Abstract
Purpose: Microwave ablation (MWA) has become increasingly popular as a minimally invasive treatment for benign and malignant liver tumors. However, few studies have demonstrated the benefits and disadvantages of MWA compared to surgical resection (SR) for large hepatic hemangiomas. This study aimed to evaluate the safety and effectiveness of MWA compared to SR for large (5–10 cm) hepatic hemangiomas.
Methods and materials: This retrospective comparative study included 112 patients with large, symptomatic hepatic hemangiomas who had been treated with MWA (n = 44) or SR (n = 68) and followed up for a median of 44 months using enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Intraoperative information, postoperative recovery time, postoperative discomfort and complications and treatment effectiveness between groups were compared using a chi-square test or an independent t-test.
Results: The operative time was significantly shorter (31.3 ± 21.76 versus 148.1 ± 59.3 min, p < .001) and the blood loss (10.2 ± 60.6 versus 227.9 ± 182.9 mL, p < .0001) and rate of prophylactic abdominal drainage [1 (2.3%) versus 57 (83.8%), p < .001] were significantly lower in the MWA group than in the SR group. Postoperative recovery of the MWA group in regard to indwelling catheter time, normal diet time, incision cicatrization time and hospital stay (p < .001) was significantly better than the SR group. However, no statistically significant difference in effectiveness was noted between the groups (p = .58).
Conclusions: MWA may be as effective as SR, and potentially safer for treating large, symptomatic hepatic hemangiomas. To confirm our findings, large-sample, multicentered, randomized controlled trials are needed.
Introduction
Hepatic hemangioma is the most common benign neoplasia of the liver, with an incidence rate of 0.4%–7.3% at autopsy [Citation1]. Fortunately, the vast majority of hepatic hemangiomas are relatively small and asymptomatic, and require no specific treatment [Citation2,Citation3]. However, for patients with large hepatic hemangiomas (≥5 cm), which have a tendency to enlarge and cause obvious symptoms, or for patients with serious worries because of hemangiomas, active medical or surgical intervention has been required [Citation4–6].
Traditionally, surgical resection (SR) has been the most effective and preferred treatment for large hepatic hemangiomas [Citation7]. Although SR can remove the tumor completely, it is more invasive, requires a relatively long hospitalization period, and has a high risk of complications [Citation2,Citation6,Citation7]. In recent years, minimally invasive procedures have been used to treat hepatic hemangiomas, such as radiation therapy, transcatheter arterial embolization (TAE) and radiofrequency ablation (RFA) [Citation8,Citation9]. TAE and radiation therapy have not been considered curative therapies for hepatic hemangiomas because of their poor results and serious complications [Citation10,Citation11]. Although RFA has been widely used over the past decade for treating hepatic hemangiomas, the effectiveness, safety and feasibility of RFA for hepatic hemangiomas ≥5 cm in diameter still remains controversial due to a smaller ablation zone, a longer operative time and a greater potential for heat-sink because of the vascular nature of the lesion [Citation12–17].
Recently, microwave ablation (MWA) has become increasingly popular as a minimally invasive treatment for benign and malignant liver cancer [Citation18]. MWA offers several advantages over RFA, such as faster heating over a larger volume and being less susceptible to the heat-sink effect [Citation19,Citation20]. In addition, the efficacy and safety of MWA for tumors has been reported to be greater than for RFA [Citation18,Citation21]. Due to the large volume of polar molecules such as ions, water and proteins in large hepatic hemangiomas, MWA can theoretically produce a greater necrotic volume compared to RFA [Citation22].
Since 2010, ultrasound (US)-guided percutaneous MWA has been used in the treatment of large hepatic hemangiomas. US guidance has been used as the navigation tool for ablation treatments with the advantages of simple, fast and real-time image guidance. US-guided percutaneous MWA has been reported to be effective, safe and feasible to perform [Citation23]. However, comparative outcomes for treating large hepatic hemangiomas in relation to MWA and SR procedures have not been reported. Therefore, we aimed to compare the effectiveness and safety of SR and MWA for large hepatic hemangiomas 5–10 cm in diameter.
Materials and methods
Patient cohort
The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. The medical records of patients with large hepatic hemangiomas (5–10 cm in diameter) who had undergone SR or MWA at our hospital from August 2010 to December 2016 were retrieved and reviewed. At our hospital, patients provided informed consent for review and analysis of their preoperative medical records.
