Abstract
Purpose: Desmoids are locally infiltrative, nonmalignant soft tissue tumors. Surgery, radiation therapy, and chemotherapy have been the mainstay of treatment, but relapse is common and side effects can result in significant morbidity. MR-HIFU is increasingly recognized as an alternative treatment modality. We assessed the success rate of MR-HIFU for the treatment of extra-abdominal desmoids at our institute.
Materials and methods: Five patients with relapsing desmoid tumors (three males, two females; age range 40–79 years) were treated using the Sonalleve system in an outpatient setting without general anesthesia. Changes in total tumor volumes were measured with a tumor tracking software. Adverse events were documented.
Results: MR-HIFU was successful in all patients without severe side effects. Follow up ranged from 13 to 60 months. Three patients required more than one treatment session. In 3 patients with small lesions (mean = 9.7 mL), complete ablation was achieved with no evidence of viable tumor on follow up MRI at an average of 35.7 months, while in two patients with larger lesions (mean = 46 mL) the targeted tumor volumes decreased by 73% at 14 months. Skin injuries comprised first- and second-degree burns and were observed with short distance to skin (mean = 0.9 cm) and proximity to bone (i.e. ribs). Skin burns healed within weeks with topical treatment.
Conclusion: MR-HIFU shows good mid-term result for extra-abdominal desmoids with complete response for small lesions and stabilization of larger lesions. MR-HIFU for desmoids can be performed under regional anesthesia/sedation as outpatients.
Introduction
Desmoid tumors (also known as aggressive fibromatosis) are rare locally infiltrative soft tissue tumors arising from musculoaponeurotic tissues with no known potential for metastasis. Tumor-related destruction of vital structures leads to morbidity and in case of organ destruction to mortality. Although they can occur at any age, most desmoids arise in individuals between the age of 15 and 60 years [Citation1]. Based on location, desmoids are divided into extra-abdominal (trunk and extremities), also called superficial desmoids, and intra-abdominal tumors. Risk factors include prior trauma or surgery and pregnancy. In 5–15% of patients there is an association with familial adenomatous polyposis (FAP) called Gardner syndrome. These patients tend to develop abdominal desmoids (intra-abdominal or abdominal wall) [Citation2,Citation3]. With the increasing use of prophylactic colectomy, desmoids are now a more important cause of morbidity and mortality in this patient group [Citation4].
The treatment of desmoids, including surgery, radiation und medical treatments, has been historically frustrating because there was no single best option with definitive success. Multiple studies suggest that close observation is a viable option for asymptomatic patients if no vital structures are endangered [Citation5–7], therefore, a watch and wait strategy is initially advocated. However, treatment may be pursued in patients with progressive disease, symptoms, risk of involvement of adjacent structures or cosmetic concerns. Treatment options include surgery with or without radiation therapy and systemic medical therapy. Surgery carries a high risk of local recurrence even after complete resection with negative margins [Citation8,Citation9]. Radiation therapy in the long term carries risks of fibrosis and strictures, and of secondary malignancy, a particular concern in younger patients. In addition, radiation therapy seems to be less effective in children [Citation10].
Recently, local therapeutic options like percutaneous cryoablation, radiofrequency ablation, and high-intensity focused ultrasound (HIFU) have been described [Citation11–13].
MR-HIFU is increasingly being recognized as an alternative treatment modality for prostate cancer, uterine fibroids, bone tumors, breast tumors, and even neurological disorders of the brain [Citation14]. In addition, there have been applications for visceral organ tumors, e.g. the liver [Citation15].
In 2011, Wang et al. were the first to describe ultrasound-guided HIFU therapy as a treatment option for extra-abdominal desmoid tumors with good success [Citation16]. A few years later Avedian et al. introduced HIFU with MR-guidance and thermal mapping [Citation17]. In addition to other advantages, MR-guidance allows immediate acquisition of post-interventional images with contrast to assess residual viability of tumor and thus treatment success.
Interestingly, there are only few reports on this relatively novel treatment option of MR-HIFU in patients with desmoids. The purpose of this study was to assess the success rate of MR-HIFU for the treatment of extra-abdominal desmoid tumors at our institute.
Materials and methods
We retrospectively analyzed patients referred for MR-HIFU treatment at our institute between the years 2011 and 2017 with histopathologically proven desmoid tumor. In total, five patients with relapsing desmoid tumors (three males, two females; age range 40–79 years) were treated and followed. Patients had provided written informed consent to use their data for research purposes before treatment. Additional institutional review board approval was not required for retrospective studies up to five patients. Treatments were performed under analgosedation with propofol and regional or local anesthesia, based on tumor location and patient preference (see ). Procedures were performed by a single physician with 8 years of experience in MR-HIFU interventions. A Sonalleve MR-HIFU system with an in-.2-MHz transducer (Profound Medical Corp, Mississauga, Canada) was used with a 1.5-T MR system (Philips, Amsterdam, Netherlands). Procedure planning was typically done using a sagittal 3D T2w TSE Image (TR 1000 ms, TE 130 ms field of view AP 250 mm FH 250 mm RL 132 mm, bandwidth 832 Hz). The size and the number of the sonications were adjusted to the size of the desmoid tumors. The initial choice of sonication power was based on the tissue temperature response to a low power test sonication, and subsequently adjusted based on MR thermometry during treatment. Overall the following parameter were used: frequency 1.4 MHz, 40–140 W, and treatment or feedback cells sized between 4 mm and 16 mm, corresponding to volumes of 0.1–5.4 ml. A tissue peak temperature of 60 °C was targeted. The whole tumor was targeted in one session with no margins from the edge of the tumor. Post-contrast images after treatment were used to assess the non-perfused tumor areas and thus treatment effect (). Patients were all discharged home the same day with a prescription for NSAIDs.
