Abstract
Purpose: To compare the efficacy and complication rates of radiofrequency ablation (RFA) and repeat surgery in the treatment of locally recurrent thyroid cancers.
Materials and methods: A total of 221 patients with locally recurrent thyroid cancers who underwent either RFA (n = 96) or repeat surgery (n = 125) between March 2008 and March 2017 were retrospectively enrolled (range of follow-up, 1–10 years). Each cohort consisted of 70 patients after propensity score adjustment. Patients with more than three recurrent lesions were excluded. The primary and secondary end points were recurrence-free survival and complication rates, respectively. Recurrence-free survival curves were compared via the log-rank test. The complications—voice changes, hypocalcemia, and immediate procedural complications—were compared between the groups. In addition, pretreatment serum thyroglobulin (Tg) levels and those at the last follow-up were also compared between the two groups to examine therapeutic efficacy.
Results: After propensity score matching, both groups showed no significant differences in baseline characteristics. The recurrence-free survival rates were comparable between the RFA and surgery groups (p = .2). There were no significant differences in mean serum Tg levels and their mean decrease after treatment between the groups (p = .891 and p = .963, respectively). Immediate procedural complications and voice changes also showed no significant between-group differences (p = .316, p = .084, respectively). Hypocalcemia occurred only in the repeat surgery group (n = 18). Overall complications were significantly more frequent in the repeat surgery group (RFA, n = 7; surgery, n = 27; p < .001).
Conclusion: RFA may be an effective and safe alternative to repeat surgery in the treatment of a small number of locally recurrent thyroid cancers.
Introduction
Differentiated thyroid cancers account for approximately 90% of endocrine tumors [Citation1]. While the mortality rate in patients with differentiated thyroid cancer has consistently been very low over the past three decades [Citation2], its recurrence rate has reportedly been highly variable, ranging from 20 to 59% [Citation3,Citation4]. Regarding the management of recurrent thyroid cancers, surgery followed by radioactive iodine therapy and thyroid hormone therapy is the mainstay of treatment [Citation5], with reports of >97% of patients receiving surgery [Citation6]. Nevertheless, repeat surgeries are often more challenging due to postoperative tissue adhesion and structural distortion, which increase the risk of complications [Citation7–9]. Recently, minimally invasive image-guided thermal ablation techniques, such as percutaneous laser ablation [Citation10,Citation11], microwave ablation [Citation12], and radiofrequency ablation (RFA), have shown good efficacy in controlling recurrent thyroid cancers. Among them, RFA is the main non-surgical alternative option at our institution.
RFA has several advantages, such as minimal invasiveness and reduced mortality, and it allows for real-time imaging guidance and outpatient-based procedures [Citation13]. The dose–response relationship for irreversible cell damage induced by RFA in thyroid tissues has been studied [Citation14]. Furthermore, RFA has been shown to be safe, feasible, and effective in treating benign thyroid nodules [Citation15–19] and even low-risk small papillary thyroid carcinomas [Citation20].
Recently, owing to its increasing applicability, RFA has been clinically used as an alternative treatment for recurrent thyroid cancers in patients at high risk of complications from surgery or those refusing to undergo repeated surgeries. Previous studies regarding the treatment of local recurrent thyroid cancers using RFA included relatively small numbers of patients, ranging from 8 to 73, and short mean follow-up periods of 9.4–61.3 months [Citation4,Citation21–24]. We attempted to validate findings from previous studies by selecting a larger patient cohort and follow-up.
The aim of this study was to assess whether percutaneous ultrasound (US)-guided RFA is as effective and safe as repeat surgery in the treatment of locally recurrent thyroid cancers.
Materials and methods
Our institutional review board approved this retrospective study, and the requirement for informed consent was waived; written informed consent for treatment, however, was obtained for all patients prior to each procedure and operation.
