Abstract
Objective: To assess the feasibility of microwave ablation (MWA) in treating ectopic secondary hyperparathyroidism (SHPT) in patients with chronic renal failure.
Methods: In this retrospective study, MWA was used to manage 22 SHPT nodules in 20 patients. The laboratory test results, including intact parathyroid hormone (iPTH), serum calcium, phosphorus and alkaline phosphatase (ALP) levels; clinical symptoms; complications before, at one day after MWA, and at the end of follow-up were recorded and compared. Both echogenicity and size of SHPT nodules on ultrasound were documented before and after MWA.
Results: iPTH levels decreased from 1106 ± 396 pg/mL to 264 ± 251 pg/mL (p < .001). Serum calcium and phosphorus levels decreased from 2.53 ± 0.21 mmol/L to 2.14 ± 0.25 mmol/L (p < .001) and from 1.96 ± 0.52 mmol/L to 1.76 ± 0.49 mmol/L (p < .05), respectively. There was no significant change in ALP levels across the different measurements (p = .895). No significant differences were detected in iPTH, serum calcium and phosphorus levels, which were all in the normal range during the follow-up period (3–26 months, mean: 15.49 months) after MWA (p = .186). The echogenicity of SHPT nodules changed from hypoechogenicity to uneven hyperechogenicity with a volume decrease in the majority of the nodules. Mild symptoms of Horner’s syndrome occurred in one patient (5%), which improved during the follow-up period. A hematoma was encountered during ablation (5%). Hypocalcemia occurred in four patients one day after MWA (20%). No other complications were associated with MWA.
Conclusion: MWA is a feasible option to treat ectopic SHPT nodules for destroying parathyroid gland tissue in ectopic SHPT with long-lasting clinical effects.
Introduction
Secondary hyperparathyroidism (SHPT)-induced renal osteodystrophy is a major complication in patients with chronic renal failure. Successful management of SHPT may prove impossible based on medical therapy alone owing to contraindications to drug treatment, medication intolerance and noncompliance.
Currently, some of these patients are treated with parathyroidectomy [Citation1–4], but treatment of ectopic SHPT nodules remains a challenge. Ectopic parathyroid results from defective migration during early development and contamination of the parathyroid tissue during parathyroid resection [Citation5]. The prevalence of ectopic parathyroidism ranges from about 2% and 43% in various anatomical series and 14% and 16% in patients with primary and SHPT, respectively [Citation6]. Traditionally, when the ectopic SHPT was located in the mediastinum, it was treated by mediastinotomy or thoracoscopic approach [Citation7–10]. The remaining ectopic nodules located in the submandibular region, neck or suprasternal fossa are often refractory to surgical resection [Citation11,Citation12] due to anesthesia risks in SHPT patients with poor health condition and pre- and post-surgical complications [Citation13].
Image-guided ablation treatments including radiofrequency ablation (RFA), laser, high-intensity focused ultrasound and percutaneous ethanol injection are widely used in neck tumors, such as benign and malignant thyroid nodules and metastatic lymph nodes, with promising clinical efficiency [Citation14–17]. Solbiati et al. initially reported that the percutaneous alcoholic ablation of enlarged parathyroid glands can be used in cases of SHPT when surgery is contraindicated or problematic [Citation18]. Afterwards, minimally invasive procedures were developed for sporadic cases of hyperparathyroidism (HPT) [Citation19–23]. However, clinical studies employing these techniques are extremely limited [Citation22]. Machi recommended that ectopic parathyroid hyperplasia be treated with RFA [Citation24], but this has not been supported by other researchers due to the difficulty in targeting ectopic glands [Citation20].
Promising results have been reported with the use of microwave ablation (MWA), a minimally invasive ablation technique, to treat incipient and recurrent SHPT nodules [Citation25,Citation26]. The aim of the present study was to determine the feasibility of MWA in terms of short-term efficacy and safety in chronic renal failure patients with ectopic SHPT.
