Abstract
Background: Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) has been used to treat various peritoneal malignancies. Cisplatin and mitomycin C (MMC) are agents commonly used in these procedures and, individually, each has been associated with acute kidney injury (AKI). There is limited literature on the complications associated with the use of both agents in HIPEC. Therefore, we sought to determine the incidence of nephrotoxicity and electrolyte abnormalities in patients undergoing laparoscopic HIPEC using this chemotherapeutic combination.
Methods: We retrospectively evaluated patients undergoing laparoscopic HIPEC for gastric or gastroesophageal adenocarcinoma using both cisplatin and MMC. Sodium thiosulfate was given for renal protection and kidney function was evaluated daily up to postoperative day #2. Details regarding patient characteristics, selection criteria, chemotherapeutic regimen, perioperative lab values and anesthetic management were collected.
Results: Twenty-three patients underwent 31 laparoscopic HIPEC procedures. Fifteen (65%) were male and the median age was 57 (range 21–75). Thirteen procedures were associated with an elevation in creatinine (Cr) with the median difference between POD#2 and baseline being 0.09 mg/dL (range 0–0.43). The glomerular filtration rate median difference between POD#2 and baseline was −17 mL/min/1.37 sq. m (range −42 to 11). No cases demonstrated AKI, defined as a 50% increase in Cr levels above baseline. An 84% incidence of postoperative hypophosphatemia (26/31) and 94% incidence of postoperative hypocalcemia (29/31) was observed.
Conclusion: The laparoscopic approach to HIPEC using both cisplatin and MMC in our cohort was not associated with an increased incidence of AKI. The incidence of hypophosphatemia and hypocalcemia needs further evaluation to determine the exact etiology.
Precis’ statement: We retrospectively studied the association of AKI with the combined use of cisplatin and MMC in laparoscopic HIPEC.
Introduction
Hyperthermic intraperitoneal chemotherapy (HIPEC) along with cytoreductive surgery (CRS) has shown to improve survival in patients with peritoneal malignancies [Citation1]. The laparoscopic approach to this procedure has gained acceptance at limited centers worldwide and has been associated with less surgical trauma, fewer postoperative complications and reduced hospital stay [Citation2,Citation3]. Laparoscopic HIPEC has been utilized for various primary malignancies including pancreatic, gastroesophageal, colorectal, ovarian, appendiceal, lobular breast carcinomas, as well as melanomas and peritoneal mesotheliomas. Literature is sparse when it comes to dual agent intraperitoneal chemotherapy, particularly with cisplatin and mitomycin C (MMC) and especially its use in laparoscopic HIPEC.
Platinum analogs and MMC are commonly used agents in HIPEC and have been associated with potential complications; acute kidney injury (AKI) is a commonly reported complication that has been associated more so with cisplatin (reported incidence: 3.7–36%) versus MMC (5.6%) [Citation4,Citation5]. In particular, perioperative AKI has been shown to increase morbidity and mortality [Citation6]. It is associated with an increased risk of postoperative sepsis, coagulopathy, and the potential need for mechanical ventilation [Citation7]. Various electrolyte abnormalities, most commonly hypomagnesemia, as well as hypocalcemia, have been associated with nephrotoxicity secondary to systemic or intraperitoneal chemotherapy using cisplatin [Citation8–11].
We have recently begun a program investigating laparoscopic HIPEC for patients with gastric cancer metastatic to the peritoneum, including a recently completed Phase II clinical trial [Citation12,Citation13]. Laparoscopic HIPEC is used after chemotherapy and restaging with stable/improved disease and no extra-peritoneal disease or as a palliative effort for intractable ascites [Citation14]. Although the treatment algorithm is actively being revised with each trial that is completed, this novel program involving laparoscopic HIPEC without cytoreduction, represents an opportunity to identify the effects of heated chemotherapy without the influence of surgical debulking.