Patient population
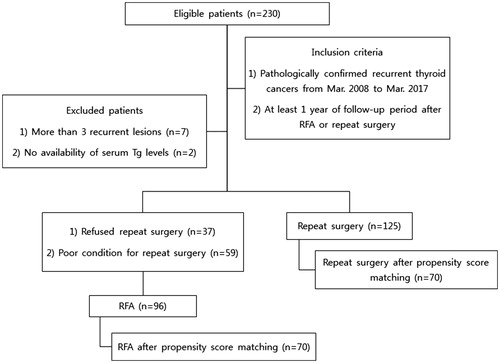
We retrospectively reviewed the medical records of patients who were diagnosed with recurrent thyroid cancers and received treatment at our institution from March 2008 to March 2017. After confirmation of recurrence, patients were recommended to undergo repeat surgery at first; RFA was recommended only to those who refused repeat surgery (n = 37) or were in a condition too poor to allow for surgery (n = 59). All selected patients met the following inclusion criteria: (a) three or fewer recurrent lesions showing high probability of malignancy on US imaging and pathologically confirmed by fine-needle aspiration biopsy (FNAB); (b) at least 1 year of follow-up period after either treatment; and (c) availability of pre- and post-treatment serum Tg levels. Among the initial cohort of 230 patients, 9 patients were excluded based on the above criteria and 221 patients were selected as the study cohort. A summary of patient enrollment process is shown in .
RFA procedure
Under US guidance, a radiologist with 10 years of RFA experience performed all RFA procedures (blinded). All patients visited the hospital as outpatients. They were injected with intravenous fentanyl citrate (2 mg/mL) prior to or during the procedure, and their blood pressure, venous oxygen saturation, pulse rate, and electrocardiography findings were closely monitored.
All US scans were performed using one of the following two systems throughout the study period—Aplio 500 Platinum (Toshiba Medical Systems, Tokyo, Japan) and HDI 5000 (Philips Medical Systems, Bothell, WA). For all RFA procedures, a radiofrequency (RF) generator (Cool-Tip RF system, Covidien, Boulder; SSP-2000, Taewoong Medical, Gyeonggi, Korea) and a thyroid-dedicated, internally cooled RF electrode (Well-Point RF electrode, Taewoong Medical, Gyeonggi, Korea) were used. Based on tumor sizes, a 7-cm-long, 18 gauge electrode with either a 0.5, 0.7, or 1.0 cm active tip was selected.
To minimize complications, all procedures were performed using multiple methods, as described previously by Lee et al. [Citation22], including the hydrodissection technique and pulled-away method in which the electrode is tilted during ablation, thereby preventing injury to the adjacent nerves and critical structures. Lidocaine (0.2%, up to 10 ml) was then injected into an area between the tumor and adjacent critical structures, such as nerves, carotid artery, jugular vein, trachea, and esophagus, to provide local anesthesia and create a protective barrier against thermal heat, which is a technique known as hydro-dissection [Citation4,Citation25,Citation26]. Firstly, the electrode tip was inserted into the deepest and most distal portion of the lesion. Gradual ablation of recurrence was applied with the moving shot technique [Citation27]. RFA was initiated with a low power (5 W in a 0.5 or 0.7 cm tip; 20 W in a 1.0 cm tip) and gradually increased to a higher power (maximum of 10 W in a 0.5 or 0.7 cm tip; 60 W in a 1.0 cm tip) until a transient hyperechogenicity was formed at the electrode tip [Citation4,Citation28] or high impedance was reached, at which point the power automatically stopped to increase. Subsequently, we conducted a fine maneuver to change the position of the electrode tip to unablated areas. Finally, cold distilled water was injected around the ablated tumor to reduce the heat. If pain became unbearable to patients during the procedure, the power was reduced or turned off for several seconds. RFA was terminated upon the change of the recurrent tumor into a transient hyperechoic zone or the development of hoarseness. The patients’ voice, discomfort, or other complications were closely monitored during and 1–2 h after RFA. All patients underwent follow-up US with FNAB after 2 months; a repeat RFA was conducted when remnant recurrence was pathologically confirmed. Of 96 patients, 17 patients (17.8%) and 5 patients (5.2%) underwent two and three RFA sessions, respectively; the remaining 74 patients (77.1%) underwent one RFA session.