Materials and methods
General clinical data
This was a retrospective study. Between March 2014 and March 2016, the data of 20 chronic renal failure patients (12 male and eight female) with ectopic SHPT treated with MWA were analyzed in China-Japan Friendship Hospital. One hundred and sixty-seven other patients with SHPT in situ nodules underwent parathyroidectomy during the same period. Regarding the surgical history of the 20 enrolled patients, parathyroidectomy had been performed once in 10 patients, twice in four patients, and six patients had no history of parathyroidectomy. Additional, three patients had a history of failed renal transplantation. The total 22 SHPT nodules located in ectopic position were diagnosed by both ultrasound and 99mTc sestamibi scan (MIBI) in the 20 enrolled patients. All nodules met the inclusion criteria and were ablated. The study was approved by the Institutional Review Board of China-Japan Friendship Hospital and written informed consent was obtained from all participating patients.
The eligibility criteria for MWA procedure in this study were as follows: (1) a clear history of SHPT and medication intolerance; (2) intact parathyroid hormone (iPTH) levels greater than 800 pg/mL [Citation27] or less than 800 pg/mL but with uncontrolled hypercalcemia; (3) at least one enlarged, possible ectopic SHPT nodule was seen on ultrasound and was accessible to MWA treatment; (4) the needle path was unobstructed by any major blood vessels, nerves or esophagus; (5) radionuclide concentration was seen in the nodules in both early and delay phases of the MIBI scan; (6) no bleeding tendency, i.e., prothrombin time less than 25 s, prothrombin activity higher than 40%, and platelet count greater than 40 cells ×109/L; (7) no serious coexisting complications such as cardiac insufficiency or hypertension. Exclusion criteria included recurrent laryngeal nerve injury after laryngoscopic examination in patients presenting with voice changes. If the criteria for MWA were not fulfilled initially, it was performed once the unmet conditions had been corrected.
Procedure and follow-up
Both ultrasound and MIBI were performed to locate the ectopic SHPT nodules [Citation6,Citation28,Citation29]. Ultrasound examination was performed with real-time color Doppler using a 10.0/3.5 MHz transducer to identify the ectopic SHPT nodule (Aplio 500, Toshiba, Japan, ). The ultrasound examination revealed suspected SHPT nodules along with the surrounding anatomical structures. Contrast-enhanced ultrasound (CEUS) (Sonovue, Bracco Company, Milan, Italy) was used to evaluate with both the enhancing characteristics of the SHPT nodules pre-ablation () and the curative effect post-ablation (). The MIBI scan (SymbiaT2, Siemens, Munich, Germany) was conducted prior to ablation () due to its high sensitivity (85%) and specificity (100%) in the diagnosis of SHPT [Citation30].
Figure 1. Microwave ablation of ectopic secondary hyperparathyroidism nodule in the mandibular area. (A) Hypoechogenic nodule (thin arrow) without blood signal beside the carotid artery (thick arrow) in pre-ablation CDFI ultrasound scan in the 53-year-old male patient. (B) A uniform hyper-enhanced nodule (thin arrow) beside the carotid artery (thick arrow) was displayed in contrast-enhanced ultrasound pre-ablation. (C) Nodule with radioactivity (black arrow) in late phase of MIBI scan. (D) Hyperechoic region inside nodule (thin arrow) beside the carotid artery (thick arrow) during ablation. (E) A non-enhanced area covered the nodule (thin arrow) beside the carotid artery (thick arrow), which suggested complete ablation. (F) Small and iso-echogenic SHPT nodule (thin arrow) beside the carotid artery (thick arrow) was displayed in ultrasound one day after ablation.
