Abstract
Objective: The aims of this study were to compare the clinical outcomes between ultrasound-guided percutaneous microwave ablation (US-PMWA) and surgical resection (SR) in patients with recurrent intrahepatic cholangiocarcinoma (ICC) and to identify the prognostic factors associated with the two treatment methods.
Methods: This retrospective study was institutional review board approved. A total of 121 patients (102 men and 19 women) with 136 ICCs after hepatectomy from April 2011 to January 2017 were reviewed. Fifty-six patients underwent US-PMWA and 65 patients underwent SR. Survival, recurrence and liver function were compared between the two groups. Effect of changes in key parameters [i.e., overall survival (OS) and recurrence-free survival (RFS)] was statistically analyzed with the log-rank test. Univariate and multivariate analysis were performed on clinicopathological variables to identify factors affecting long-term outcome.
Results: The OS and RFS after MWA were comparable to that of SR (p = .405, and p = .589, respectively). Estimated 5-year OS rates were 23.7% after MWA and 21.8% after SR; for RFS, estimated 3-year RFS rates were 33.1% after MWA and 30.6% after SR. Major complication rates in SR group were higher than that in MWA (p < .001) (SR, 13.8% vs. MWA, 5.3%). Multivariate analysis showed tumor number (p = .012), ALBI grade (p = .007), and metastasis (p = .016), may become OS rate predictors.
Conclusions: US-PMWA had comparable oncologic outcomes with SR and could be a safe and effective treatment for recurrent ICC after hepatectomy.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a subtype of the cholangiocarcinoma, according to anatomical location of the malignancy [Citation1]. As the second most common primary liver cancer following hepatocellular carcinoma (HCC), a vast majority of the ICC occur sporadically without any apparent cause [Citation2,Citation3]. Surgical resection (SR) is the first-line treatment and feasible only in 20–40% of all patients [Citation4]. However, the incidence of recurrence is very high and the 5-year survival rate after hepatectomy is only 20–40% [Citation5,Citation6]. Park et al. [Citation7] previously reported that the cumulative1-, 3- and 5-survival rates of 63 patients who underwent hepatic resection for ICC were 68.2%, 50.5% and 31.8%, respectively. Yamashita et al. reported 356 patients with ICC who underwent curative surgery and a total of 214 patients (60%) had recurrence. Five-year survival rate was 44% after SR for recurrent ICC [Citation8].
Image-guided thermal ablation was an alternative therapeutic efficiency treatment option for ICC [Citation9–11]. Recently, as one of the most recent in the field of thermoablative techniques, microwave ablation has been led frequently reported in terms of its therapeutic effectiveness for solid malignancies, that was associated with many theoretical advantages such as higher intratumoral temperature, larger ablation volume, less operation time, less dependence on the electrical conductivities of tissues [Citation12–14]. Zhang et al. [Citation15] previously reported that clinical and survival outcomes of percutaneous microwave ablation (PMWA) for ICC and 1-, 3- and 5-years overall survival (OS) rate were 93.5%, 39.6% and 7.9%, respectively.
Repeated SR can prolong survival for patients with recurrent ICC, but its implementation is frequently limited by poor liver function and multiple tumors recurrence. Ultrasound-guided PMWA (US-PMWA) yields local tumor control and median survival time that is similar to that obtained with SR in patients with recurrent HCC and colorectal liver metastasis had been reported [Citation16–18]. However, to our knowledge, no comparison of these two therapies for recurrent ICC has been reported. The purpose of this study was to compare the efficacy and safety of SR and US-PMWA for recurrent ICC treatment.
Materials and methods
Study design and patients selection
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of our hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived. The medical records of all patients with initial recurrent ICC after hepatectomy between April 2011 and January 2017 were reviewed. Among them, 121 consecutive patients (102 men, 19 women; mean age, 54.2 ± 10.7 years; range,18–78 years) underwent either US-PMWA (n = 56) or SR (n = 65) and were included in this study according to the following criteria: (1) first intrahepatic recurrent ICC after curative hepatectomy; (2) a single tumor maximum size smaller than 5 cm and tumor number smaller than 3; (3) no major vascular invasion or extrahepatic metastasis; or (4) Child–Pugh class A or B; (5) refused liver transplantation. The exclusion criteria were as follows: (1) repeated recurrent ICC; (2) serious medical comorbidities, including heart, lung and renal function dysfunction; (3) had severe coagulation disorders (i.e., prothrombin time >25s, prothrombin activity <40%, and platelet count <50 cells × 109/L.) or (4) active severe infection.