Reoperation
Repeat surgeries were performed, under general anesthesia, by seven experienced thyroid surgeons (n = 125; 2–12 years of experience). Patients were positioned semi-erect, and neck dissection (modified radical, central, or selective) or excision was performed on neck compartments with recurrences and any nearby compartments if no comprehensive neck dissection had been previously performed.
Study endpoints
Primary end-point
The primary endpoint was recurrence-free survival (without local recurrence at the site of RFA or repeat surgery). We defined local recurrence as subsequent recurrences occurring at the same site of RFA treatment or surgery. Recurrences at different cervical levels after treatment were considered as regional recurrence because our primary focus was to compare the therapeutic effects of RFA and repeat surgery on local recurrence sites.
Secondary end-point
Complication rates (i.e. voice change, hypocalcemia, immediate procedure-related complications) were the secondary end-point of the study. Voice changes were defined as hoarseness or inability to produce high-tone speech after RFA or reoperation. We considered voice changes lasting over one year as permanent voice changes and those recovering within a year as transient; voice changes were monitored on follow-up outpatient visits. Furthermore, we considered voice changes lasting longer than a month, including permanent changes, as major complications and voice changes lasting a month as minor complications, as described in a previous study [Citation29]. In the same manner, hypocalcemia was also divided into transient and permanent hypocalcemia, considered as minor and major complications, respectively. Any immediate side effects, such as vasovagal reaction, hypertension, or vomiting during the procedure, were also considered as minor complications. The distinction between major and minor complications was based on previous recommendations [Citation30,Citation31].
Follow-up protocol
All patients were followed-up by regular monitoring of serum Tg levels, US of the neck, and clinical evaluation 6–12 months after treatment. Additional imaging modalities, including computed tomography (CT) and positron emission tomography CT, were also used when recurrences were detected on US or serum Tg levels were elevated. US or FNAB was performed for all patients who underwent RFA after 1 or 2 months. All local and regional recurrences detected were pathologically confirmed by FNAB.
Statistical analysis
Statistical analyses were performed with the R statistical packages (version 3.4.4, R Foundation, Vienna, Austria; www.R-project.org). Data measurements were expressed as mean ± SD. Statistical significance was set at p < .05. Categorical variables were analyzed using a chi-squared test or Fisher’s exact test and continuous results were analyzed by unpaired t tests or Mann–Whitney U-tests after testing for normality via the Shapiro–Wilk test. Kaplan–Meier curves and the log-rank test were used to compare recurrence-free survival between groups.
We conducted propensity score matching analysis using a logit model to minimize the effects of potential confounders on selection bias. The model was adjusted for the following variables: sex; age at time of RFA or repeat surgery; number, size and location of recurrent tumors; previous history of neck dissection; and follow-up time. Both cohorts were trimmed by removing 81 patients (RFA, n = 26; surgery, n = 55). Finally, a total of 140 patients (one-to-one matched 70 patients from each cohort) were compared, and recurrent-free survival and complication rates were subsequently recalculated and compared.
Results
Patient characteristics
The baseline characteristics of patients in the RFA and surgery groups are summarized in . No significant differences in sex, age, and number of recurrent tumors were found between the two groups. The number of patients who had total thyroidectomies as the initial surgery before recurrence was significantly greater in the RFA group than in the surgery group [93 of 96 (96.9%) and 110 of 125 (88%), respectively; p = .017]. The number of patients without neck dissection during their initial surgery was greater in the RFA group [46 of 96 (47.9%)] than in the surgery group [35 of 125 (28%); p = .002]. Furthermore, patients in the RFA group had more radical neck dissections than did those in the surgery group [37 of 96 (38.5%) and 21 of 125 (16.8%), respectively; p < .001]. However, patients in the surgery group had more central neck dissections [64 of 125 (51.2%) and 12 of 96 (12.5%), respectively; p < .001]. The RFA group had more centrally located recurrent tumors than the surgery group [64 of 96 (66.7%) and 54 of 125 (43.2%), respectively; p < .001]. The mean size of recurrent tumors was larger in the surgery group (1.3 ± 0.7 cm) than in the RFA group (1.0 ± 0.8; p = .005). After propensity score matching, all baseline characteristics, except for the type of initial surgery, showed no significant differences between the two groups.