In total, 112 patients with large hepatic hemangiomas (5–10 cm in diameter) were analyzed in this study, comprising 44 patients who had undergone MWA and 68 patients who had undergone SR.
The inclusion criteria for patients undergoing SR comprised the following: (1) a minimum of 1 hepatic hemangioma between 5 cm and 10 cm in diameter; (2) abdominal pain and anemia symptoms; (3) a hemangioma that had enlarged >0.5 cm within 12 months; (4) liver and renal function test results that were normal, and; (5) willingness to undergo SR.
The inclusion criteria for patients undergoing MWA comprised the following: (1) a minimum of 1 hepatic hemangioma between 5 cm and 10 cm in diameter; (2) abdominal pain and anemia symptoms; (3) a hemangioma that had enlarged >0.5 cm within 12 months; (4) the distance between the hepatic hemangioma and visceral organs such as the gallbladder, colon, and stomach was not less than 0.5 cm; (5) liver and renal function test results that were normal; (6) not having received any other prior treatment, and; (7) having declined SR as a form of treatment.
Diagnosis of hepatic hemangioma
Currently, imaging examinations are the main methods used to diagnose hepatic hemangioma. In this study, a diagnosis of hepatic hemangioma was made based on at least two coincident radiologic findings using contrast-enhanced US, computed tomography (CT) and magnetic resonance imaging (MRI). On contrast-enhanced US, hepatic hemangiomas show characteristic puddling, with peripheral nodular enhancement in the early vascular phase followed by a progressive and centripetal fill-in during the late vascular phase. On an enhanced CT, a hepatic hemangioma shows a characteristic pattern, and the presence of a tumor is represented clearly, showing a nodular peripheral puddling pattern in the early phase and a fill-in pattern subsequently. The MRI features involving hepatic hemangiomas have been reported to be typically of low signal intensity and are well defined on T1-weighted images, and of very high intensity in T2-weighted images [Citation24]. The number, size and location of the hemangiomas were determined prior to treatment.
Treatment
Preoperative routine tests were performed for all patients, including a routine blood test, blood coagulation function, liver and kidney function tests, and a viral marker test, in addition to MRI and CT scanning.
US-guided percutaneous MWA
MWA was performed percutaneously under general anesthesia using a real-time US-guided and water-cooled MWA system (a 2450 MHZ MTC-3C microwave generator, Vision Medical, Nanjing, China) according to the manufacturer's recommendations. Vital signs were continuously monitored during and within 1 h following the procedure. The microwave energy was delivered using a 15-gauge electrode (25 cm in length; Vision-China Medical Devices R&D Center, Nanjing, Jiangsu Province, China). The power and MWA time were set based on tumor size and location, according to the manufacturer’s instructions.
According to our MWA protocol, one or two electrodes were inserted into the tumor, parallel to each other at an inter-electrode distance of 2.5–3.0 cm in the same plane, using a 3.5-MHz probe (MyLab 60; Esaote, Genoa, Italy). The microwave generator was switched on and the temperature of the electrode tip was set to maintain 100 °C for 3–10 min. The distal margin of the lesion was ablated first, and then the electrodes were withdrawn 1.5–2 cm for multipoint ablations until the tumors were completely covered by the ablation zone. Following the MWA procedure, another one or two needle tracts were ablated at the same or adjacent inter-costal space, if necessary. Patients with multiple hepatic hemangiomas, or with two lesions >10 cm in diameter, underwent two MWA procedure sessions. During the MWA, preoperative preventive measures (preoperative catheterization, a 20 mg furosemide intravenous injection, and a 125 ml sodium bicarbonate solution intravenous infusion when 50% of the hemangiomas had been ablated) were performed to prevent the incidence of acute kidney injury (AKI) after October 2013.
All patients were closely monitored post-MWA for procedure-related acute or chronic complications. Liver and kidney protection, small-dose and short-term dexamethasone or methylprednisolone, and hemostasis were applied after the MWA. Peripheral blood tests, and liver and renal function examinations, were performed on day 1 and day 3 post-MWA.
Surgical resection
Varying types of resection were performed according to the location and size of the hemangiomas for patients who underwent SR, including 34 patients with hemangioma excision, 4 patients with left liver resection, 8 patients with left lateral segmentectomy, 10 patients with irregular hepatectomy, 6 patients with multiple excision methods, 4 patients with laparoscopic excision and 2 patients with laparoscopic left lateral segmentectomy. The surgical incisions were planned, based on the location of the hemangiomas. In total, 52 patients had oblique incisions below the right costal margin, 10 patients had median upper abdominal incisions and 6 patients underwent laparoscopic treatment through 5 laparoscopic holes.
