Abstract
Background: Central intraductal papilloma (IDP) has a low risk of cancer evolution; therefore, surgical treatment of IDP is controversial. We sought to validate ultrasound (US)-guided percutaneous microwave ablation (MWA) for minimally invasive treatment of IDP.
Methods: Thirteen women with central IDP, including six with nipple discharge, underwent US-guided core needle biopsy and MWA from December 2016 to November 2017. Lesions histologically diagnosed as benign IDP were included. The hydro-dissection technique was used to protect the nipple during the entire ablation procedure. We evaluated and recorded data of complete ablation, volume reduction, and complications.
Results: MWA was successfully performed in all patients, with 100% complete ablation, assessed by magnetic resonance imaging or contrast-enhanced US. Mean tumor size was 13.5 ± 4.1 (7.0–20.0) mm; the mean ablation time was 1.4 (0.7–10.3) min. At the median 13.7-month follow-up, mean lesion sizes at 3, 6, and 12 months after MWA were all significantly smaller than that at baseline. Total volume reduction rates were 52.3 ± 18.2% (range, 24.2–81.8%), 72.6 ± 23.1% (range, 39.4–95.9%), and 92.9 ± 7.5% (range, 75.0–100%) at 3-, 6-, and 12-month follow-up, respectively, with significant differences (p < .01). Three lesions with diameters 7 mm, 9 mm, and 12 mm disappeared completely at 3, 6, and 6 months after MWA, respectively, on US imaging. Nipple discharge disappeared immediately after MWA. Cosmetic effects were reported as excellent by all patients and no complications were observed.
Conclusion: US-guided MWA of central IDP proved feasible and effective, with considerable volume reduction and satisfactory cosmetic outcomes.
Introduction
Intraductal papilloma (IDP) of the breast is a benign tumor, affecting 2–3% of the female population. IDP is the most common pathological finding in 30–50-year-old women who have pathological nipple discharge, accounting for 40–70% of cases [Citation1–3]. IDP is characterized by the presence of a proliferating arborescent fibrovascular core lined with an outer epithelial layer and an inner myoepithelial layer [Citation3]. IDP can be divided into central papilloma (large-duct papilloma), commonly involving a single lesion near the nipple, and peripheral papilloma (small-duct papilloma), commonly involving multiple lesions and with a higher risk of cancer evolution [Citation4]. For IDP with associated atypia or malignancy diagnosed using core needle biopsy, the recommended treatment is excision. However, the treatment choice for IDP without core biopsy-diagnosed atypia or malignancy remains controversial [Citation5]. With the desire for minimal invasive therapy and better cosmetic outcome, apart from traditional excision, alternative techniques have been used for IDP treatment, such as image-guided vacuum-assisted biopsy (VAB) [Citation6], providing a minimally invasive modality for these lesions.
Thermal ablation achieves rapid progress in the treatment of tumor in multiple organs including the liver, kidney, lung, thyroid, pancreas, breast, and others [Citation7–11]. However, unlike their wide application in the liver and kidney, ablation techniques are a relatively new minimally invasive treatment for breast lesions. As both are minimally invasive techniques, ablation shares potential advantages with VAB of a very low risk of bleeding and breast shape change. VAB techniques are recommended for treating benign lesions <2.5 cm and fewer than three lesions, owing to the potential risk of hematoma, residual tumor, and skin dimpling [Citation12]. Microwave ablation (MWA) is a promising ablation technique with higher thermal efficiency. MWA has demonstrated encouraging results for benign and malignant breast tumors, with especially favorable effects for the management of benign lesions adjacent to the nipple, skin, and pectoral muscles [Citation13,Citation14].
Minimal invasion and cosmetic outcomes are the new focus in breast surgical treatment. In central IDP of the breast, most patients have clinical signs of yellow, brown, or bloody discharge from the nipple, and occasionally, a palpable lump near the areola. Although central IDP commonly involves a single lesion, ablation at a location adjacent to the breast nipple is challenging owing to concerns about thermal injury. To our best knowledge, no articles on ablation treatment of IDP have been published. Therefore, we performed this pilot study to prospectively analyze the skilled application and clinical outcome of percutaneous MWA in central IDP of the breast under ultrasound (US) guidance.