After encountering a high incidence of postoperative hypocalcemia and hypophosphatemia in patients who had undergone laparoscopic HIPEC with both cisplatin and MMC at our institution and considering the growing utilization of the laparoscopic approach worldwide, we sought to describe our institution’s experience with this procedure. We focused on the incidence and potential factors contributing to AKI. We hypothesized that the combination of these agents was having a compounding nephrotoxic effect potentially leading to these electrolyte derangements.
Methods
After institutional review board approval (Protocol #14-1087), we retrospectively evaluated 31 laparoscopic HIPEC cases involving dual agent chemotherapy with cisplatin 200 mg and MMC 30 mg that were performed at our institution by the same surgeon between September 2016 and October 2017. For this study, we included adult patients with a diagnosis of gastric or gastroesophageal adenocarcinoma with stage IV disease based on positive peritoneal cytology or carcinomatosis. Patients did not undergo laparoscopic cytoreduction although multiple biopsies were often performed prior to HIPEC administration. We excluded patients who had evidence of metastatic disease outside of the peritoneum, such as to the liver, lungs, or bone.
Data collection
Patient demographics including age, gender, American Society of Anesthesiologists (ASA) physical status, body mass index (BMI) and percent body surface area (BSA) were gathered from the patient’s electronic medical record. Perioperative creatinine (Cr) levels, glomerular filtration rate (GFR) [based on the Modification of Diet in Renal Disease Study Equation] and hemoglobin (HGB) were collected from the clinic visit prior to surgery (baseline) up to postoperative day #2 (as most patients were discharged home on POD #2). Data consisting of intraoperative factors such as estimated blood loss, need for transfusion, urine output, and surgical/anesthesia duration was also collected.
Laparoscopic HIPEC technique
Criteria for consideration for laparoscopic HIPEC included Eastern Cooperative Oncology Group performance status ≤ 2, leukocyte count ≥ 3000/uL, absolute neutrophil count ≥ 1500/uL, platelet count ≥ 100,000/uL, serum creatinine ≤ 1.5 mg/dL, and aspartate transaminase and alanine transaminase levels ≤ 5 times the institutional upper limit of normal. Previous chemotherapy or chemoradiotherapy was permitted, with a minimum of 3 weeks without treatment prior to laparoscopic HIPEC. All patients underwent general anesthesia. After a preoperative bolus of 1 L 0.9% normal saline, a diagnostic laparoscopy was performed at the beginning of the HIPEC procedure. Working ports were placed in bilateral mid-abdominal quadrants. Peritoneal washings were obtained for cytology, and biopsies were obtained of any suspicious lesions. Once the diagnostic portion of the procedure was concluded, the bilateral ports were exchanged for inflow and outflow catheters placed under laparoscopic visualization. Crystalloid perfusate was then circulated using an extracorporeal circulation device at a flow rate of 700–1500 mL/min. Once the outflow temperature of the perfusate was greater than 39 °C, fixed doses of MMC (30 mg) and cisplatin (200 mg) were instilled. To limit the systemic toxicity of cisplatin, a loading dose of sodium thiosulfate (STS; 7.5g/m2, up to a maximum of 12.5 g) was infused over 20 min prior to the addition of cisplatin to the peritoneal perfusion circuit. This was immediately followed by a maintenance intravenous infusion of STS [25.56 g/m2] over the next 12 h. The target inflow temperature was 41–42 °C, and the target outflow temperature was 39–40 °C. The abdomen was constantly manipulated for 60 min to assure even distribution of the perfusate. The target urinary output was above 0.5 mL/kg/h. Abdominal washout was then performed with 3 L of crystalloid solution. Pneumoperitoneum was reestablished, residual perfusate was removed, the abdomen was inspected, and the outflow cannulas were removed under direct vision.
Endpoints
AKI was defined as an increase in Cr ≥50% above baseline Cr levels per the NCI-CTCAE criteria (version 5.0). Our laboratory normal reference range for calcium and phosphorus levels was 8.4–10.2 mg/dL and 2.5–4.5 mg/dL, respectively.