Criteria for outcomes assessment
Treatment outcomes were evaluated according to information concerning surgical time, intraoperative blood loss, postoperative recovery, the incidence of complications, postoperative liver and renal function test results, the complete cure rate, and postoperative recurrence. All patients who had undergone SR and MWA were followed up, using enhanced CT or MRI, for 12 months postoperatively. Complete cure was defined as no obvious enhancement of the lesions on enhanced CT or MRI scans. In cases of complete cure, subsequent CT or MRI examinations were repeated every three months, for 12 months. Twelve months later, all patients were telephoned every six months for follow-up. In cases of incomplete cure, repeat treatment was considered if the residual tumor had progressed within six months of follow-up. At the end of July 2017, the follow-up period ended.
Statistical analysis
Continuous parameters were expressed as means ± standard deviation (SD), while categorical variables were expressed as numbers and percentages. For statistical analysis, a chi-square test was used for the comparison of categorical variables, and an independent t-test was employed for continuous variables. All statistical analyses were conducted with IBM SPSS version 19 for Windows. Statistical significance was defined as a p value of <.050.
Results
Patient characteristics
A total of 112 patients met the enrollment criteria and were included in the study. MWA and SR procedures comprised the primary treatment in 44 and 68 patients, respectively. Patient characteristics are summarized in . There was no significant difference between the two treatment groups with regard to patient age, sex, tumor number, tumor size, tumor location, or other indices of liver and renal function, such as albumin (Alb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB) and serum creatinine (SCr) (p > .050).
Table 1. Baseline characteristics of patients with hepatic hemangiomas ≥5 cm.
Intraoperative and postoperative data
For both groups, the patients had successful operations without serious intraoperative complications or death. The operating time in the SR group (148.1 ± 59.3 min) was significantly longer than that of the MWA group (31.3 ± 21.76 min, p < .001).
During surgery, although intraoperative blood loss in the SR group was significantly higher than in the MWA group (227.9 ± 182.9 versus 10.2 ± 60.6, p < .001), there was no significant difference in the intraoperative blood transfusion rate between the two groups (p > .25). Moreover, as a routine precaution to monitor bleeding and bile leakage post-SR (57/68, 83.8%), an indwelling abdominal tube was required less frequently for patients in the MWA group (p < .001) ().
Table 2. A comparison of operation data between the MWA and SR procedure groups.
Patients in the MWA group had a faster recuperation time than patients in the SR group. The return-to-normal bowel function time was significantly less in the MWA group than in the SR group (0.0 ± 0.2 days vs 3.0 ± 2.2 days, p < .001). Moreover, surgical drains were not a standard requirement post-MWA but were routinely used post-SR. Patients in the MWA group did not require a skin incision, whereas patients in the SR group had sutures removed 5.8 ± 3.7 days postoperatively (p < .001).
The average hospitalization period for the MWA group was 4.8 ± 1.9 days, which was significantly less than for the SR group (9.7 ± 2.8 days, p < .001) ().
Complications between SR and MWA patients
As shown in , short-term postoperative minor complications were identified in both groups and involved pain, excessive wound exudate, cough, low-grade fever (≤38 °C), coprostasis, and gastrointestinal discomfort. All patients recovered within 3–9 days after symptomatic treatment, and there was no statistically significant difference between the groups (p > .050). Additionally, AKI (Grade I) was identified in three patients in the MWA group, with an absolute increase in SCr of not less than 26.4 µmol/L, or a percentage increase in SCr of not less than 50% within 48 h [Citation25]. However, these three patients with stage I AKI had an increased SCr of <100% (), and recovered within 3–7 days after symptomatic treatment ().
Figure 1. Data concerning three patients in the MWA group with acute kidney injury (AKI). (A) The increased percentage of SCr post-MWA within 48 h. (B) Postoperative recovery time for three patients in the MWA group with AKI.
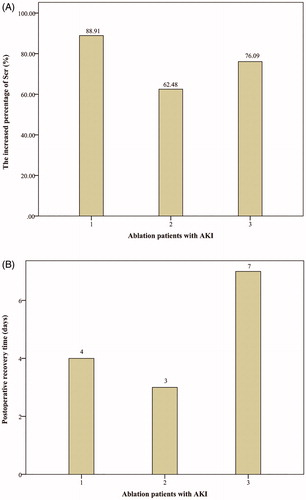
Table 3. The pain/discomfort and complication rates for patients following MWA and SR procedures.