Materials and methods
Patient enrollment
This prospective study was approved by our institutional review board, and medical records and imaging studies were reviewed. Written informed consent for the procedure was obtained from each enrolled patient. The data of the present study belong to our study registered in ClinicalTrials.gov with identifier number NCT 02860104. From December 2016 to November 2017, a total of 15 patients were diagnosed with central IDP (distance from the nipple <5 mm) without atypia using US-guided core needle biopsy performed in our department. In this study, we recruited 13 patients who underwent US-guided percutaneous MWA; two patients declined to participate in the study. The mean age of treated patients was 50.1 ± 15.5 (27–78) years. All patients were diagnosed using US and magnetic resonance imaging (MRI) and were assessed as Breast Imaging Reporting and Data System (BI-RADS) category 3. Because of potential malignancy in IDP, all patients refused to undergo surveillance for lesions. Information collected for each patient included demographics, maximum diameter of IDP, number of IDPs, color and duration of nipple discharge, and location of IDP. We also measured and recorded ablation variables including session, time, and power; complications; volume reduction; discharge disappearance; complete ablation; and satisfaction with cosmetic outcomes. Indications for MWA of these 13 patients were the following: 10 patients had psychological stress from concerns about malignant transformation owing to the occurrence of IDP (including yellow discharge from the nipple in four patients and no discharge in the remaining six patients), one patient had pain symptoms, and in two patients had bloody nipple discharge. Six patients (6/13, 46.2%) reported palpable IDP prior to MWA.
Pre-procedure evaluation
The eligibility criteria included the following: (a) IDP confirmed using core needle biopsy of the solid component of the lesion; (b) symptoms of nipple discharge and local pain or discomfort considered most likely related to the breast lesion; (c) the patient was unwilling or refused to undergo surgical excision and VAB; and (d) with the presence of an appropriate route for percutaneous puncture under US guidance. The exclusion criteria included the following: (a) patients who were pregnant or breastfeeding; and (b) patients with evidence of coagulopathy.
Prior to the procedure, the number and location of lesions were evaluated with conventional US and contrast-enhanced imaging (US and MRI). The maximum diameter of lesions was measured based on US imaging. The lesion volume was obtained by multiplying the three diameters of each lesion by 0.525 (ellipsoid volume). US-guided biopsy was performed prior to MWA using an automated biopsy gun with a 16 G cutting needle (Bard Biopsy, Tempe, AZ), and two to three separate punctures were performed. The data were extracted and analyzed by two physicians (J. Y., 10-year experience in interventional radiology and W. H., 3-year experience in radiology). All IDP lesions displayed a hypoechoic nodular lesion in a dilated duct, which was adjacent to and connected to the nipple, visible on US imaging ().
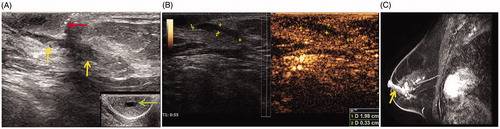
Figure 1. Imaging before microwave ablation (MWA) in a 63-year-old woman with left central intraductal papilloma (IDP). (A) Ultrasound (US) scan before MWA shows the dilated duct with hypoechoic IDP (yellow arrow) connected to the nipple (red arrow). The dilated duct is in the middle of nipple (green arrow). (B) Contrast-enhanced US before MWA shows both the dilated duct and lesion hypo-enhancement with a size 2.0 × 0.3 × 0.4 cm. (C) Sagittal contrast-enhanced magnetic resonance imaging (MRI) shows hyperintense duct (arrow) in the left breast before MWA in the arterial phase.