Recurrent ICCs were diagnosed in all patients based on the most current clinical guidelines at the time of treatment. For patients in the SR group, the recurrent ICC diagnosis was confirmed by postoperative histopathology. For patients in the MWA group, the recurrent ICCs were diagnosed based on pathologic findings of needle biopsy samples before MWA. Reasons for choosing MWA instead of SR were as follows: (1) insufficient liver remnant; (2) psychological resistance to invasive treatment; (3) refusal of general anesthesia; (4) high risk for complications of resection associated with trouble location or old age.
The following demographic and clinicopathologic parameters were obtained from each patient: age, gender, comorbidities, cirrhosis, etiology, albumin–bilirubin (ALBI) grade, maximum tumor diameter, tumor number, pathologic differentiation and laboratory examination (i.e., CA-199, ALT, AST). ALBI grade instead of CTP grade had been the most important criterion for evaluating liver function in this study, which was associated with many advantages such as simple, objective and evidence-based. ALBI score was calculated before treatment using the appropriate clinical parameters and ALBI grade were defined as follows: [log bilirubin (μmol/L)×0.66] + [albumin (g/L)× −0.085], (grade 1, 2 and 3= ≤–2.60, >–2.60 to –1.39 and >–1.39, respectively) [Citation19]. The procedural or treatment variables collected were postoperative hospital stay, operation time, estimated blood loss, complications, cost, date and site of recurrence or metastasis, and date of and status on the last follow-up. We also recorded the reasons for death.
Preventive transcatheter arterial chemoembolization (TACE)
Preventive TACE was defined as TACE that was performed 1–2 months after the initial surgery with no signs of residual tumor evident by hepatic artery angiography. TACE operation was performed by two interventional radiologists in the interventional radiography department. Local anesthesia of 1% lidocaine (Yi you, Beijing, China) was subcutaneous injection and hepatic angiography using a 4-F guiding catheter (65 cm in length) was performed through a right common femoral approach to map liver vascular anatomy, arterial tumor supply, and eventual arteriovenous shunts. The lipiodol and chemotherapeutic drugs including oxaliplatin combined with fluoropyrimidine were advanced into the segmental hepatic artery feeding the lesion.
US-guided MWA procedure
A KY2000 MW ablation system (Kangyou Medical Instruments, Nanjing, China) consisting of two independent MW generators, two flexible coaxial cables and two water-pumping machines, which could drive two 15-gauge cooled-shaft antennae simultaneously was used. Before treatment, all patients were scanned using contrast-enhanced computed tomography (CT)/magnetic resonance imaging (MRI) and ultrasound, and an appropriate puncture route was chosen by ultrasound. After local anesthesia with 1% lidocaine, US-guided biopsy was performed in 2–3 separate punctures using an automatic biopsy gun with an 18-gauge cutting needle. Subsequently, the antenna was inserted percutaneously into the tumor and placed at designated sites under ultrasound guidance. One antenna was inserted into the center of tumors less than 1.7 cm, and multiple antennae were inserted into tumors 1.7 cm or larger. Two antennae were used simultaneously during MW ablation to achieve a larger ablation zone. A power output of 50 W for 10 min was routinely used during MWA. After all the punctures, intravenous anesthesia with a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE) and ketamine (Shuang he Pharmaceuticals, Beijing, China) was administered via the peripheral vein. If the heat-generated hyperechoic water vapor did not completely encompass the entire tumor, prolonged microwave emission was applied until the desired temperature was reached. When the tumor was located not more than 5 mm from the skin surface or adjacent to bowel, gallbladder or other important tissues, the hydrodissection was executed. In addition, microwave needle tract need to be ablated during the needle withdrawal. For tumors with subcutaneous invasion, an ice bag was placed on the skin to avoid scalding during MW ablation.