Major complications (Grade III) were observed in 5 patients in the SR group. There were 3 patients with perihepatic effusion, 2 patients with pleural effusion, 1 patient with an incisional hernia and 1 patient with seroperitoneum, including 2 patients with two types of major complications. These patients received symptomatic treatments and finally recovered. In the MWA group, 1 patient developed a diaphragmatic hernia (Grade III) and required a surgical diaphragmatic hernia repair, and recovered subsequently. The occurrence rate for serious complications (Grade III) was higher in the SR group; however, there was no statistically significant difference between the groups (p > 0.050) ().
The clinical serology index levels were analyzed between the groups. Compared to SR patients, patients in the MWA group had a significantly higher hemoglobin (Hgb) (p < .001), Alb (p < .001), ALT (p = .001), AST (p < .001), DB (p = .015) and SCr (p = .003) levels on day 1 postoperatively (). Compared to preoperative levels, the Hgb and Alb levels on day 1 postoperatively decreased in both groups. The decreased range in the SR group was significantly higher than in the MWA group (p < .050) (). The ALT, AST and DB levels significantly increased postoperatively; however, these levels decreased significantly within three days post-hepatoprotection and post-symptomatic treatments in both groups ().
Figure 2. A comparison of the Hgb and Alb values pre- and post-treatment. (*Using an independent t-test, the p value was calculated to evaluate the changes in factors between MWA and SR. The value marked in the picture presents the changes in factors pre- and post-treatment). A, the Hgb value; B, the Alb value.
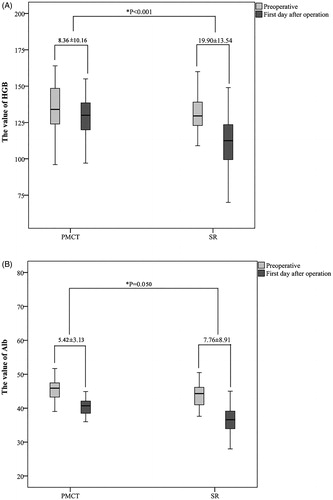
Figure 3. The postoperative change in biochemical parameters concerning liver and renal function (value = mean ± SD). A p value was calculated using a paired-samples t-test. (A) The ALT value (B) The AST value (C) The DB value (D) The SCr value.

Table 4. Comparing postoperative biochemical parameters between MWA and SR procedures.
Effectiveness of SR and MWA techniques for patients
Complete tumor ablation (n = 41 patients, 93.2%) was confirmed using spiral CT or MRI one month postoperatively, ( shows the MRI comparison of 1 patient), while complete resection was achieved in 65 (95.6%) patients, with no significant difference between the groups (p > .050) (). Three incompletely ablated patients were not treated further because of the small-size and slow-growing nature of their hemangiomas, and because the symptoms due to the hemangioma had resolved for all 3 patients. At the end of the follow-up period, all patients were alive, their original symptoms had completely disappeared, and no long-term complications had occurred in either group.
Figure 4. An MRI of one patient (n = 1 hemangioma, 6.3 cm in diameter) pre-MWA, one month post-MWA, and 12 months post-MWA. (A) Pre-MWA. (B) One month post-MWA. (C) 12 months post-MWA.
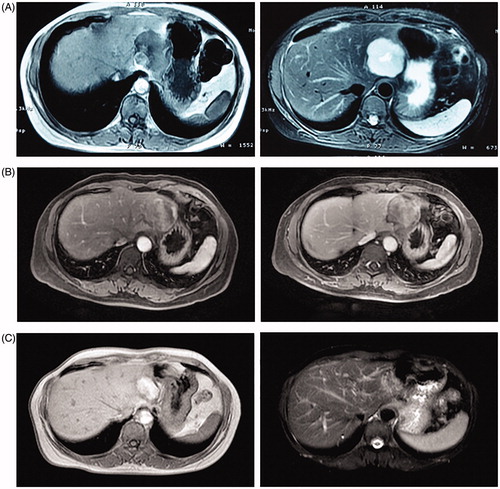
Table 5. A comparison of effectiveness of treatment between MWA and SR procedures.