US-guided MWA
US guidance was performed using a GE LOGIQ E9 scanner (R4; GE Medical Systems Ultrasound & Primary Care Diagnostics, Wauwatosa, WI) with a 6.0–15.0 MHz matrix linear array multi-frequency transducer. The microwave unit (KY-2000; Kangyou Medical, Nanjing, China) is capable of producing 100 W of power at 2450 MHz. The needle antenna has a diameter of 1.6 mm (16 G) and a length of 10 cm. The active tip length is 3 mm. After local anesthesia with a mixture of 2% lidocaine and 1% ropivacaine (1:1, with total dose 20 ml) administered subcutaneously around the dilated duct and the nipple, a hydro-dissection technique was first performed, using a PTC needle (HAKKO, Nagano, Japan) with diameter 0.7 mm (22 G) and length 7 cm, inserted between the dilated duct and the nipple under US guidance. A total 20–100 ml saline was infused slowly to protect the nipple during the entire ablation procedure. After hydro-dissection, the antenna was percutaneously inserted into the solid nodule in the dilated duct under US guidance and then inserted to ablate the dilated duct apart from the nipple (). Finally, the antenna was inserted to ablate the superficial dilated duct adjacent to the nipple. For every applicator tip site, the microwave emitting duration was 10–30 s and the power output was 20 W. For all IDPs, only one antenna was used, moved perpendicular to the long axis of the IDP, to perform ablation until the hyperechoic area completely encompassed the entire IDP.
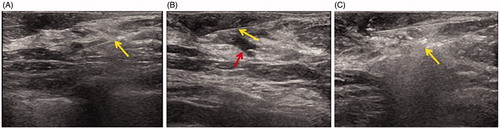
Figure 2. US imaging during MWA in the patient. (A) US shows 16 G core needle biopsy (arrow) of the hypoechoic lesion. (B) US showing the 22 G fine needle (yellow arrow) at the depth of the nipple for saline infusion (red arrow). (C) US showing the 16 G MWA needle (arrow) ablating the dilated duct and intraductal papilloma with the hyperechoic zone.
Follow-up and imaging analysis
Immediately after MWA, contrast-enhanced US was performed to evaluate treatment efficacy. The US scan equipment was the same as that used for ablation guidance. The US contrast agent used was Sonovue (Bracco Company, Milan, Italy). Contrast-enhanced MRI was performed using a dedicated four-channel phased array breast coil on a 1.5 T MRI scanner (Signa Excite HD Twin, General Electric Healthcare, Milwaukee, WI) with a 40 mT/m gradient and a 150 mT/m/s slew rate, for 1–3 d after MWA. Technique success (TS) referred to the IDP being treated according to protocol and being covered completely by the ablation zone after the MWA procedure. Technique effectiveness (TE), namely complete ablation, was defined as the absence of enhancement of any areas of lesions on a follow-up enhanced imaging study performed 1 month after MWA [Citation15]. If nodular enhancement in the dilated duct or nipple discharge was noted, this was thought to indicate the presence of residual unablated IDP. In this case, further ablation was considered if the patient still met the criteria for MWA. In patients with TE evaluated using contrast-enhanced MRI/US, routine US was repeated, owing to its convenience and low cost, to monitor the breast at 3 and 6 months after MWA and then at 6-month intervals. With suspected new lesions in a follow-up US scan, contrast-enhanced MRI/US can be used to evaluate any new lesions as well as the ablated IDP.
Clinical symptoms, cosmetic outcomes, and complications were recorded during the follow-up. However, our central concern was the complete necrosis of lesions and the volume reduction ratio (VRR), which was calculated using the following equation: VRR (%)= [(initial volume − final volume)×100]/initial volume. The ratio was assessed using US measurements taken pretreatment and at the last follow-up visit.