Table 1. Baseline characteristics of study population.
Post-treatment follow-up of serum Tg levels
The posttreatment serum Tg levels are summarized in . The mean pretreatment (RFA, 18.2 ± 95.5; surgery, 21.8 ± 116; p = .394) and post-treatment (RFA, 0.8 ± 2.8; surgery, 1.8 ± 5.8; p = .891) serum Tg levels were not significantly different between the two groups. The number of patients with post-treatment Tg levels >1 ng/mL was also not significantly different [RFA, 15 of 95 (15.8%); surgery, 16 of 124 (12.9%); p = .543). The mean decrease in the serum Tg level in patients in the RFA and repeat surgery groups was 14.7 ± 27.7 ng/mL and 51.8 ± 187.8 ng/mL, respectively (p = .963).
Table 2. Serum Tg level changes and recurrence-free survival of both groups.
Comparison of complications between RFA and surgery groups
The surgery group had significantly more voice changes [23 of 125 (18.4%)] than did the RFA group [8 of 96 (8.3%); p = .033]. No hypocalcemia was observed in the RFA group, whereas 28.8% (36 of 125) of patients in the surgery group had hypocalcemia (p < .001); 10 and 26 incidences of transient and permanent hypocalcemia, respectively, were noted. No significant difference in immediate procedural complications was found between the two groups [RFA, 2 of 96 (2%); surgery, 0 of 125 (0%); p = .192]; the two complications in the RFA group were elevated blood pressure and dyspnea during the RFA. The number of major complications in the surgery group [39 of 125 (31.2%)] was significantly greater than that in the RFA group [3 of 96 (3.1%), p < .001], while the number of minor complications was comparable between the groups (p = .184). Overall, the number of total complications was greater in the surgery group [52 of 125 (41.6%)] than in the RFA group [10 of 96 (10.2%); p < .001; ]. During the follow-up, a few recurrences—all pathologically confirmed as metastatic papillary thyroid carcinomas—occurred after RFA or repeat surgeries. Subsequent local recurrences were not significantly different between groups [RFA, 1 of 96 (1%); surgery, 6 of 125 (4.8%); p = .138]. In addition, regional recurrence, which was defined as subsequent recurrence at different cervical levels, was not significantly different between the two groups [RFA, 11 of 96 (11.2%); surgery, 7 of 125 (5.6%); p = .126; ). After propensity score matching, only the incidences of voice change (both transient and permanent) were no longer significantly different between the two groups (p = .084). No life-threatening complications were observed throughout the entire follow-up period.
Table 3. Complications after RFA and repeat surgery.
Comparison of recurrence-free survival between RFA and surgery groups
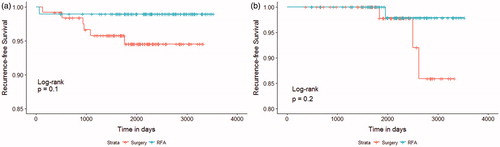
In the RFA group, all recurrent lesions showed complete disappearance without regrowth on follow-up US or CT ( and ). The mean follow-up periods for the RFA and surgery groups were 76.8 ± 23.7 and 69.1 ± 20.4 months, respectively (). The Kaplan–Meier survival curves of both groups revealed no significant difference in recurrence-free survival rates (p = .1) (). The 3-year and 6-year recurrence-free survival rates, as estimated by Kaplan–Meier survival curves, were 90.8 and 89.5% in the RFA group and 95.8 and 94.5% in the surgery group, respectively. After propensity score matching, the recurrence-free survival curves remained similar between the two groups (p = .2; ); and the 3- and 6-year recurrence-free survival rates were 100% (n = 66) and 97.9% (n = 38) for the RFA group and 100% (n = 63) and 97.8% (n = 30) for the surgery group, respectively. There were no patients with distant metastases during follow-up period in both RFA and surgery groups.
