of smoking, alcohol use, preoperative hypertension and diabetes. History of Hepatitis B or C virus infection, preoperative liver function as graded by Child-Pugh Classification, liver cirrhosis, and type and size of liver tumor were all similar between the two groups as well.
Table 1. Preoperative patient/tumor characteristics.
Intraoperative outcomes
As shown in , no significant difference was found in terms of operative time (169.85 ± 52.92 vs. 191.40 ± 43.70 min, Control group vs. MWA group, p > .05) and hepatic portal occlusion time (19.79 ± 8.99 vs. 17.59 ± 13.10 min, Control group vs. MWA group, p > .05) between groups. Of note, the MWA group had significantly less blood loss compared with the Control group (380.0 ± 28.48 vs. 301.3 ± 16.05 mL, Control group vs. MWA group, p > .05). However, the amount of blood transfusion and the hemoglobin on the 1st postoperative day were comparable between the two groups.
Table 2. Intraoperative outcomes.
Postoperative outcomes
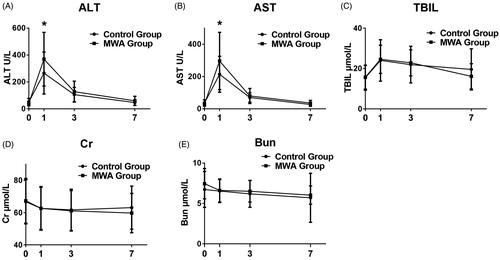
Postoperative liver function recovery, as indicated by serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL), was determined by blood test on days 1, 3 and 7 after surgery. shows that although both ALT and AST levels on day 1 were significantly higher in the MWA group than in the Control group, no differences were found on days 3 and 7. No significant differences of the TBIL levels were found between the two groups (). In addition, the two groups had a similar amount of human albumin solution consumption during the first 3 days after surgery (44.55 ± 32.51 vs. 47.50 ± 36.90 g, Control group vs. MWA group, p > .05). Postoperative renal function, as determined by serum creatinine and blood urea nitrogen, was compared, but no differences were found at all time points ().
As shown in , eight patients in the Control group and nine patients in the MWA group experienced transient pyrexia lasting fewer than 3 days; however, no patient had hepatic or abdominal abscess. One patient in each group had mild biliary leakage, and both recovered after continuous drainage. Two patients in the Control group and one patient in the MWA group were transferred to the ICU due to serious lung infections. The two groups had similar incidences of intrahepatic tumor recurrence and distant tumor occurrence at 1 year after surgery. The lengths of hospital stay were not significantly different between the Control and MWA groups (16.91 ± 4.45 vs. 16.16 ± 4.31 days, Control group vs. MWA group, p > .05). All patients recovered well, and no patient died in hospital. Finally, although an approximate additional one thousand dollars were paid for the utilization of MWA, the total in-hospital costs were not significantly higher in the MWA group (9198.90 ± 2357.20 vs. 10160.21 ± 1767.16 USD, Control group vs. MWA group, p > .05).
Table 3. Postoperative outcomes.
Discussion
In addition to liver transplantation, complete surgical resection has been accepted as a key potential curative treatment for primary liver cancers such as HCC and ICC. With a better understanding of tumor biological characteristics and technological improvements, overall postoperative outcomes have improved greatly. However, both short-term and long-term complications after surgery remain major concerns. Thus, recent technological innovations such as laparoscopy, navigation technology, intraoperative real-time fluorescence guidance and novel local treatments such as MWA, RFA and HIFU have attracted attention for optimal management of primary and metastatic liver tumors.
Compared with other local treatment technologies such as RFA, warmer temperatures can be generated and larger ablations can be achieved by MWA, as it is not impaired by tissue desiccation or charring. Livers are full of ductal structures, including the portal vein, artery and bile duct, giving rise to a pronounced cooling effect. However, MWA can overcome this heat sink effect, giving rise to a higher ablation efficacy. More importantly, MWA has become a novel safe and effective strategy for treating small-sized liver tumors, showing tumor progression and long-term survival comparable to that of surgical resection. In addition, novel applications of MWA in liver surgery have been reported [Citation5,Citation10].