Statistical analysis
Continuous variables are summarized with standard descriptive statistics, including mean, standard deviation, media, and range. Categorical variables are summarized as number and frequency. The paired Student t-test was performed to compare the tumor size at baseline versus that at 3-month and 6-month follow-ups. The Student t-test was also used to compare VRR at 3 months and 6 months after MWA. In addition, the Wilcoxon signed-rank test was used to compare tumor volume before and after MWA. Data were reported as mean ± standard deviation or median. All statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL, USA) by one physician (Y. J.). Differences with a p values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
In this preliminary pilot study, US-guided MWA was performed successfully for 13 IDPs in 13 patients. Median follow-up was 13.7 (range, 9.0–20.6) months. All IDPs were <5 mm from the nipple. Ahn et al. developed a scoring system to predict malignancy in IDP based on clinical, radiological, and pathological factors including bloody nipple discharge (scoring 3.4), size on imaging ≥15 mm (scoring 1.9), BI-RADS ≥4 b (scoring 1.6), peripheral location (scoring 2.7), and palpable lesion (scoring 1.8) as independent predictors of malignancy in benign papilloma without atypia [Citation16]. We used a total score ≤4 for five factors as the recommended cutoff value, as an auxiliary method to determine the probability of a benign lesion. ‘Likely benign’ lesions were evaluated using a combination of the scoring system and results of core needle biopsy. All 13 lesions had a total score of ≤4 ().
Table 1. The clinical features of patients and masses.
Therapeutic parameters
All patients with IDP underwent one-session treatment with MWA. Based on US and contrast-enhanced US or MRI imaging evaluation, both TS and TE were achieved in 13 of 13 (100%) IDPs. Nipple discharge and duct dilation disappeared in all the patients immediately after MWA (). The median duration to reach TS on US was 1.4 (range 0.7–10.3) min. The ablation power in all MWA sessions was 20 W. The mean fluid infusion volume between the IDP and nipple was 47.2 ± 13.9 mL (range, 35–80 mL). After MWA, the echogenicity of the 13 treated IDPs gradually decreased and became heterogeneously hypoechoic in about 5 min on US imaging. Non-enhancement was visible on contrast-enhanced US and MRI after MWA. During the follow-up period, no recurrence was observed.
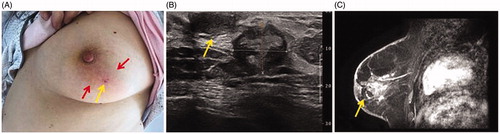
Figure 3. Image after MWA in the patient. (A) After MWA, only a pinhole-sized scar is visible on the skin (yellow arrow); around the ablation zone, skin flushing (red arrow) can be seen, which resolved in 1 week after 3-day antibiotic treatment. (B) US showing the heterogeneous ablation zone (measure markers) adjacent to the nipple (arrow) with a size 1.7 × 1.3 cm at 6 months after MWA. (C) Contrast-enhanced MRI image showing hypointense MWA treatment zone (arrow) in the arterial phase.
Volume reduction
The ablated IDPs gradually decreased in zone over time. Changes in the IDP volume between pretreatment with MWA and at each follow-up are summarized in . The baseline mean size of the 13 IDPs was 13.5 ± 4.1 mm (range, 7–20 mm) and the median volume was 0.4 ml (range, 0.1–1.0 ml). The maximum IDP lesion size and the lesion volume at 3, 6, and 12 months after MWA were significantly smaller than those at baseline (p < .05). However, there was no significant difference in the maximum lesion size after MWA between 3 and 6 months (p = .07; 95% confidence interval (CI): –0.2, 5.1) and between 6 and 12 months (p = .06; 95% CI: –0.1, 5.6). There was a significant difference for lesion volume after MWA between 3 and 12 months (p = .003; 95% CI: 0.08, 0.3) and between 6 and 12 months (p = .03; 95% CI: 0.008, 0.1). In total, the VRR was 52.3 ± 18.2% (range, 24.2%–81.8%), 72.6 ± 23.1% (range, 39.4%–95.9%), and 92.9 ± 7.5% (range, 75.0–100%) at the 3-, 6-, and 12-month follow-ups, respectively, with significant differences (p = .008 for 3 versus 6 months and p = .005 for 6 versus 12 months). Three lesions with maximum diameters of 7 mm, 9 mm, and 12 mm disappeared completely at 3, 6 and 6 months, respectively, after MWA with US and MRI imaging.
Table 2. The effect of IDPs volume reduction after MWA.