Figure 2. Ultrasound (US; a) and computed tomography (CT; c) images obtained at the initial radiofrequency ablation procedure in a 51-year old woman with recurrence at right cervical level 4 (a and c, respectivelyarrows). The recurrent lesion completely disappeared at the 98-month follow-up, as observed on US (b), and had also disappeared at the 5-month follow-up, as observed on CT (d; arrows).
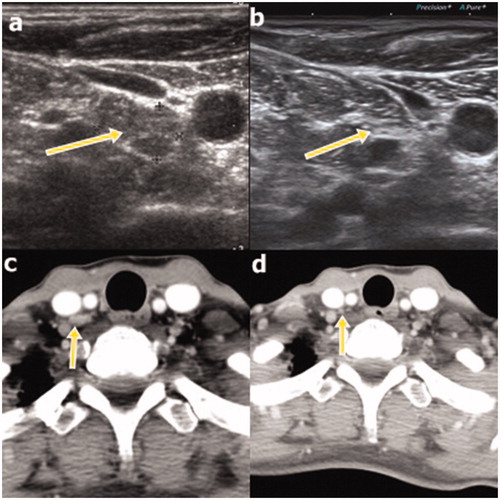
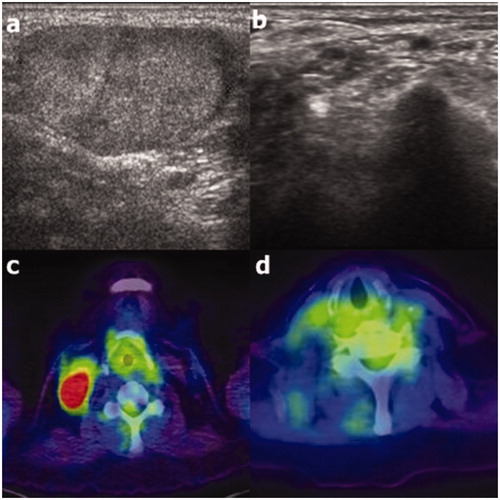
Figure 3. Ultrasound (US; a) and positron emission tomography-computed tomography (PET-CT; c) images of recurrence at right cervical level 4 in an 81-year old female patient. At the 122-month follow-up, complete disappearance of the recurrence was observed on US (b); it was also noted at the 39-month follow-up on PET-CT (d).
Discussion
In our study, the recurrence-free survival rates were comparable between the two groups. Moreover, no significant difference was found in post-treatment serum Tg levels between the two groups, indicating that their therapeutic efficacies were similar. Regarding safety, patients who were treated with RFA had fewer overall complications than those who underwent repeat surgery.
Our findings of comparable local recurrence-free survival between both groups are consistent with a previous study by Kim et al [Citation24]. In addition, the 3-year recurrence-free survival rates were high in the RFA group (Kim et al.: 92.6%; current study: 100%) and reoperation group (Kim et al.: 92.2%; current study: 100%). However, in contrast to the study by Kim et al., the RFA group in our study showed a higher local recurrence-free survival curve than the repeat surgery group. Nevertheless, findings regarding the post-treatment serum Tg levels (i.e. mean decreases in serum Tg were comparable between groups), which we measured to assess therapeutic effect, were consistent with the study by Kim et al.