Statistical analysis
Descriptive statistical analysis was used to summarize clinical data. The mean, standard deviation, median and range were used to summarize continuous covariates. Frequencies and percentages were used to summarize categorical covariates. A summary of Cr, GFR and HGB was provided longitudinally to assess mean response trajectories over time. Changes between baseline and select postoperative days of interest were assessed using the Wilcoxon signed-rank test. A p value <0.05 was considered statistically significant.
Results
Twenty-three different patients consisting of 8 females and 15 males underwent a total of 31 laparoscopic HIPEC procedures. Five patients had one repeat surgery while one patient had three repeat procedures using the same dual chemotherapeutic regimen. The median age of our patient population was 57 years old (range: 21–75). An ASA 3 status was given to the patient in 30/31 cases. The median BMI and BSA, at first surgery, were 24.8 kg/m2 (range: 20.3–36.1) and 1.9 m2 (range: 1.4–2.2), respectively. A summary of patient characteristics is provided in .
Table 1. Summary of patient characteristics.
The mean dose of cisplatin was 106.6 ± 10.9 mg/%BSA and the mean dose of MMC was 16 ± 1.6 mg/%BSA. The duration of chemotherapy instillation at goal inflow/outflow temperature was 60 min for all cases. The median surgery time was 150 min (range: 123–182) and the median anesthesia time was 209 min (range: 186–238).
The average estimated blood loss was 27.5 mL ± 22.15. For five cases, no blood loss was documented. No blood products were ever transfused for any of the cases. The median intraoperative urine output was 325 mL (range: 140–1985), resulting in an overall average of 2.15 mL/kg/h. The amounts of fluids administered along with the number of hypotensive events are mentioned in . The average amount of crystalloids administered was 1603 mL and most (27/31) patient cases did not receive any colloids. Of the 31 cases leading to a total of 4219 blood pressure measurements, patients had as few as 54 measurements and as many as 207. Zero episodes of hypotensive events (mean arterial pressure < 55 mmHg) occurred for the majority of patients (n = 18; 58.1%). Only 3.2% (n = 1) experienced, a single hypotensive event, followed by 19.4% (n = 6) experiencing two events, with the remaining six patients experiencing three or more events. None of the patients experienced more than 10 hypotensive events. Assuming any single episode corresponds to 1 min of hypotension, the average time a patient experienced hypotension during a case was approximately 1.4 min (SD = 2.4; Median = 0; Range = 0–10).
Table 2. Summary of clinical characteristics.
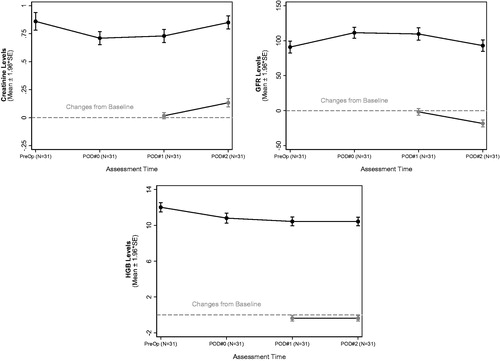
While 13 cases were associated with an elevation in Cr, 18 procedures were associated with an improvement (decrease) in postoperative Cr levels. shows the median difference between POD#2 and baseline for Cr was 0.09 mg/dL (range 0–0.43) and the median difference between POD#2 and baseline for GFR was −17 mL/min/1.37 sq. m (range −42 to 11). The median drop in HGB was 0.2 g/dL (range −2.2 to 1.1). shows the trend in each of these values from baseline to POD#2. Not a single case demonstrated AKI. Additionally, the incidence of postoperative hypocalcemia, hypophosphatemia, and hypomagnesemia was found to be 94%, 84% and 9.7% respectively ().
Table 3. Longitudinal summary of Creatinine, GFR and HGB.
Table 4. Assessments of calcium, phosphate and magnesium.