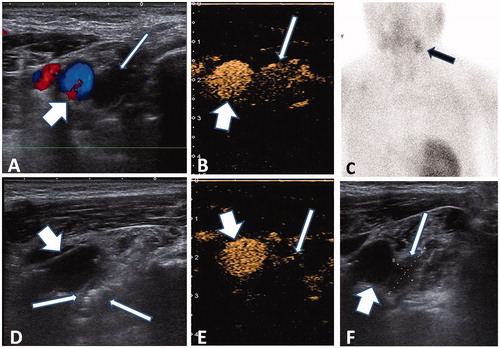
MWA was performed as part of an inpatient regimen by a radiologist who had over six years’ experience in MWA of thyroid and parathyroid nodules. Before ablation, intravenous access was obtained via an antecubital vein. Patients were placed in the supine position with the neck extended. After sterilizing the neck, local anesthesia was administered through a puncture site using 1% lidocaine hydrochloride. A lidocaine and normal saline mixture (1:3 ratio) was injected into the area around the hyperplastic nodule for heat insulation, nerve isolation and local anesthesia [Citation20]. We used a microwave generator and a 16-gauge internally cooled antenna with a 0.3-cm tip (Intelligent Basic Type of Microwave Tumor Ablation System, Nanjing ECO Microwave System Co. Ltd, Nanjing, China). The antenna was inserted freehand into the parathyroid nodule under US-guidance using a 10 MHz linear probe for nodules in the neck and 10.0/3.5 MHz probe for nodules in the suprasternal fossa. Before each ablation, the needle tip was monitored by ultrasound to ensure its location inside the nodule. During ablation, the needle tip was held in a quiescent state for 30 s during microwave irradiation (30 W) and repeat radiation was administered 2–3 times with an interval of 5 s to prevent heat injury to the surrounding critical structures [Citation31] (). The antenna was separately inserted into different units for ablation. Power was suspended if there was pain intolerance. The ablation was terminated when transient hyperechoic zones were identified inside the whole nodule. Ten minutes after ablation, CEUS was used to evaluate the effect of ablation [Citation24]. If the non-enhanced zone covered the ablated nodule, a complete ablation had been achieved (). In case of nodular enhancement inside the nodule, additional ablation was immediately performed.
An anesthesiologist assisted the radiologist in case of pain, vasovagal reactions or uncontrollable hematoma pressing on the trachea. The total ablation time was recorded.
At the end of the procedure, mild compression with bagged normal saline (4 °C) was applied to the site of the needle path for 20 min. All patients remained under observation for 2 h after the procedure.
Ultrasound examination was performed at 2 and 24 h after ablation to identify possible hematomas or other complications, and then repeated at 3-month intervals for 1 year. If the patient suffered hoarseness for longer than 24 h, laryngoscopy was implemented to exclude recurrent laryngeal nerve injury. Major and minor complications were those defined by the Society of Interventional Radiology [Citation32]. Levels of iPTH, serum calcium, phosphorus and alkaline phosphatase (ALP) were tested on days 1 and 7, and at 1, 3, 6, 9 and 12 months after ablation.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 for Windows (IBM SPSS Statistics, Armonk, NY). The serum calcium, phosphate and ALP levels before and after ablation were expressed as mean ± standard deviation (SD) and the paired-samples t-test was performed between the two moments. The values of iPTH were expressed as median (interquartile range) given their abnormal distribution and paired Wilcoxon’s signed-rank test was performed between the two moments. Values of p < .05 were considered statistically significant.
Results
The age range of the enrolled patients was 42–67 years (53.2 ± 10.3 years). The underlying nephropathy causes included nephritis in 14 cases (70%), hypertensive nephropathy in three cases (15%), obstructive nephropathy in one case (5%) and polycystic kidney in two cases (10%). Sixteen patients were undergoing hemodialysis and four were undergoing peritoneal dialysis, with a dialysis time range of 1–20 years (9.6 ± 6.8 years). The maximum diameter of the 22 ectopic nodules was 1.0–3.3 cm (mean ± SD: 1.68 ± 0.55 cm). Regarding the location of the ectopic SHPT, 18 (81.8%) occurred in the suprasternal fossa, one (4.5%) occurred outside of the left carotid artery, one (4.5%) occurred inside the thyroid and two (9.1%) occurred in the mandibular area. One patient had coronary disease as a comorbidity (1/20, 5%). SHPT-associated complications included soft tissue calcification (2/20, 10%), Achilles tendon rupture (2/20, 10%), anemia (4/20, 20%) and hypertension (10/20, 50%).