Figure 1. (a) MRI (T1 FS SPIR sequence with contrast) of popliteal fossa before treatment. (b) Subtraction sequence with contrast directly post-HIFU. (c) Post-processing of lesion with tumor tracking tool. Note that due to (unfortunate) different angulation of MR-sequences pre- and post-contrast the bone is not in the same plane as the tumor in the pre-contrast sequence.
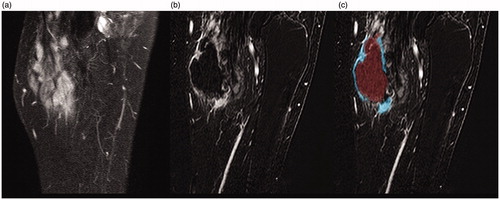
Table 1. Summary of patient data incl. prior treatments, effect of HIFU, and adverse effects.
Clinical and imaging follow-up was performed after 3 and 6 months and then every 12 months. Changes in total tumor volumes were measured with a tumor tracking software (IntelliSpace V8.0, Philips, Amsterdam, Netherlands) on post-contrast images. Patients with residual contrast enhancing desmoid tissue were considered for re-treatment. Adverse events were documented using the CTCAE system; additionally, burns were graded according to the American Burn Association (ABA) in first, second, third, and fourth degree.
Results
Two patients had desmoids located in the rectus abdominis muscle, one in the deltoid muscle, one in the intercostal muscles and one in the popliteal fossa (). All patients had received prior treatment (surgery or radiation therapy) with disease relapse. In the patient with a large desmoid in the popliteal fossa, the deeper part of the desmoid could not be treated by MR-HIFU because of encasement of neurovascular structures. Three patients showed contrast enhancing nodules during follow-up, indicating insufficient thermal ablation, and therefore required additional treatment sessions (within 2 months, 10 months, and 3 years, respectively; see ). The number of sonications ranged from 4 to 17 and sonication duration from 20 min to 123 min, depending on the tumor size. Average treatment temperature was 64.5 °C (range 62°–75°). Follow-up periods range from 13 to 60 months.
MR-HIFU was successful in all patients with good treatment effect and without severe side effects. In three patients with small desmoids (mean = 9.7 ml, range 3–23 ml), complete ablation was achieved with no evidence of viable tumor on follow up MRI () at an average of 35.7 months (range 20–60 months), while in two patients with larger lesions (mean= 46 ml, range 22–70 ml), targeted tumor volumes decreased by 73% at 13 and 15 months, respectively. No patient required analgesia beyond NSAIDs. Slight skin redness (first-degree burn) was noted in 2/5 patients while second-degree skin burn was observed in another two patients and thought to correlate with distance to skin (average 0.9 cm [0.5–1.5 cm]) as well as proximity to bone (i.e. ribs). The patient without skin burn had a skin-tumor distance of 2.5 cm. Skin burns were all less than 2 cm in diameter and healed after a few weeks with topical cream-based therapy.
Discussion
Desmoids are locally infiltrative tumors without potential for metastasis and with a predilection for recurrence after surgical excision, making them a very attractive target for local therapies. Our study adds to the evolving evidence that MR-HIFU treatment of superficial desmoids is a viable treatment option because of its favorable features, i.e. definitive therapeutic effect, repeatability, low adverse effects and no evidence of triggering new lesions.
In this study, we used the volumetric MR-HIFU Sonalleve system for treatment of patients, while previous studies mostly used the ExAblate 2000 (InSightec, Haifa, Israel) HIFU system, which uses a different ablation strategy. Volumetric ablation allows controlled heating of a larger volume per sonication by rapid spiral wise movement of the focal spot. Additionally, thermal ablation is controlled utilizing feedback cells that modify the sonication parameters and generate a spatially controlled temperature profile at the treatment location [Citation18].
Our observed volume reduction is similar to previously published studies that reported a volume decrease between 39% and 100% [Citation16,Citation17,Citation19–22].
Since all MR-HIFU were performed without general anesthesia on an outpatient basis, repetition of the treatment was well accepted by our patients.