Cosmetic outcomes and complications
Cosmetic outcomes for the skin texture, pigmentation, and wound, based on major or minor complications, are normally reported as excellent, good, acceptable, and poor [Citation13]. In our study, cosmetic outcomes were reported to be excellent by all patients (100%) and by physicians who performed the ablations. All ablated IDPs softened gradually during the follow-up period. Eight (8/13, 61.5%) patients reported that the ablated lesions were not palpable at all and 12 (12/13, 92.3%) patients reported that the volume reduction by the last follow-up was comparable to that just after MWA. Only one patient reported no noticeable change in the lesion volume.
Overall, the procedure proved to be well tolerated by all of our patients, with only mild sensations of heat and pain in the ablation site; however, no patients requested the procedure be stopped during ablation. No sedatives or tranquilizers were needed before or after ablation. Wound scars were only pinhole-sized with no skin or nipple retraction, ecchymosis, hematoma, or burns. Five patients reported that their psychological stress disappeared immediately after MWA. In women who experienced swelling after fluid infusion, the breast returned to normal 1 d after MWA. One patient complained of skin flushing around the ablation zone 2 d after MWA, with mild pain, but she had no fever or abnormal white blood cell counts and the condition resolved within 1 week after antibiotic administration for 3 d. Another patient complained of mild pain at the ablation site for 2 months.
Discussion
IDPs are breast lesions that occur in the ducts of mammary glands owing to epithelial and myoepithelial hyperplasia surrounding a fibrovascular stalk [Citation4,Citation17]. IDPs are pathologically diagnosed as benign, or atypical or malignant [Citation18]. IDPs comprise central IDP and peripheral IDP, with most having a central location and involving a single lesion [Citation1]. Peripheral IDPs arise in the terminal duct lobular units; thus, these IDPs are often clinically occult and discovered incidentally upon imaging examination. Central IDPs originate in the large ducts, such as the segmental or subsegmental duct. These frequently present with serous or serosanguineous nipple discharge and display a solid component in the dilated duct, visible upon imaging examination.
In our study, we focused on ablation treatment of central IDP without atypia. For IDP with atypia diagnosed with a core needle biopsy, excision is generally recommended to rule out a potential concurrent malignant neoplasm; IDP with atypia has a confirmed upgrade rate as high as 40.8% [Citation16,Citation19]. However, for IDPs without atypia, the recommendations for excision versus observation vary. IDP without atypia is regarded as having a low potential for malignancy, with an upgrade rate to carcinoma of 6.0% per 100 excised IDPs [Citation20], 6.8% per 250 excised benign IDPs without atypia, and 7% according to recent meta-analysis results [Citation19,Citation21]. The recommendation for IDPs diagnosed with core needle biopsy or VAB is to therapeutically excise the lesion seen on imaging using VAB, and no longer using open surgery, with follow-up surveillance imaging for 5 years [Citation22]. These recommendations provide the basis for minimally invasive treatment of this disease, with 6-month follow-up intervals recommended in the guidelines [Citation23]. There have been an increasing number of reports on the effectiveness of US-guided or mammography-guided (stereotactic) VAB in treating IDP of the breast with no clinical or pathological signs of increased risk for malignant transformation [Citation6,Citation24,Citation25]. Further clinical observation after biopsy shows a recurrence rate of 0–15.4% [Citation25–27]. A recent large series including 1578 patients, with a median follow-up of 34 months in those with benign papilloma, reported a recurrence rate of 4.4% after VAB [Citation27]. VAB requires a core needle with 7–11 G diameter and a relatively long duration, ranging between 10 and 20 min. After biopsy, a stress dressing is routinely applied to the biopsy site for 24–48 h to reduce bleeding risk; potential hematoma and recurrence are the main challenges of VAB for IDP.