All RFA-treated recurrent lesions showed complete disappearance without regrowth on follow-up US or CT, which is consistent with previous studies with reported volume reduction ratios ranging from 89.7 to 98.4% in locally recurrent thyroid cancers treated by RFA [Citation4,Citation22,Citation24]. We assume the treated lesions to be recurrence-free because of the lack of visible regrowth and stable serum Tg levels during the observed follow-up period.
Our results for complications were also in agreement with those of Kim et al., who had reported that the total number of complications was lower in the RFA group [Citation24]; however, in this current study, significantly lower overall complication rates were observed in the RFA group. A meta-analysis on evaluating safety of RFA in the treatment of recurrent thyroid cancers reported that overall and major complication rates were 10.98 and 6.71%, respectively [Citation32], which are similar to our findings of 10.4 and 3.1% of overall and major complication rates, respectively. In addition, hypocalcemia was observed exclusively in the repeat surgery group and not observed in the RFA group, which was also in agreement with prior studies [Citation4,Citation24,Citation25,Citation33]. Furthermore, unlike the study by Kim et al., the incidence of voice changes was significantly higher in the repeat surgery group (18.4%), which is slightly higher than, but comparable to, prior results of permanent recurrent laryngeal nerve paralysis rates after repeat surgeries (1–12%) [Citation34]. However, this difference was no longer observed—after propensity score matching. Several cases of voice changes after RFA on surgical beds were previously reported [Citation4,Citation25,Citation35], which suggests that RFA is less safe when applied on the surgical bed. Baek et al. explained that the recurrent laryngeal nerve is not well visualized on US on the surgical bed, making it more vulnerable to thermal injury [Citation4]. However, while a higher proportion of RFA procedures was performed in the central cervical regions in our study, only a few patients experienced voice changes (8.6%). This suggests that it is probably still safe to perform RFA on surgical beds, especially when careful attention is paid to the recurrent laryngeal nerve along with the provision of additional protective procedures to minimize recurrent laryngeal nerve injury (e.g. hydrodissection, pulled-away method, and cold distilled water injection after RFA).
There are a few possible reasons for the higher incidence of overall complications in the repeat surgery group. Presumably, the overall complications were minimized by accurately targeting lesions using the real-time US-guided RFA approach, which was followed by conducting the several additional protective procedures described above. In addition, patients who underwent repeat surgery likely had more adhesions and structural distortions, which might have led to a higher number of complications than that observed in those treated with RFA. Finally, the difference in complication rates may be attributable to the operator-dependent nature of RFA and repeat surgeries, and therefore, varying outcomes in complications are conceivable.
It is interesting to note that a significantly higher proportion of patients in the RFA group had radical neck dissections as their initial surgical treatment. However, patients in the repeat surgery group had more central neck dissections initially. We assumed that patients who were initially treated with radical neck dissections may opt for a non-surgical alternative, such as RFA, while those with previous central neck dissections may tolerate more extensive repeat surgeries, especially if they have recurrence in lateral neck compartments. In fact, based on our initial results, a higher proportion of patients in the repeat surgery group had recurrences in lateral neck compartments (56.8 versus 33.33%); however, this difference was non-significant after adjusting with propensity score matching.
There was only one local recurrence after RFA, which was confirmed by FNAB only 2 months after RFA. During >5 years of follow-up, the disappearance of lesions was confirmed by US images. However, our definition of recurrence was strictly based on pathologic confirmation, and therefore, this case was regarded as a case of local recurrence. In addition, there were 11 regional recurrences observed in the RFA group. The aim of this study (i.e. evaluation of treatment efficacy) was based on the incidences of local recurrence, and we assumed that regional recurrences at sites that were different from those of initial therapy could occur due to other confounding factors not related to RFA.