HIFU can be performed with ultrasound or MR guidance. The advantage of MR guidance is the thermal mapping which allows visual feedback of the temperature within the desmoid as well as in the near- and far-field enabling adjustments to the sonication parameters. The dense (T2 hypointense) tissue of desmoids seem to be favorable for HIFU therapy by absorbing sufficient energy with rather low sonication energy if e.g. compared to fibroid treatment (typically less than 100 Watt were used). The precision of MR-HIFU was studied by Avedian et al. using four cadaver extremities and reported an ablation margin accuracy of 3–8 mm [Citation17], which matches our clinical experience. In addition, the MR imaging allows post-treatment contrast enhanced images which can delineate untreated desmoid tissue. Careful evaluation of immediate post-treatment images is important in order not to misinterpret the enhancing hyperemic rim around the treatment area with residual desmoid. It is advisable to use subtraction technique between pre- and post-contrast T1 weighted images not only to detect the enhancing areas better but also not to be confused by potentially non enhancing T1 hyperintense areas.
MR-HIFU is a non-invasive alternative compared to surgery or radiation therapy. The most feared complications of HIFU therapy are neural injuries because of the thermal sensitivity of neuronal tissue. In order to avoid neural damage, rigorous planning is necessary and as in one of our cases certain areas close to neural structures cannot be treated. The most common complication is thermal injuries in the near field, which cannot always be avoided due to the superficial position of the desmoids. Two of our five patients suffered a grade 2 skin burn of less than 2 cm diameter which healed with topical treatment. Typically the risk of a skin burn increases if the shortest distance between desmoid and skin is less than 10 mm or if there is proximity to bone as with the ribs in one of our cases due to extensive reflection of the ultrasound waves. One possible solution in such cases would be to use an active cooling system to protect the skin or to decrease the sonication power. We did not observe any injuries in the far field, but there is a report of thermal injuries in the far field especially after repeated sonications due to greater tumor volume [Citation21]. Since none of the factors applied to our patients we did not take any dedicated precautions regarding possible far-field injuries.
Besides possible skin damage there is very little discomfort after MR-HIFU of desmoids. Post-procedural pain is usually mild and readily treatable with non-steroidal anti-inflammatory drugs (NSAIDs). In fact, patients with desmoid related pain reported a decrease of their complaint after HIFU therapy after a few weeks which has also been described in the literature [Citation21].
Our study is limited by sample size, retrospective design, and lack of a randomized comparison with other treatment options. The latter, however, is difficult because superficial desmoids are rare and enrollment in a randomized comparative study would be challenging because the morbidity of radiation therapy and surgery is much higher than MR-HIFU.
In conclusion, MR-HIFU offers a non-invasive outpatient treatment option for relapsing superficial desmoids after surgery with promising midterm results. Because of its morbidity MR-HIFU may also be considered as a primary treatment option for superficial desmoids.
Ethical approval
For this type of study formal consent is not required. Informed consent was obtained from all individual participants included in the study. Consent for publication was obtained for every individual person’s data included in the study.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Reitamo JJ, Häyry P, Nykyri E, et al. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–673.
- Koskenvuo L, Peltomäki P, Renkonen-Sinisalo L, et al. Desmoid tumor patients carry an elevated risk of familial adenomatous polyposis. J Surg Oncol. 2016;113:209–212.
- Fallen T, Wilson M, Morlan B, et al. Desmoid tumors – a characterization of patients seen at Mayo Clinic 1976–1999. Fam Cancer. 2006;5:191–194.
- Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–1062.
- Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–2593.
- Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. JCO. 2011;29:3553–3558.
- Bonvalot S, Ternès N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol. 2013;20:4096–4102.
- Merchant NB, Lewis JJ, Woodruff JM, et al. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86:2045–2052.
- Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. JCO. 2003;21:1390–1397.
- Rutenberg MS, Indelicato DJ, Knapik JA, et al. External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer. 2011;57:435–442.
- Kujak JL, Liu PT, Johnson GB, et al. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol. 2010;39:175–182.
- Schmitz JJ, Schmit GD, Atwell TD, et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10-year experience. AJR Am J Roentgenol. 2016;207:190–195.
- Ilaslan H, Schils J, Joyce M, et al. Radiofrequency ablation: another treatment option for local control of desmoid tumors. Skeletal Radiol. 2010;39:169–173.
- Quadri SA, Waqas M, Khan I, et al. High-intensity focused ultrasound: past, present, and future in neurosurgery. Neurosurg Focus. 2018;44:E16.
- Jean-Francois A, et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: technical and clinical consensus. J Ther Ultrasound. 2013;1:13.
- Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia. 2011;27:648–653.
- Avedian RS, Bitton R, Gold G, et al. Is MR-guided high-intensity focused ultrasound a feasible treatment modality for desmoid tumors? Clin Orthop Relat Res. 2016;474:697–704.
- Köhler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36:3521–3535.
- Zhao WP, Han ZY, Zhang J, et al. Early experience: high-intensity focused ultrasound treatment for intra-abdominal aggressive fibromatosis of failure in surgery. BJR. 2016;89:20151026.
- Shi Y, Huang Y, Zhou M, et al. High-intensity focused ultrasound treatment for intra-abdominal desmoid tumors: a report of four cases. J Med Ultrasonics. 2016;43:279–284.
- Ghanouni P, Dobrotwir A, Bazzocchi A, et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol. 2017;27:732–740.
- Bucknor MD, Rieke V. MRgFUS for desmoid tumors within the thigh: early clinical experiences. J Ther Ultrasound. 2017;5:4.