Ablation provides a step forward to minimize the invasiveness of breast tumor treatment. In recent years, several ablation techniques including radiofrequency ablation, cryoablation, laser ablation, high-intensity focus ultrasound, and MWA have been increasingly applied in treating malignant and benign breast tumors, as well as intraductal papilloma, with satisfactory result regarding tumor necrosis and volume reduction [Citation28–31]. Zhang et al. recorded MWA of 11 intraductal papilloma, however, there were no details of technique and efficacy available in the study [Citation28]. Based on our experience of the MWA technique, to our knowledge, this is the first study of percutaneous thermal ablation for central IDP adjacent to the nipple using US guidance and evaluating the technical feasibility and safety of MWA. The present study showed that MWA is a safe and effective method for central IDP, with median ablation duration of 1.4 min. There was no recurrence or malignancy observed in the post-ablation bed during the median 13.7 months of follow-up. In addition, there was no thermal skin injury, nipple deviation or collapse, hematoma, and no gross changes in the breast. The lesion achieved a satisfactory reduction effect, especially in the 12 months after MWA. Several valuable experiences can be shared, as follows. (1) As a potentially malignant disease, strict ablation indications of IDP should be followed. All lesions should be pathologically diagnosed using core needle biopsy. In addition, we used a scoring system developed by Ahn et al., with good sensitivity and negative predictive value associated with a score ≤4 for a likely benign diagnosis, to assist clinicians in decision-making [Citation16]. (2) Careful US scanning and US combined with MRI scans are necessary to identify the size, border, and location of the IDP before ablation. (3) For lesions adjacent to the nipple, hydro-dissection with saline and a lower microwave power of 20 W are very important for avoiding thermal damage to other central ducts. (4) Combined with multi-modality imaging, including US, contrast-enhanced US, and MRI for accurate assessment of ablation, subsequent follow-up surveillance is necessary. MRI is an especially good choice for evaluating the effect of ablation, with greater sensitivity in detecting residual or progressed lesions. (5) The dilated duct in central IDP is usually connected to the nipple, so the orientation of the needle puncture and pull back is recommended to be perpendicular to the long axis of the dilated duct, to avoid nipple injury owing to thermal gas along the duct. Finally, the solid component of the IDP is suggested to be ablated first, followed by the duct wall.
Our study has some limitations. First, as a preliminary study of thermal ablation for benign central IDP without atypia, the study population was relatively small. Thus, this technique requires further testing in larger populations. Second, because the central IDP is located in a major duct, the influence of ablation on lactation was not investigated in the study as no patients were breastfeeding during follow-up. Lastly, a longer follow-up period is warranted to evaluate the long-term complete ablation effect and volume reduction efficacy for IDPs.
In summary, our prospective results showed that US-guided percutaneous MWA provides a safe, feasible, minimally invasive treatment option for benign central IDP without atypia. Larger sample sizes and longer follow-up are warranted, to assess treatment efficacy. Clinical trials including other treatment options should also be performed, to evaluate use of MWA in patients with central IDP.
Acknowledgments
The data of the present study belonged to our registered study in Clinical-Trials.gov and the identifier number is NCT 02860104.
Disclosure statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. All authors have made a substantial contribution to the information or material submitted for publication. All have read and approved the final manuscript.
Additional information
Funding
References
- Wei S. Papillary lesions of the breast: an update. Arch Pathol Lab Med. 2016;140:628–643.
- Brookes MJ, Bourke AG. Radiological appearances of papillary breast lesions. Clin Radiol. 2008;63:1265–1273.
- Paterok EM, Rosenthal H, Sabel M. Nipple discharge and abnormal galactogram. Results of a long-term study (1964–1990). Eur J Obstet Gynecol Reprod Biol. 1993;50:227–234.
- Ohuchi N, Abe R, Takahashi T, et al. Origin and extension of intraductal papillomas of the breast: a three-dimensional reconstruction study. Breast Cancer Res Treat. 1984;4:117–128.
- Jaffer S, Nagi C, Bleiweiss IJ. Excision is indicated for intraductal papilloma of the breast diagnosed on core needle biopsy. Cancer. 2009;115:2837–2843.
- Wei H, Jiayi F, Qinping Z, et al. Ultrasound-guided vacuum-assisted breast biopsy system for diagnosis and minimally invasive excision of intraductal papilloma without nipple discharge. World J Surg. 2009;33:2579–2581.
- Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015;4:176–187.
- Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483–498.
- Chen CK, Chou HP, Sheu MH. Image-guided lung tumor ablation: principle, technique, and current status. J Chin Med Assoc. 2013;76:303–311.