Discussion
This study aimed to compare the safety and therapeutic effectiveness of SR and MWA procedures for patients with large hepatic hemangiomas. Our data showed that US-guided MWA used to treat large hepatic hemangioma is feasible and easier to perform, and results in fewer complications, a faster recovery rate, and a shorter hospital stay than SR treatment. Moreover, the complete ablation rate was comparable between both groups.
Although SR has been reported to be the most effective treatment for large hepatic hemangiomas, it remains a highly invasive procedure involving a long-term recovery period [Citation26]. With a benign tumor, more minimally invasive treatments are needed [Citation27]. Alternatively, TAE or radiation therapy may be used; however, these treatments are not curative. RFA and MWA, the most common local thermal ablation methods, have been used in the treatment of large hepatic hemangiomas. Previously, we reported on the effectiveness and safety of MWA as a new therapeutic modality for large hepatic hemangioma, and found that MWA could potentially be regarded as the first-line therapy [Citation23]. However, more information was required concerning the effectiveness and safety of MWA for large hepatic hemangiomas compared to SR. The present study systemically compared the technical and clinical outcomes of large hepatic hemangiomas treated using SR and MWA procedures.
As a minimally invasive treatment, MWA for large hepatic hemangiomas has many advantages. First, in the process of MWA, the ablation area covered the hemangioma specifically, so damage to healthy liver tissue was minimal. Second, compared to SR, MWA could be performed within a shorter operation time, required no abdominal incision, had a faster recovery time, and a shorter hospitalization time. Third, a well-performed MWA of a hepatic hemangioma led to complete tumor necrosis at the same effectiveness rate as SR. There was no significant difference in the complete cure rate between patients in the SR and MWA groups in our study. Additionally, residual hepatic hemangioma tissues in the MWA group were completely ablated after a second MWA undertaken one month later.
However, sound knowledge and the capacity for skilled operation of the MWA technology were required for the safe and effective treatment outcome. In the process of treatment, exploring, improving and identifying new treatment strategies are ongoing. For 17 patients treated prior to October 2013, the following principles concerning ablation of large hepatocellular carcinoma were used. Hemangiomas were treated using multi-electrodes, on high power, with long-term ablation >1 cm peripherally, according to the safety margin requirements of malignant tumors. Although the complete ablation rate was high, acute renal dysfunction after MWA was noteworthy. Three patients treated with MWA sustained AKIs, according to the parameters of the renal function tests for SCr, urea, and nitrogen, and uric acid levels increased continuously. Therefore, preoperative preventive measures (preoperative catheterization, a 20 mg furosemide intravenous injection, and a 125 ml sodium bicarbonate solution intravenous infusion when 50% of the hemangiomas were ablated) were undertaken to reduce the incidence of AKI, after October 2013. Subsequently, no patient sustained an AKI following MWA treatment. Additionally, due to the highly vascular properties of hepatic hemangiomas and the mechanism of MWA, a large number of tumor cells and healthy liver tissues were damaged during the ablation process, resulting in hemolysis and elevated serum aminotransferase and bilirubin levels. On day 1 post-MWA, the Hgb, Alb, ALT, AST, DB and SCr levels were significantly higher than those of patients in the SR group (p < .050), and the levels recovered within three days post-hepatoprotection and symptomatic treatments (). During the MWA procedure, we found that the hemangiomas significantly reduced within a shorter timeframe with rapid evaporation of the blood components inside the tumor due to the high temperature, with the ablation time being almost 50% shorter than the previous average time. The effectiveness rate concerning MWA used in the treatment of hemangioma did not decrease, while the degree of liver function damage reduced significantly.
In relation to benign tumors, we considered that the ultimate goal of MWA for large hepatic hemangioma should differ from that of malignant tumors. Increasing disease control and quality of life should be the aim for treating benign tumors. For patients with hemangiomas located in high-risk areas such as the diaphragmatic dome, the hilus or in areas near hollow organs, a small residual range was permissible. If the residual tumor had increased rapidly in size within the follow-up period, a second MWA could be considered, due to the minimally invasive, safe, effective and repeatable characteristics of MWA. In our study, three patients did not receive further treatment because of the small-size and slow-growing nature of their hemangiomas, for which the residual tissue for one patient showed almost no change in three years.
This study had some limitations. First, this was a retrospective analysis, and there might have been selection bias. Second, the resection methods varied, which might have affected the occurrence rate regarding surgical complications. Third, the data were derived from a single center, and the feasibility for MWA and SR largely depended on the operator’s technique and experience. Fourth, the study was limited due to the small sample size and the short follow-up period. Further prospective studies are required.