Discussion
Various mechanisms have been proposed as culprits for AKI including ischemic-reperfusion injury due to intraoperative hypotension, fluid shifts, direct effects of nephrotoxic drugs and inflammation [Citation15,Citation16]. Patient factors such as age, baseline estimated GFR, preoperative albumin, anemia, intraoperative blood loss and hypotension have also been described as being associated with AKI and reported incidences of AKI amongst various studies range from 2% to 22% [Citation17–21]. Using the NCI-CTCAE criteria to define AKI we found no patients developing this complication.
There are various considerations regarding our findings. First, laparoscopic HIPEC is currently considered at our institution for patients with gastric cancer and metastatic disease limited to the peritoneum, most often as part of a clinical trial. As the peritoneum is the most common site of metastatic disease at diagnosis, and also the most common site of recurrence after potentially curative surgery, HIPEC may represent a novel treatment strategy to improve the survival of patients with advanced gastric cancer and therefore worthy of study in clinical trials and retrospective reports [Citation22,Citation23]. Due to the well-documented significant rate of morbidity and mortality with cytoreduction and gastrectomy, we sought to initiate our studies in patients with relatively low burdens of peritoneal disease until the benefit of HIPEC in gastric cancer is clearer. Therefore, we can speculate that our patient population might include a group of patients with relatively low preoperative risk to develop AKI due to disease characteristics and clinical trial inclusion criteria.
Second, multiple strategies to mitigate the incidence of perioperative AKI have been proposed. Intraoperative goal-directed fluid management and initiating hemodynamic monitoring in the pre/intraoperative period have been shown in studies to reduce the incidence of AKI, but optimal endpoints are unknown [Citation24]. In our patients, typically less than 2 L of intraoperative fluid was administered. Preoperative initiation of a normal saline bolus and intra/postoperative STS were utilized for each patient as part of our AKI mitigation strategy. STS is a neutralizing agent for cisplatin that protects against renal damage and has been shown to be renoprotective when administered concurrently with cisplatin [Citation25].
Furthermore, it is worth considering that our cases did not involve CRS. Therefore, the procedures were of a relatively shorter duration, had minimal blood loss, no needed blood transfusions, and subsequently fewer fluid shifts. This helps support the idea that AKI is due not only to the chemotherapeutic agent itself, but also the extent of the procedure.
Another important finding of our work is the high incidence of hypophosphatemia and hypocalcemia observed postoperatively. Although hypocalcemia has been associated with the nephrotoxicity seen with systemic and intraperitoneal chemotherapy using cisplatin, hypophosphatemia is not a typical finding seen with its use, with only one case report linking the two [Citation26]. Considering that these electrolyte abnormalities were not evident on preoperative laboratory assessments, an association could be made between the postoperative values and some aspect about the procedure itself. A proposed etiology could be sub-clinical renal insult or a specific reaction to the combination of the cisplatin and MMC [Citation27]. Magnesium deficiency, which can significantly influence the serum levels of other electrolytes such as potassium, calcium, and phosphate, has been associated with 40–90% of patients being treated with cisplatin [Citation28]. However, it was only observed in 9.7% of our cases.
Clinical manifestations of hypocalcemia such as fatigue, tetany, seizures and myocardial dysfunction were not observed in the postoperative period prior to repletion [Citation29]. Phosphate depletion can also affect many different organ systems secondary to increased affinity of HGB for oxygen and decreased intracellular adenosine triphosphate. Since signs and symptoms of these electrolyte derangements can potentially be masked by the sedative effects of a recent anesthetic, it is important to be able to anticipate and preemptively correct these abnormalities as needed.
Limitations of our study include its retrospective nature and the small number of patients in our cohort. However, it is a significant finding that there was no observable incidence of AKI with the use of these potentially nephrotoxic drugs. The criteria for AKI can also influence the incidence, as some might underestimate subtle changes in renal function. Additionally, ionized (free) calcium was not always obtained perioperatively, to verify the low serum calcium levels that were found in the majority of cases. Therefore, a low protein state such as hypoalbuminemia could have potentially contributed to this observed derangement. Although we only found one case where the patient had hypoalbuminemia preoperatively, albumin levels were not measured postoperatively. Obtaining urinary levels of calcium and phosphorus to check for fractional excretion could potentially help decipher if these ions are lower due to a renal wasting phenomenon [Citation30].