During hepatectomy, liver transection is one of the most important steps, significantly influencing both short- and long-term outcomes. Since the finger-fracture technique and the clamp-crushing technique using small forceps [Citation11] were described, many methods have been reported [Citation1]. The principle of these methods during parenchyma transection is to leave vital structures intact, coagulate small vessels and seal small biliary ducts. To achieve these objectives, various instruments with varying energy sources have been developed, including water jet dissectors, ultrasonic dissectors (CUSA), and saline-linked radiofrequency sealers. In addition, use of the harmonic scalpel and stapling devices in liver parenchyma transection has been reported. However, no single instrument is satisfactory. Many studies with conflicting results have been reported concerning the advantages and the drawbacks of each technique [Citation12–15]. A meta-analysis revealed that no alternative transection method showed significant benefit in terms of blood loss, parenchymal injury, transection time and hospital stay, compared with the clamp-crushing technique [Citation16].
Microwave technology has been used recently to reduce bleeding from the liver parenchyma by coagulating the tissue before liver transection [Citation7–9]. Francone et al. [Citation17] found that thermoablative precoagulation of the transection line for laparoscopic ultrasound-guided liver resection can achieve minimal blood loss and reduce the need for perioperative transfusions during parenchymal transection. By contrast, an earlier study compared CUSA, microwave tissue coagulation and clamp crushing for liver resection in patients with hepatocellular carcinoma. No significant differences were found in terms of the amount of blood loss and operative time; however, a higher incidence of bile leakage was identified in the microwave tissue coagulation group [Citation18]. Imura et al. described a new technique of ultrasound-guided microwave coagulation of the Glissonean pedicle feeding the liver segment or cone unit before parenchyma transection. The mean operative times were similar; however, the mean blood loss was significantly lower in the microwave coagulation group than in the conventional hepatectomy group [Citation19]. Of note, recent studies have shown massive intraoperative blood loss were related to a significantly higher complication rates post liver surgery; the presence of postoperative complications was independently associated with poor overall survival [Citation20]. In the present study, the MWA group had significantly less blood loss. However, the amount of intraoperative blood transfusion was not significantly different, probably due to the small sample size of our study. The incidence of one-year tumor recurrence was comparable between the two groups in the present study.
Postoperative bile leakage and abscess are also relatively common complications of MWA. Sasaki et al. [Citation21] reviewed a total of 1118 cases of hepatectomy using heat coagulative necrosis by microwave tissue coagulator and found that postoperative bile leakage and fluid/abscess formation were seen in 3.0% and 3.3% of cases, respectively. A similar incidence of bile leakage with no abscess occurrence was found in our study.
Hepatic portal occlusion is an important detrimental factor that may impair postoperative recovery of liver function. Thus, we compared the time of hepatic portal occlusion between groups and found no significant difference. More importantly, although the serum levels of ALT and AST were higher in the MWA group at the 1st day postoperatively, no significant differences were found at the 3rd and 7th days postoperatively. Renal function was also normal and comparable at all time points. A recent study evaluated the safety of percutaneous MWA in treating renal cell carcinoma in patients with renal dysfunction and found that after MWA, no significant elevation of renal function was observed in patients with chronic kidney diseases [Citation22].
In conclusion, this study demonstrated that precoagulation with MWA was effective and safe for hepatic parenchymal transection during liver partial resection. Although the hospitalization expenses were slightly higher, precoagulation with MWA reduced intraoperative blood loss. However, further studies with larger numbers of patients are needed to confirm our findings.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Scalzone R, Lopez-Ben S, Figueras J. How to transect the liver? A history lasting more than a century. Dig Surg. 2012;29:30–34.