Follow-up and endpoints
All patients entered the follow-up period, which consisted of contrast-enhanced CT or MRI at 1, 3 and every 3 months intervals thereafter after ablation or hepatectomy. Thereafter, the follow-up visit covered several evaluations, including routine physical examination, laboratory tests such as total bilirubin, serum albumin, prothrombin time and tumor marker levels. Technique effectiveness was defined as the absence of contrast-enhancement on imaging in any area of the mass after one month. The endpoints of this study were death and tumor recurrence. In this study, local tumor recurrence, and intrahepatic recurrence both were regarded as tumor recurrence. Local tumor recurrence was defined based on imaging findings of irregular nodular, scattered, or eccentric pattern of peripheral enhancement around the ablation zone in the patients after US-PMWA and around the resected margin in the patients after hepatectomy. Intrahepatic distant recurrence was defined as the appearance of an irregular, scattered or eccentric pattern of peripheral enhancement intrahepatic lesion, which were farther away from the ablation zone after MWA and around the resected margin after hepatectomy. Once tumor recurrence was found, a second MWA treatment was arranged. OS was calculated from the date of first session of MWA treatment to the date of death or last date of follow-up (survival or loss). Recurrence-free survival (RFS) was calculated from the date of first session of MWA treatment to the date of tumor recurrence or the last date of follow-up (no finding recurrence or loss). Major complications were defined as events which caused substantial morbidity and disability that increased the level of care, or led to hospital admission, or substantially prolonged the hospital stay [Citation20].
Statistical analysis
Data between the MWA and SR groups were compared with the Student’s t-test. Continuous variables were analyzed by the Wilcoxon signed-rank test and categorical variables were analyzed using either the Pearson’s chi-squared test or the Fisher’s exact test. OS, disease-free survival and local seeding progress-free survival were assessed by the Kaplan–Meier method with log-rank test. A Cox proportional hazards model was used to identify the significant effects of multiple factors on survival and local progress rate. Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL). For all tests, a p value less than .05 was considered to be statistically significant.
Results
Patients selection
A total of 438 patients with recurrent ICC underwent MWA or SR during the study period. As indicated in , a total of 317 patients were excluded because they met the exclusion criteria. As a result, 121 patients were included in our study, 56 patients (7 females, 48 males; average age 54.5 ± 9.3 years) in the MWA group and 65 patients (12 females, 53 males; average age 53.9 ± 17.5 years) in the SR group. The characteristics of the patients and tumors are summarized in . The mean age and sex were comparable between the two groups (p = .892 and p = .369, respectively). The mean maximum diameter and tumor number were comparable between the two groups (p = .752 and p = .866, respectively). The comorbid diseases, pathological differentiation, cirrhosis and etiology were comparable between the two groups (p = .613, p = .885, p = .707 and p = .981, respectively). Patients who underwent MWA had lower KPS score than those who received surgery (p = .014). ALBI grade was comparable between the two groups (p = .900). Preventive TACE were comparable between the two groups (p = .791). Metastasis were comparable between the two groups (p = .228).
Figure 1. Flow diagram shows exclusion criteria in patients with recurrent intrahepatic cholangiocarcinoma (RICC). SR = surgical resection, MWA = microwave ablation.
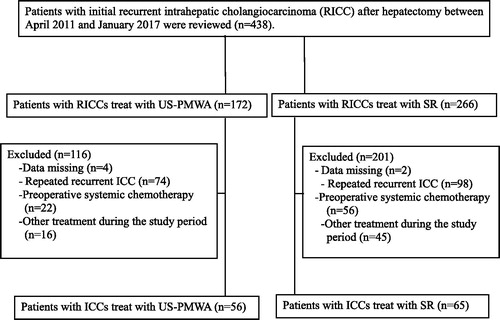
Table 1. Baseline characteristics of patients undergoing MWA and SR.
Treatment parameters
The treatment parameters are summarized in . Fifty-six patients with 62 tumors received a total of 65 treatments sessions in MWA group. Sixty-five patients with 74 tumors received a total of 75 treatments sessions in SR group. Forty-seven tumors were successfully treated in one MWA session and nine tumors were treated in two sessions. The surgical time, estimated blood loss, cost and hospitalization in the SR group were significantly higher than in the MWA group (p < .001, p < .001, p = .017 and p = .012). The pre- and postoperative liver function in two groups are summarized in .
Table 2. The changes of liver and renal function before and after treatment in ICC patients.
