Abstract
Purpose: Desmoid tumors are benign, locally aggressive soft tissue tumors derived from fibroblasts. Magnetic resonance-guided focused ultrasound (MRgFUS) is a safe and effective treatment for desmoid tumors. The purpose of this study was to retrospectively review the MRgFUS treatments of desmoid tumors at our institution to determine which technical treatment parameters contributed most significantly to the accumulation of thermal dose.
Materials and methods: The study protocol was approved by the local IRB. We retrospectively reviewed data from MRgFUS treatments performed in histologically-confirmed desmoid tumors, over a period of 18 months. Sonication parameter means were compared with ANOVA. Mixed effects and linear regression models were used to evaluate the relative contribution of different parameters to thermal dose volume.
Results: Nine-hundred thirty-six sonications were reviewed in 13 treatments. Accumulated dose per sonication was greatest for elongated sonications (0.96 cc ± 0.90) compared to short (0.88 ± 0.93 cc) and nominal (0.55 ± 0.70 cc) sonications, p < .001. 65.2% of short sonications resulted in high percentage ablations, compared to 46.0% of nominal and 35.1% of elongated sonications. Standardized beta coefficients (anticipated increased volume in cc per unit) for power, duration, energy and average temperature were 0.006, 0.057, 0.00035 and 0.03, p < .001. Regarding dose efficacy, dose area contributed the greatest to this variability – 50.7% (45.5–54.8%), followed by distance – 16.6% (12.9–20.0%).
Conclusions: A variety of sonication parameters significantly contributed to thermal ablation volume following MRgFUS of desmoid tumors, in reproducible patterns. This work can serve as the basis for future models working toward improved planning for MRgFUS treatments.
Introduction
Desmoid tumors are benign, but locally aggressive monoclonal proliferations of fibroblasts arising in muscle and connective tissues. These tumors most frequently occur sporadically in association with mutations of the beta-catenin gene but a minority are associated with hereditary cancer syndromes, such as familial adenomatous polyposis. These tumors can be variably symptomatic as a function of their local mass effect and may grow, regress or stabilize in the natural course of patients with the disease.
Conventional treatments for these tumors include close observation, surgery, radiation and systemic medical therapy. However, surgery, even with negative margins, can have recurrence rates as high as 50% and multiple recurrences following surgery can result in significant local morbidity [Citation1]. Radiation results in tumor control in ∼72–76% of adults but is associated with pain, reduced motion, pathological fractures and secondary malignancies [Citation2]. Systemic medical therapies such as tamoxifen, nonsteroidal anti-inflammatory drugs and cytotoxic chemotherapy demonstrate variable efficacy. More recently, cryoablation has been used successfully to treat patients with these tumors, however, this may not be appropriate for particularly large tumors [Citation3].
Magnetic resonance-guided focused ultrasound (MRgFUS) has emerged as a safe and effective treatment for control of desmoid tumors [Citation4–6]. In the United States, this technology was first developed and FDA-approved for treatment of uterine fibroids and given the tissue similarities between uterine fibroids and desmoid tumors, early investigators hypothesized that MRgFUS would also prove for the latter. While this has largely proven to be true, there is an incomplete understanding of MRgFUS technical parameters during thermal ablation of desmoid tumors, as the technology were initially developed for treatment of uterine fibroids. Some MRgFUS treatments of desmoid tumors result in significant non-perfused volumes, while others only result in partial ablation of a smaller percentage of the mass (). The purpose of this study was to retrospectively review MRgFUS treatments of desmoid tumors at our institution to determine which technical treatment parameters contributed most significantly to accumulation of ablative thermal dose per sonication.
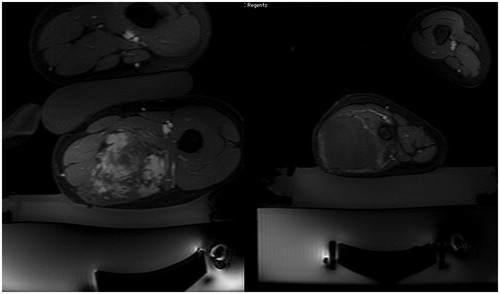
Figure 1. (A) Axial post-contrast fat saturated images following MRgFUS of desmoid tumors of the thigh in two separate patients. In the image on the left, there is persistent patchy enhancement following the treatment, consistent with a residual viable tumor. (B) In contrast, the image on the right from a different patient demonstrates only a thin peripheral rim of enhancement, compatible with a successful large volume ablation (>95%).