In conclusion, this study provided support for the use of MWA as an alternative treatment for large hepatic hemangiomas because of its low risk of complications, the likelihood of complete ablation, a faster postoperative recovery time, and an improved quality of life. Although the MWA procedure did not attain a complete cure rate, most patients with little residue did not require further treatment because of the slow-growing nature of their benign tumors. Whether MWA can be considered first-line therapy for large hepatic hemangiomas requires further confirmation through large-sample, multicentered and randomized controlled trials.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Toro A, Mahfouz AE, Ardiri A, et al. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13:327–339.
- Schnelldorfer T, Ware AL, Smoot R, et al. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg. 2010;211:724–730
- Yeh WC, Yang PM, Huang GT, et al. Long-term follow-up of hepatic hemangiomas by ultrasonography: with emphasis on the growth rate of the tumor. Hepato-gastroenterology. 2007;54:475–479
- Kumashiro Y, Kasahara M, Nomoto K, et al. Living donor liver transplantation for giant hepatic hemangioma with Kasabach-Merritt syndrome with a posterior segment graft. Liver Transpl. 2002;8:721–724
- Ferraz AA, Sette MJ, Maia M, et al. Liver transplant for the treatment of giant hepatic hemangioma. Liver Transpl. 2004;10:1436–1437.
- Hamaloglu E, Altun H, Ozdemir A, et al. Giant liver hemangioma: therapy by enucleation or liver resection. World J Surg. 2005;29:890–893.
- Miura JT, Amini A, Schmocker R, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB. 2014;16:924–928.
- Park SY, Tak WY, Jung MK, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54:559–565.
- Srivastava DN, Gandhi D, Seith A, et al. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging. 2001;26:510–514.
- Huang XQ, Huang ZQ, Duan WD, et al. Severe biliary complications after hepatic artery embolization. World J Gastroenterol. 2002;8:119–123.
- Gaspar L, Mascarenhas F, da Costa MS, et al. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29:45–50.
- Zou H, Yan J, Wu YX, et al. [The new technology of enhanced radiofrequency ablation is safe and effective for treating giant hepatic hemangioma]. Zhonghua Gan Zang Bing za Zhi. 2012;20:261–265.
- Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78–85.
- van Tilborg AA, Nielsen K, Scheffer HJ, et al. Bipolar radiofrequency ablation for symptomatic giant (>10 cm) hepatic cavernous haemangiomas: initial clinical experience. Clin Radiol. 2013;68:e9–e14.
- Sharpe EE, 3rd, Dodd GD, 3rd. Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: report of two cases and review of literature. J Vasc Intervent Radiol. 2012;23:971–975.
- Gao J, Kong J, Ding XM, et al. Laparoscopic vs computerized tomography-guided radiofrequency ablation for large hepatic hemangiomas abutting the diaphragm. World J Gastroenterol. 2015;21:5941–5949.
- Gao J, Ji JS, Ding XM, et al. Laparoscopic radiofrequency ablation for large subcapsular hepatic hemangiomas: technical and clinical outcomes. PLoS One. 2016;11:e0149755.
- Ierardi AM, Floridi C, Fontana F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med. 2013;118:949–961.
- Brace CL. Microwave ablation technology: what every user should know. Curr Prob Diagn Radiol. 2009;38:61–67.
- Ratanaprasatporn L, Charpentier KP, Resnick M, et al. Intra-operative microwave ablation of liver malignancies with tumour permittivity feedback control: a prospective ablate and resect study. HPB. 2013;15:997–1001.
- Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379–1384.
- Ziemlewicz TJ, Wells SA, Lubner MA, et al. Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol. 2014;37:1299–1305.
- Tang XY, Wang Z, Wang T, et al. Efficacy, safety and feasibility of ultrasound-guided percutaneous microwave ablation for large hepatic hemangioma. J Dig Dis. 2015;16:525–530.
- Maruyama M, Isokawa O, Hoshiyama K, et al. Diagnosis and management of giant hepatic hemangioma: the usefulness of contrast-enhanced ultrasonography. Int J Hepatol. 2013;2013:1.
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Chen MF. Hepatic resection for benign tumours of the liver. J Gastroenterol Hepatol. 2000;15:587–592.
- Rocourt DV, Shiels WE, Hammond S, et al. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg. 2006;41:1149–1152.