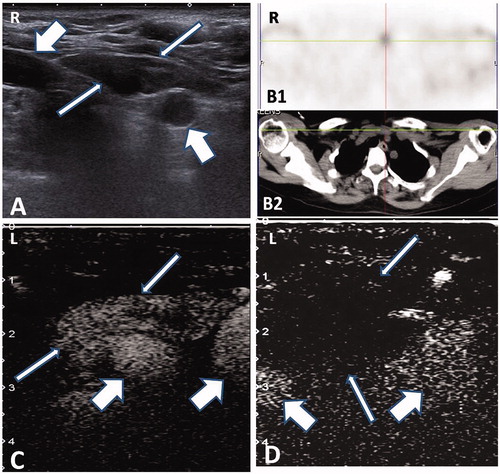
Twenty-two SHPT nodules in 20 patients that matched the inclusion criteria were treated by MWA in 20 treatment sessions. All of the 22 nodules showed radioactivity associated with early and late phases of the MIBI scan () and hyperenhancement in the arterial phase during pre-ablation CEUS (). The ablation time was 65–395 s (mean ± SD: 207 ± 91 s), and the number of puncture sites ranged from 3 to 8 (mean ± SD: 5.2 ± 1.8 sites) in a single nodule. After ablation, all nodules showed no enhancement during CEUS, indicating complete ablation ().
Figure 2. Microwave ablation of ectopic secondary hyperparathyroidism nodule in the suprasternal fossa. (A) A 49-year-old female patient had a hypoechogenic nodule (thin arrow) between the right and left carotid artery (thick arrow) in the suprasternal fossa. (B) MIBI scan (B1) and corresponding CT scan (B2) showing the radioactive nodule located on the suprasternal fossa (cross mark). (C) Ectopic SHPT nodule showing non-uniform hyper-enhancement (thin arrow) in front of the carotid artery (thick arrow) in contrast-enhanced ultrasound pre-ablation. (D) A non-enhanced area covered the nodule (thin arrow) beside the carotid artery (thick arrow), which suggested complete ablation.
The follow-up period ranged from 2 to 26 months (mean ± SD, 15.3 ± 4.9). Before MWA, the mean iPTH level in the 20 patients was 1106 ± 396 pg/mL, which decreased to 264 ± 251 pg/mL one day after MWA (p < .001) and was 408 ± 407 pg/mL by the end of the follow-up (p < .001). Toward the end of follow-up, 16 patients (80%) had iPTH levels within the range (≤600 pg/mL) recommended by the recent Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines for Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) [Citation4]. Similar changes occurred with respect to serum calcium levels, which were 2.53 ± 0.21 mmol/L before MWA, 2.14 ± 0.25 mmol/L one day after MWA and 2.27 ± 0.11 mmol/L at the end of the follow-up, with a statistically significant decrease post-ablation (p=.003, p=.012, p<.05). Hypercalcemia was present in 10 patients before MWA (10/20, 50%), in eight patients one day after MWA (8/20, 40%) and in one patient at the end of follow-up (5%). The same pattern was found in phosphorus levels: 1.96 ± 0.52 mmol/L before MWA, 1.76 ± 0.49 mmol/L one day after MWA (p=.024) and 1.51 ± 0.39 mmol/L at the end of the follow-up (p=.032). In addition, hyperphosphatemia was present in 14 patients (14/20, 70%) before and one day after MWA and in two patients (2/20, 10%) at the end of the follow-up. ALP levels were 241 ± 191 mmol/L before MWA, 238 ± 177 mmol/L one day after MWA (p=.896) and 207 ± 136 mmol/L at the end of follow-up (p=.564, ). Hypertension was fully resolved in four patients (40%) and unchanged in six patients. The anemia present in four patients did not recover after MWA. Interestingly, multiple soft tissue calcifications that were present in two cases before MWA eventually atrophied post-MWA.