Materials and methods
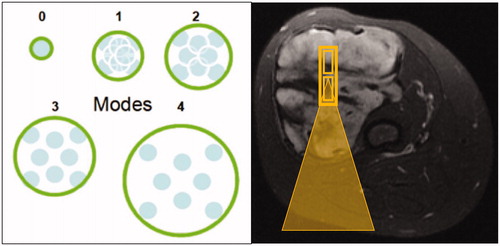
The local Institutional Review Board approved the study. We retrospectively reviewed sonication data from all MRgFUS treatments performed at our medical center on the Insightec Exablate device (Haifa, Israel) in histologically confirmed desmoid tumors over a period of 18 months (December 2014 through July 2016). Sonication parameters reviewed included transducer roll, pitch, power, duration, energy, average temperature, distance (transducer to target), sonication acoustic type (nominal, short, elongated). Nominal sonications () represent either (1) eight sub-sonications centered around a single position orthogonal to the direction of the beam trajectory or (2) a single sub-sonication. Short sonications are similar to the single sub-sonication of nominal sonications but may have variable energy depending on exact positioning. Elongated sonications are defined as ‘stacking’ of multiple sub-sonications along the long axis of the direction of the ultrasound beam.
Figure 2. (A) The schematic on the left demonstrates the orientation of sub-sonications during a nominal sonication, with sub-sonications centered around a single point perpendicular to the direction of the sound beam, at varying degrees of spacing (0: least spacing, 4: most spacing). (B) In contrast, the axial T2-fat saturated image on the right demonstrates stacking of multiple sub-sonications (small yellow rectangles) along the direction of the beam path, which is used for elongated sonications. Beam path trajectory showed by the yellow triangle.
The primary outcome assessed was the volume (in cc) of ablated tissue per sonication, which was automatically generated by the Exablate software based on the standard MRI thermometry proton resonance frequency shift sequence (multiphase multislice fast spoiled gradient echo: FOV/slice thickness/TR/TE/flip angle/echo train length = 28 × 28 cm/4 mm/210 ms/18.3 ms/35/12) which is run concurrently with each sonication. This thermometry method calculates the change in temperature per sonication and thus provides an estimate of the temperatures obtained, assuming normal body temperature. The Exablate software combines this temperature information with the length of time at a given temperature in order to calculate an estimate of the volume of tissue ablated with each sonication, based on standard time-dose modeling, while accounting for imaging artifacts [Citation7]. In addition to the primary outcome, we also qualitatively graded the amount of tissue ablated as a percentage of the target sonication volume: an ablation volume of 0–25% of the target was considered grade 1, 25–50% was grade 2, 50–75% was grade 3, and 75–100% was grade 4. Grade 3 or 4 sonications were termed ‘high efficacy’ sonications.
Statistical analysis
Mean values and standard deviations of sonication parameters and percentages were calculated across sonication types. Means were compared with one-way ANOVA and Chi-square analyses were used to compare percentages. Mixed effects models were then used to estimate the association between each technical parameter and accumulation of thermal dose (adjusting for within-patient correlation across both repeated treatments on the same patient and repeated sonications). Linear regression models were used to estimate the relative contribution of each of the parameters to the total variance explained by all parameters jointly. p values <.05 were considered statistically significant.
Results
Thirteen MRgFUS treatments of desmoid tumors were performed at our institution in seven patients over the 18 months from December 2014 through July 2016, with 936 total sonications. Average sonications per treatment were 73 ± 40 with a range of 6–140. provides an overview of sonication data organized by the type of sonication (short, nominal or elongated). Average sonication energy was greatest for elongated sonications (3311 J ± 1206 J), compared to short (1826 J ± 641 J) and nominal (1608 ± 568 J) sonications, p < .001. The amount of accumulated dose volume per sonication was greatest for elongated sonications (0.96 ± 0.90 cc) followed by short (0.88 ± 0.93 cc) and nominal (0.55 ± 0.71 cc) sonications, p < .001. Short sonications had the highest percentage of high efficacy sonications (65.2%) compared to nominal (46.0%) or elongated (35.1%), p < .001.
Table 1. Overview of sonication parameter data sorted by sonication type.
Linear mixed effects models provided beta coefficients which represent an estimate of the additional amount of thermal ablation volume accumulated, for each one unit increase in the value of a given technical parameter. These models demonstrated significant associations (p < .001) across a wide range of technical parameters with the following beta coefficients including: duration (0.057), power (0.006), energy (0.00035), average temperature (0.03) and dose area (0.0068).
Linear regression models demonstrated that all variables evaluated together explained ∼44.3% of inter-treatment variability in accumulated dose volume. Dose area contributed most significantly to this variability, accounting for 52% (95% CI: 40–64%), followed by spot energy – 14.1% (95% CI: 7.3–21.4%), and roll – 10.8% (4.1–18.6%). Regarding the presence of a high efficacy sonication or not, the technical parameters explained 70.7% of the witnessed variability. Dose area again contributed the greatest to this variability – 50.7% (45.5–54.8%), followed by distance between the transducer and target – 16.6% (12.9–20.0%) and the specific treatment – 9.2% (7.0–11.6%) ().
Table 2. Variability in thermal dose with regard to sonication technical parameters, calculated by linear and mixed effects models.