Midterm survival and recurrence outcome
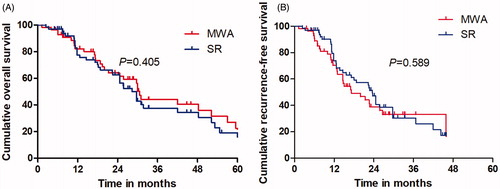
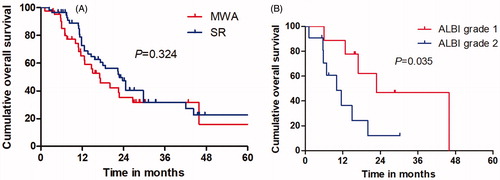
The median OS was 31.3 months (range, 3.8–62.9 months) in the MWA group and 29.4 months (range, 4.3–65.4 months) in the SR group. Mortality rate was 53.6% (30 of 56 patients) in the MWA group; Causes of death for all patients were ICC tumor progression. Eight of the patients had comorbid diseases, mainly including hypertension and/or diabetes. Forty patients (61.5%; 40 of 65 patients) died in the SR group, and the cause of death in was mainly ICC local or systemic progression. Based on the follow-up imaging, 100% technique success rate was achieved in both groups (MWA: 63 of 63 treatments; SR: 75 of 75 treatments). Twenty-eight LTP lesions (50%; 28 of 56 patients) were discovered after MWA treatment and 33 LTP lesions (50.8%; 33 of 65 patients) were discovered after SR treatment. Nine patients after MWA (16.1%; 9 of 56 patients) and eight patients after SR (12.3%; 8 of 65 patients) had distant metastasis. The 1-, 3- and 5-year OS rates in the MWA group and SR group were 81.2, 42.5 and 23.7% and 77.4, 36.4 and 21.8%, respectively (), showing no significant statistical difference (p = .405). The 1-, 3- and 5-year RFS rates in the MWA group and SR group were 70.3, 33.1 and 0% and 76.7, 30.6 and 0%, respectively (), showing no significant statistical difference (p = .589). There was no significant difference between the survival of patients with ALBI grade 1 between MWA group and SR group (p = .381), (). OS of patients with ALBI grade 2 in MWA group was higher than that in SR group (p = .035), ().
Complication
There were no treatment-related deaths in this study. Twelve patients were treated for major complications. There was one patient in the MWA group and two patients in the SR group that developed hepatic failure. In the MWA group, two patients developed liver abscesses and one in ascites. In the SR group, six patients developed ascites and three patients developed jaundice. The major complications incidence rate was higher in the SR group (9 of 65; 13.8%) than in the MWA group (3 of 56; 5.3%) (p < .001).
Univariate and multivariate analyses
Univariate and multivariate logistic regression analyses were performed to identify predictors influencing the long-term outcome of patients with recurrent ICC who underwent US-PMWA and SR. The univariate analysis showed statistically significant differences in terms of OS rates, depending on the age (χ2 = 3.795; p = .039), KPS (χ2 = 9.511; p = .012), tumor number (χ2 = 0.881; p = .008), metastasis (χ2 = 1.118; p = .026), chemoradiation (χ2 = 4.625; p = .032) and ALBI grade (χ2 = 2.021; p = .042), (). Multivariate analysis showed that the factors that significantly affected the OS rate were tumor number (HR = 4.959; p = .012), metastasis (HR = 3.987; p = .016) and ALBI grade (HR =2.367; p = .007), ().
Table 3. Univariate analysis of prognostic factors for survival.
Table 4. Multivariate analysis of prognostic factors with cox proportional hazards model.
Discussion
The optimal treatment for recurrent ICC after resection has not been established. Guidelines by the European Association for the Study of the Liver on the management of ICC suggest that SR or thermal ablation may be attempted in cases of intrahepatic recurrence [Citation21]. Although the general consensus is to stop further treatment in cases of ICC recurrence, many methods are still performed including chemotherapy, radiotherapy, hepatectomy, radiofrequency ablation (RFA) and TACE. Several studies have demonstrated the importance to improving the survival outcome for ICC patients with recurrence with more effective treatments [Citation8,Citation22,Citation23].
In this study, we analyzed the OS and RFS of 121 consecutive patients undergoing SR or MWA. Comparison of the clinical efficacy between MWA and traditional SR for the treatment of recurrent ICC was performed. With approximately the same liver function, treatment with SR and MWA had comparable survival outcomes in ICC patients and had a similar recurrence rate. After repeat SR for recurrent ICC, 5-year OS was 21.8%, which was higher than without any treatment in other published series. Park et al. [Citation24] reported that the 5-year OS rate was only 15% when a recurrent tumor was found after SR. Song et al. [Citation25] reported that the median survival time for patients with recurrent cholangiocarcinoma was significantly better in patients that received repeated SR (18.9 months) versus those who did not (7.7 months).