- Yu J, Liang P. Status and advancement of microwave ablation in China. Int J Hyperthermia. 2016;1:1–10.
- Fleming MM, Holbrook AI, Newell MS. Update on image-guided percutaneous ablation of breast cancer. AJR Am J Roentgenol. 2017;208:267–274.
- Hahn M, Krainick-Strobel U, Toellner T, et al. Interdisciplinary consensus recommendations for the use of vacuum-assisted breast biopsy under sonographic guidance: first update 2012. Ultraschall Med. 2012;33:366–371.
- Yu J, Chen BH, Zhang J, et al. Ultrasound guided percutaneous microwave ablation of benign breast lesions. Oncotarget. 2017;8:79376–79386.
- Zhou W, Zha X, Liu X, et al. US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology. 2012;263:364–373.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705.e4.
- Ahn SK, Han W, Moon HG, et al. Management of benign papilloma without atypia diagnosed at ultrasound-guided core needle biopsy: scoring system for predicting malignancy. Eur J Surg Oncol. 2018;44:53–58.
- Ueng SH, Mezzetti T, Tavassoli FA. Papillary neoplasms of the breast: a review. Arch Pathol Lab Med. 2009;133:893–907.
- Rizzo M, Linebarger J, Lowe M, et al. Management of papillary breast lesions diagnosed on core-needle biopsy: clinical pathologic and radiologic analysis of 276 cases with surgical follow-up. J Am Coll Surg. 2012;214:280–287.
- Khan S, Diaz A, Archer KJ, et al. Papillary lesions of the breast: to excise or observe? Breast J. 2017;24:350–355.
- Maráz R, Boross G, Ambrózay E, et al. Selective ductectomy for the diagnosis and treatment of intraductal papillary lesions presenting with single duct discharge. Pathol Oncol Res. 2013;19:589–595.
- Wen X, Cheng W. Nonmalignant breast papillary lesions at coreneedle biopsy: a meta-analysis of underestimation and influencing factors. Ann Surg Oncol. 2013;20:94.
- Rageth CJ, O’Flynn EA, Comstock C, et al. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2016;159:203–213.
- Canadian Association of Radiologists. CAR practice guidelines and technical standards for breast imaging and intervention. Ottawa: Canadian Association of Radiologists; 2012.
- Kibil W, Hodorowicz-Zaniewska D, Popiela TJ, et al. Mammotome biopsy in diagnosing and treatment of intraductal papilloma of the breast. Pol Przegl Chir. 2013;85:210–215.
- Quinn-Laurin V, Hogue JC, Pinault S, et al. Vacuum-assisted complete excision of solid intraductal/intracystic lesionses and complex cysts: is follow-up necessary? Breast. 2017;35:42–47.
- Bonaventure T, Cormier B, Lebas P, et al. Benign papilloma: is US-guided vacuum-assisted breast biopsy an alternative to surgical biopsy? J Radiol. 2007;88:1165.
- Li S, Wu J, Chen K, et al. Clinical outcomes of 1,578 Chinese patients with breast benign diseases after ultrasound-guided vacuum-assisted excision: recurrence and the risk factors. Am J Surg. 2013;205:39.
- Zhang W, Li JM, He W, et al. Ultrasound-guided percutaneous microwave ablation for benign breast lesions: evaluated by contrast-enhanced ultrasound combined with magnetic resonance imaging. J Thorac Dis. 2017;9:4767–4773.
- Garcia-Tejedor A, Guma A, Soler T, et al. Radiofrequency ablation followed by surgical excision versus lumpectomy for early stage breast cancer: a randomized phase II clinical trial. Radiology. 2018;289:317–324.
- Mauri G, Sconfienza LM, Pescatori LC, et al. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: a systematic review and meta-analysis. Eur Radiol. 2017;27:3199–3210.
- Mauri G, Sconfienza LM, Sardanelli F. Imaging-guided percutaneous ablation: a step forward to minimize the invasiveness of breast cancer treatment. Radiology. 2019;290:849–850.