In conclusion, we did not encounter any episode of AKI in our cohort of patients treated with cisplatin and MMC. Certain unknown risks of this combined drug regimen might exist, which could include the potential electrolyte derangements we observed. Future studies are needed to corroborate these findings and to investigate the clinical outcomes associated with dual agent chemotherapy in laparoscopic HIPEC procedures.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Huang C-Q, Min Y, Wang S-Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–55683.
- Passot G, Bakrin N, Isaac S, et al. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur J Surg Oncol. 2014;40:957–962.
- Esquivel J, Averbach A, Chua TC. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with limited peritoneal surface malignancies. Ann Surg. 2011;253:764–768.
- Tan GHC, Shannon NB, Chia CS, et al. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Hyperthermia. 2018;34:595–600.
- Ye J, Ren Y, Wei Z, et al. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: a retrospective cohort study. Surg Oncol. 2018;27:456–461.
- Park JT. Postoperative acute kidney injury. Korean J Anesthesiol. 2017;70:258–266.
- Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858.
- Baradaran A, Tavafi M, Ardalan MR, et al. Cisplatin; nephrotoxicity and beyond. Ann Res Antioxid. 2016;1:e014.
- Somashekhar SP, Prasanna G, Jaka R, et al. Hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: a single institution Indian experience. Natl Med J India. 2016;29:262–266.
- Hakeam HA, Breakiet M, Azzam A, et al. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail. 2014;36:1486–1491.
- Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev. 1999;25:47–58.
- Badgwell B, Blum M, Das P, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24:3338–3344.
- Badgwell B, Blum M, Das P, et al. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg Endosc. 2018;32:512.
- Newhook TE, Agnes A, Blum M, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy is safe for patients with peritoneal metastases from gastric cancer and may lead to gastrectomy. Ann Surg Oncol. 2019.
- Agarwal A, Dong Z, Harris R, et al. Cellular and molecular mechanisms of AKI. J Am Soc Nephrol. 2016;27:1288–1299.
- Hallqvist L, Granath F, Huldt E, et al. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2018;35:273–279.
- Cata JP, Zavala AM, Van Meter A, et al. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34:538–544.
- Kusamura S, Baratti D, Younan R, et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14:2550–2558.
- Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–869.
- Bouhadjari N, Gabato W, Calabrese D, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin: amifostine prevents acute severe renal impairment. Eur J Surg Oncol. 2016;42:219–223.
- Saxena A, Valle SJ, Liauw W, et al. Limited synchronous hepatic resection does not compromise peri-operative outcomes or survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2017;115:417–424.
- Ikoma N, Chen HC, Wang X, et al. Patterns of initial recurrence in gastric adenocarcinoma in the era of preoperative therapy. Ann Surg Oncol. 2017;24:2679.
- Ikoma N, Blum M, Chiang YJ, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23:4332.
- Hobson C, Singhania G, Bihorac A. Acute kidney injury in the surgical patient. Crit Care Clin. 2015;31:705–723.
- Pfeifle CE, Howell SB, Felthouse RD, et al. High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol. 1985;3:237–244.
- Elazzazy S, El-Geed HA, Al Yafei S. Severe hypophosphatemia induced after first cycle of the ESHAP protocol for Hodgkin's lymphoma: a case report. Int Med Case Rep J. 2013;6:1–5.
- Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012; 16:313.
- Oronsky B, Caroen S, Oronsky A, et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations, and management. Cancer Chemother Pharmacol. 2017;80:895–907.
- Goltzman D. Clinical manifestations of hypocalcemia. In Mulder JA, editor. UpToDate. [Retrieved 2018 Sep 12]. Available from: https://www.uptodate.com/contents/clinical-manifestations-of-hypocalcemia
- Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10:1257–1272.