Similarly, Takahashi et al. [Citation23] reported on ICC patients treated with thermal ablation therapy. The median time to LTP and OS were 7.1 months and 23.6 months among 20 patients. In this study, the median OS was 31.3 months tumors recurring after SR were eradicated by US-PMWA, which has a longer median OS time than that of thermal ablation for the above-mentioned primary ICC. Zhang et al. compared clinical outcomes of thermal ablation to repeated hepatic resection for recurrent ICC. They found SR and thermal ablation groups did not differ in terms of OS and DFS [Citation26]. This result was similar to our findings but only one thermal ablation method (i.e., US-PMWA) was utilized in this study.
Many laboratory tests related to liver function reserves or tumor presence including ALT, AST, serum albumin and total bilirubin levels were reviewed in our study. Preoperative laboratory indices related to liver function were found to be similar between the SR group and MWA group. However, postoperative laboratory indices for the SR group were significantly higher than those of MWA. Possible explanations for this result may be US-PMWA as a minimally invasive treatment, with less damage to liver function, especially in patients with poor liver function. Previous study had reported that PMWA is a safe and effective treatment for ICC and Child–Pugh class A could significantly influence prognosis. This suggests that better assessment of liver function is a key element in managing ICC patients who underwent MWA [Citation15]. ALBI grade as a biomarker might be better at stratifying liver reserve compared to Child–Pugh score for patients with early-stage ICC who underwent US-PMWA and could provide an important reference for the treatment of patients and assessment of their outcomes [Citation27–30]. In this study, ALBI grade was found to be an independent factor associated with OS, which exhibited reliable discriminative ability for assessment of OS after US-PMWA. Among patients with ALBI grade 1, OS rate in SR group was compared with the MWA group. However, among patients with ALBI grade 2, OS rate in the SR group was less than in the MWA group. As part of a good prognosis liver function was less affected by MWA, and median OS time in MWA group was longer than SR group.
Univariate and multivariate analyses indicated that tumor number, presence of metastasis and ALBI grade were significant prognostic factors of OS in patients treated with repeated SR or MWA. Although cancers graded Child–Pugh A after MWA have been reported previously to be associated with better survival in ICC patients with early recurrence, Child–Pugh grade was not a significant prognostic factor in our study. Similarly, the non-effect of CTP grade in our data may be due to subjective clinical assessment of ascites and hepatic enccephalopathy by different clinicians impacting results. Compared with repeated SR, MWA has the advantage of being less invasive and being associated with fewer complications. Nevertheless, two patients in our MWA group did develop liver abscesses. Yu et al. [Citation31] reported a high incidence of liver abscess in patients treated with MWA for intrahepatic metastatic cholangiocarcinoma with bilioenteric anastomosis. Further research is needed to determine how best to avoid this problem. Moreover, shorter hospital stays, surgical time, as well as less cost and estimated blood loss in US-PMWA treatment reflect the safety and efficacy of this minimal invasive method. Importantly, US-PMWA has more repeatability than SR.
There are several limitations to our study that should be noted. First, this is was single-center retrospective study with a relatively small sample size. Such a limitation might have reduced its statistical power in comparative analysis so that some associations were not detected. Second, the success of MWA was assessed by radiographic findings versus pathologic margin-free status. Therefore, despite intermediate follow-up being reported, it may have taken longer for radiographic techniques to detect MWA failures. Third, KPS score in the MWA group was lower than that in SR group and whether poor physical conditions affect prognosis requires further explore.
In conclusion, the present results indicate that US-PMWA has similar safety and efficacy as repeated SR. Both methods provide an effective treatment for recurrent ICC. US-PMWA should be preferred in any cases when the patients with liver function of ALBI grade 2.
Disclosure statement
No conflict of interest exits in the submission of this manuscript. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111.
- Burt AD, Alves V, Bedossa P, et al. Data set for the reporting of intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma and hepatocellular carcinoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology. 2018;73:369–385.