The strength of our study lies in its large cohort of 221 patients with a mean follow-up period of 6 years (range: 1–10 years). To minimize selection bias, 81 patients were trimmed via propensity score matching, and the remaining cohort of 140 patients was analyzed, overcoming the limitations of previous studies [Citation4,Citation21–24,Citation36]. The results of this study further validated the role of RFA as a non-surgical alternative to repeat surgery. There are a few other minimally invasive image-guided thermal ablation techniques for treating recurrent thyroid cancers, such as laser and microwave ablation. Laser ablation has been shown to yield good therapeutic results for both recurrences and incidental papillary thyroid microcarcinoma [Citation10,Citation37]. Similarly, microwave ablation showed promise for treating cervical metastatic lymph nodes from papillary thyroid carcinomas [Citation12]. Currently, RFA is used as the sole non-surgical alternative in our institution, and the possibility of employing other techniques remains. Finally, RFA has been reported to provide better quality of life than open surgery for benign thyroid nodules [Citation38,Citation39]; future studies on assessing quality of life in recurrent thyroid cancer patients treated with various minimally invasive techniques would be needed.
There are a few limitations to this study. In addition to the inherent bias accompanied with the retrospective nature of our patient cohort, the outcomes of both RFA and surgery are operator-dependent. Only one radiologist and seven different surgeons performed all procedures and operations at a single center. These factors could have added selection bias to the results. Further studies conducted by multiple radiologists and surgeons in a multicenter setting could overcome such limitations.
Conclusion
In conclusion, the effectiveness of RFA for controlling locally recurrent thyroid cancers was similar to that of repeat surgery, with RFA resulting in fewer overall complications. RFA could be an effective and safe alternative to repeat surgery for treating patients with less than three recurrences of thyroid cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lin Y, Li T, Liang J, et al. Predictive value of preablation stimulated thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio in differentiated thyroid cancer. Clin Nucl Med. 2011;36:1102–1105.
- Morris LG, Sikora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–891.
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. Am J Roentgenol. 2011;197:W331–W336.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–573.
- Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19:1373–1380.
- Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metabol. 1992;75:714–720.
- American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39:1023–1030.
- Mauri G, Cova L, Tondolo T, et al. Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metabol. 2013;98:E1203–E1207.
- Teng D, Ding L, Wang Y, et al. Safety and efficiency of ultrasound-guided low power microwave ablation in the treatment of cervical metastatic lymph node from papillary thyroid carcinoma: a mean of 32 months follow-up study. Endocrine. 2018;62:648–654.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26:420–428.
- Holmer C, Lehmann KS, Knappe V, et al. Bipolar radiofrequency ablation for nodular thyroid disease–ex vivo and in vivo evaluation of a dose-response relationship. J Surg Res. 2011;169:234–240.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hypertherm. 2016;15:1–5.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:11–22.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049.
- Li XL, Xu HX, Lu F, et al. Treatment efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation for benign thyroid nodules. BJR. 2016;89:20150858.
- Kim JH, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33:212–219.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10:C144–C149.
- Lee SJ, Jung SL, Kim BS, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol. 2014;15:817–826.
- Guenette JP, Monchik JM, Dupuy DE. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Intervent Radiol. 2013;24:672–679.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276:909–918.
- Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244:296–304.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125.
- Shin JE, Baek JH, Lee JH. Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: current status and challenges. Curr Opin Oncol. 2013;25:14–19.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Goldberg SN, Charboneau JW, Dodd GD, 3rd, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345.
- Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update: supplement to the consensus document. J Vasc Intervent Radiol. 2014;25:1706–1708.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:920–930.
- Park KW, Shin JH, Han BK, et al. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol. 2011;18:2564–2568.
- Kim MK, Mandel SH, Baloch Z, et al. Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1214–1216.
- Dupuy DE, Monchik JM, Decrea C, et al. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001;130:971–977.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25:163–170.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Valcavi R, Tsamatropoulos P. Health-related quality of life after percutaneous radiofrequency ablation of cold, solid, benign thyroid nodules: a 2-year follow-up study in 40 patients. Endocr Practice. 2015;21:887–896.
- Yue WW, Wang SR, Li XL, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Scientif Reports. 2016;6:37838.