- Meloni MF, Chiang J, Laeseke PF, et al. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 2017;33:15–24.
- Yeasmin S, Nakayama K, Ishibashi M, et al. Microwave endometrial ablation as an alternative to hysterectomy for the emergent control of uterine bleeding in patients who are poor surgical candidates. Arch Gynecol Obstet. 2009;280:279–282.
- Nakayama K, Rahman MT, Rahman M, et al. Microwave endometrial ablation is a highly efficacious way to emergently control life-threatening uterine hemorrhage. Arch Gynecol Obstet. 2011;283:1065–1068.
- Wai OK, Ng LF, Yu PS, et al. Post biopsy liver hemorrhage successfully controlled by ultrasound-guided percutaneous microwave ablation. J Clin Imaging Sci. 2016;6:34.
- Zhou H, Wu J, Ling W, et al. Application of microwave ablation in the emergent control of intraoperative life-threatening tumor hemorrhage during hepatic surgeries. Int J Hyperthermia. 2018;34:1049–1052.
- Abdelraouf A, Hamdy H, El Erian AM. Initial experience of surgical microwave tissue precoagulation in liver resection for hepatocellular carcinoma in cirrhotic liver. J Egypt Soc Parasitol. 2014;44:343–350.
- Christian DJ, Khithani A, Jeyarajah DR. Making liver transection even safer: a novel use of microwave technology. Am Surg. 2011;77:417–421.
- Percivale A, Griseri G, Gastaldo A, et al. Microwave assisted liver resection: clinical feasibility study and preliminary results. Minerva Chir. 2012;67:415–420.
- Takao Y, Yoshida H, Mamada Y, et al. Transcatheter hepatic arterial embolization followed by microwave ablation for hemobilia from hepatocellular carcinoma. J Nippon Med Sch. 2008;75:284–288.
- Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180:285–290.
- Palibrk I, Milicic B, Stojiljkovic L, et al. Clamp-crushing vs. radiofrequency-assisted liver resection: changes in liver function tests. Hepatogastroenterology. 2012;59:800–804.
- Li M, Zhang W, Li Y, et al. Radiofrequency-assisted versus clamp-crushing parenchyma transection in cirrhotic patients with hepatocellular carcinoma: a randomized clinical trial. Dig Dis Sci. 2013;58:835–840.
- Kim J, Ahmad SA, Lowy AM, et al. Increased biliary fistulas after liver resection with the harmonic scalpel. Am Surg. 2003;69:815–819.
- Hanyong S, Wanyee L, Siyuan F, et al. A prospective randomized controlled trial: comparison of two different methods of hepatectomy. Eur J Surg Oncol. 2015;41:243–248.
- Rahbari NN, Koch M, Schmidt T, et al. Meta-analysis of the clamp-crushing technique for transection of the parenchyma in elective hepatic resection: back to where we started?. Ann Surg Oncol. 2009;16:630–639.
- Francone E, Muzio E, D’Ambra L, et al. Precoagulation-assisted parenchyma-sparing laparoscopic liver surgery: rationale and surgical technique. Surg Endosc. 2017;31:1354–1360.
- Nakayama H, Masuda H, Shibata M, et al. Incidence of bile leakage after three types of hepatic parenchymal transection. Hepatogastroenterology. 2003;50:1517–1520.
- Imura S, Shimada M, Utsunomiya T, et al. Ultrasound-guided microwave coagulation assists anatomical hepatic resection. Surg Today. 2012;42:35–40.
- Chok KS, Ng KK, Poon RT, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87.
- Sasaki K, Matsuda M, Hashimoto M, et al. Liver resection for hepatocellular carcinoma using a microwave tissue coagulator: Experience of 1118 cases. World J Gastroenterol. 2015;21:10400–10408.
- Lin Y, Liang P, Yu XL, et al. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with renal dysfunction. Int J Hyperthermia. 2017;33:440–445.