- Antwi SO, Mousa OY, Patel T. Racial, ethnic, and age disparities in incidence and survival of intrahepatic cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol. 2018;17:604–614.
- Neeff HP, Holzner PA, Menzel M, et al. [Intrahepatic cholangiocarcinoma: Results after 84 resections]. Chirurg. 2018;89:374–380. German.
- Wright GP, Perkins S, Jones H, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. 2018;25:83–90.
- Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105:848–856.
- Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–1830.
- Yamashita YI, Shirabe K, Beppu T, et al. Surgical management of recurrent intrahepatic cholangiocarcinoma: predictors, adjuvant chemotherapy, and surgical therapy for recurrence: a multi-institutional study by the Kyushu Study Group of Liver Surgery. Ann Gastroenterol Surg. 2017;1:136–142.
- Sommer CM, Kauczor HU, Pereira PL. Locoregional therapies of cholangiocarcinoma. Visc Med. 2016;32:414–420.
- Xu HX, Wang Y, Lu MD, et al. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078–1084.
- Butros SR, Shenoy-Bhangle A, Mueller PR, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging. 2014;38:490–494.
- Han Y, Shao N, Xi X, et al. Use of microwave ablation in the treatment of patients with multiple primary malignant tumors. Thorac Cancer. 2017;8:365–371.
- Medhat E, Abdel AA, Nabeel M, et al. Value of microwave ablation in treatment of large lesions of hepatocellular carcinoma. J Dig Dis. 2015;16:456–463.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology. 2014;270:880–887.
- Zhang K, Yu J, Yu X, et al. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma[J]. Int J Hyperthermia. 2018;34(3):1–6.
- Ren H, Liang P, Yu X, et al. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: a pilot study. Int J Hyperthermia. 2011;27:249–254.
- Yu J, Liang P, Yu XL, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25:1119–1126.
- Wang J, Liang P, Yu J, et al. Clinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastases. Oncol Lett. 2014;8:323–326.
- Hiraoka A, Michitaka K, Kumada T, et al. ALBI score as a novel tool in staging and treatment planning for hepatocellular carcinoma: advantage of ALBI grade for universal assessment of hepatic function. Liver Cancer. 2017;6:377–379.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Yang HI, Sherman M, Su J, et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. JCO. 2010;28:2437–2444.
- Lurje G, Bednarsch J, Roderburg C, et al. Intrahepatic cholangiocarcinoma - current perspectives and treatment algorithm. Chirurg, 2018;12:76–82.
- Takahashi EA, Kinsman KA, Schmit GD, et al. Thermal ablation of intrahepatic cholangiocarcinoma: Safety, efficacy, and factors affecting local tumor progression[J]. Abdom Radio, 2018;4:1–6.
- Park HM, Yun SP, Lee EC, et al. Outcomes for patients with recurrent intrahepatic cholangiocarcinoma after surgery. Ann Surg Oncol. 2016;23:4392–4400.
- Song SC, Heo JS, Choi DW, et al. Survival benefits of surgical resection in recurrent cholangiocarcinoma. J Korean Surg Soc. 2011;81:187–194.
- Zhang SJ, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20:3596–3602.
- Yantong Y, Shan L, Zhijie C, et al. A model prediction of long-term prognosis in patients with centrally located hepatocellular carcinoma undergoing hepatectomy. Eur J Surg Oncol. 2018;44:1595–1602.
- Chm H, Chiang CL, Fas L, et al. Comparison of platelet-albumin-bilirubin (PALBI), albumin-bilirubin (ALBI), and child-pugh (CP) score for predicting of survival in advanced hcc patients receiving radiotherapy (RT). Oncotarget. 2018;9:28818–28829.
- Hou YL, Gao MD, Guo HY, et al. Diagnostic value of albumin-bilirubin grade combined with serum ammonia in cirrhosis with hepatic encephalopathy. Zhonghua Yi Xue Za Zhi. 2018;98:127–131. Chinese.
- Ho SY, Liu PH, Hsu CY, et al. Prognostic performance of ten liver function models in patients with hepatocellular carcinoma undergoing radiofrequency ablation. Sci Rep. 2018;8:843.
- Yu MA, Liang P, Yu XL, et al. Liver abscess as a complication of microwave ablation for liver metastatic cholangiocarcinoma after bilioenteric anastomosis. Int J Hyperthermia. 2011;27:503–509.