Discussion
MRgFUS has been demonstrated to be a highly effective and safe approach for the treatment of desmoid tumors. MRgFUS devices originally designed to ablate uterine fibroids have proven to be reliable treatment options for patients with desmoid tumors in recent years. Similar to uterine fibroids, these soft tissue tumors are derived from fibroblasts and readily absorb focused sound energy. However, one of the main current limitations of MRgFUS of desmoid tumors is that it is difficult to know the ideal technical parameters for a treatment before sonications are initiated. Over the course of a series of dose verification steps at the beginning of the treatment and additional MR thermometry feedback from the first prescribed sonications in the treatment plan, the treating clinician is able to make adjustments to the power, duration, shape and angle of sonications, as well as other features of the overall sonication scheme. As these feedback systems were originally developed for ablation of uterine fibroids within the pelvis, we hope that the preliminary data in our study can lead to future studies that will allow us to increase our confidence in optimal and specific treatment plans for desmoid tumors prior to initiation of treatment. Development of this type of improved treatment planning would facilitate better treatment efficiency, allowing clinicians to decrease the risk of either undertreatment or overtreatment. Overtreatment, in particular, can dramatically increase the risk of skin burns, the most common complication during MRgFUS of desmoid tumors of the extremities.
Our results demonstrate the effect of changes of specific technical parameters during MRgFUS of desmoid tumors on the size of the ablation volume as well as the amount of variability in treatment outcome attributable to these parameters based on our cohort of patients. Of note, the beta coefficients from our linear mixed effects models provide an estimate of how much additional tumor volume would be ablated for an increase in each respective technical parameter. For example, in our cohort, an increase in the energy level by 1000 J could be expected to increase the net ablation volume by 0.35 cc (0.00035 × 1000). While a number of these factors are clearly inter-dependent, for example, the beta coefficient for energy is approximately the product of those calculated for power and duration, as one would expect, these coefficients provide some sense of the impact of manipulating these parameters during MRgFUS of desmoid tumors. This is the first study demonstrating this type of relationship between different technical settings and ablation volume during MRgFUS ablation of soft tissue tumors.
One particularly interesting result in our study was that elongated sonications, which on average require the most energy (3311 ± 1206 J) only resulted in a 9% increased ablation volume (0.96 ± 0.90 cc) compared to short sonications (0.88 ± 0.93 cc) which used ∼55% of the energy required on average by elongated sonications. This effect may be related to the narrow orientation of elongated sonications, which makes them less energy efficient (more prone to heat loss), than more compact sonication shapes. As the risk of skin burn and injury to adjacent critical structures rises rapidly with the amount of energy used per sonication, this result suggests that short sonications may offer a better combination of sonication energy and ablation volume, albeit while extending the overall duration of treatment. However, further studies are needed to better understand this result.
Our study had several limitations. While we reviewed a large number of sonications, these were performed across 13 treatments in seven patients, so both treatment specific conditions and patient-specific characteristics may partially bias our results. We have assumed that desmoid tumors across patients have similar acoustic characteristics but these may vary somewhat from treatment to treatment. Regarding the results in terms of elongated sonications versus the other two sonication types, we should note that the elongated sonications may be more susceptible to thermometry error: because they are so narrow, some of the accumulated thermal doses may end up off slice if the imaging slice is not adequately aligned. Additionally, our main outcomes relied on the MR thermometry feedback provided during each treatment which can be affected by a range of imaging artifacts and may perform differently based on tumor tissue content (e.g., T2 hyperintense versus T2 hypointense desmoid tumors). MR thermometry is at best an estimate of the amount of direct thermal ablation per sonication and may be affected by. Finally, we did not account for additional sonication parameters such as the number of elements used or electronic steering. Overall, larger studies are needed to further improve the accuracy of our understanding of the effects of these technical parameters and their contribution to variability.
Conclusions
In conclusion, a variety of technical parameters contribute to ablation size during MRgFUS of desmoid tumors, along with the variability of the treatment result. Short sonications may offer a better combination of sonication energy and ablation volume, albeit while extending the overall duration of treatment. Further large cohort studies are needed to improve the efficiency of treatment planning for MRgFUS of desmoid tumors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Melis M, Zager JS, Sondak VK. Multimodality management of desmoid tumors: how important is a negative surgical margin? J Surg Oncol. 2008;98:594–602.
- Rutenberg MS, Indelicato DJ, Knapik JA, et al. External‐beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer. 2011;57:435–442.
- Schmitz JJ, Schmit GD, Atwell TD, et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10-year experience. AJR Am J Roentgenol. 2016;207(1):190–195.
- Ghanouni P, Dobrotwir A, Bazzocchi A, et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol. 2017;27(2):732–740.
- Avedian RS, Bitton R, Gold G, et al. Is MR-guided high-intensity focused ultrasound a feasible treatment modality for desmoid tumors? Clin Orthop Relat Res. 2016;474:697–704.
- Bucknor MD, Rieke V. MRgFUS for desmoid tumors within the thigh: early clinical experiences. J Ther Ultrasound. 2017;5:4.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Thermal dose requirement for tissue effect: experimental and clinical findings. Proceedings of SPIE International Society for Optical Engineering. 2003;4954:37.