Table 1. Biochemical parameters before and after MWA.
According to the results of the ultrasound follow-up, the echogenic characteristics of all the SHPT nodules changed from uniform or uneven hypoechogenicity to uneven hypo- or normal echogenicity (). The volume of most nodules decreased. Four ablated nodules (18.2%) disappeared in ultrasound, 14 (63.6%) decreased in volume by more than 50% and four (18.2%) had decreased by less than 50% at the end of the follow-up.
A mild form of Horner’s syndrome occurred in a patient (5%) with ectopic SHPT nodule located outside of the left carotid artery after MWA. The patients had no discomfort during ablation, but had blepharoptosis three days later, especially post meridiem. However, this symptom improved mildly during follow-up. A hematoma occurred during ablation of a nodule in the suprasternal fossa in one patient (5%). A 2 U hemocoagulase atrox injection (Penglai Nuokang Pharmaceutical Co. Ltd, Yantai, China) was immediately administered intravenously and the hematoma was controlled. These two cases were defined as major complications. Hypocalcemia occurred in eight patients one day after MWA and was corrected by calcium supplementation in most cases, except one case with slight hypocalcemia at the end of follow-up. Transient mild to moderate pain was encountered as a side effect in three patients (3/20, 15%) during ablation, but receded spontaneously after ablation. Hypocalcemia and pain were regarded as minor complications. No other complications were reported during the follow-up period.
Discussion
Hyperparathyroidism includes primary, secondary and tertiary types and usually result from parathyroid gland hyperplasia that produces excess parathyroid hormone (PTH). Although the etiologies differ, parathyroid hyperplasia, laboratory investigation findings and clinical symptoms are similar [Citation33]. So, removal of the hyperplastic parathyroid glands by resection and ablation is an efficient treatment choice for the three types of HPT.
Ectopic SHPT, mostly seen in the secondary and tertiary types, may occur in different areas. Roy et al. found that ectopic parathyroid glands were predominantly located in the thymus (38%), followed by the retroesophageal region (31%) and then the intrathyroidal region (18%) [Citation28]. In the present study, the ectopic nodules occurred in the suprasternal fossa (81.8%), inside the thyroid (4.5%), the mandibular area (9.1%) [Citation34] and outside of the left carotid artery (4.5%). Eighteen patients presented with a single ectopic nodule (90%) and two patients presented with two nodules (10%), which were consistent with a prior report [Citation28].
The present pilot study enrolled 20 cases, representing the first experience with the MWA technique in patients with ectopic SHPT. Twenty sessions were performed to successfully manage 22 SHPT nodules. These nodules did not require any further treatments such as percutaneous ethanol injection therapy, high-intensity focused ultrasound or laser ablation [Citation22,Citation23,Citation35]. There was a marked decrease in serum iPTH levels in all patients one day after MWA. At the end of follow-up, most patients (80%) had iPTH levels within the range recommended by the recent KDIGO CKD-MBD guidelines [Citation4]. The results were inferior to those of thoracoscopy and open surgery [Citation7–10,Citation36]; however, MWA has a shorter operation time, fewer complications and wider indications. Although the values of iPTH slightly increased at the end of the follow-up period compared with levels 1 day after MWA, this difference was not significant. Concomitantly, the levels of serum calcium and phosphorus also improved, with both levels decreasing significantly after MWA. Moreover, the shrinkage of soft tissue calcification post-ablation in two cases also suggests an improvement in calcium-phosphorus metabolism after MWA.
MWA treatment resulted in the lowering of iPTH, serum calcium and phosphorus levels. These promising results demonstrate MWA may inactivate HPT-related cells. Thus, MWA constitutes a non-surgical alternative to parathyroidectomy of ectopic SHPT nodules.
In the present study, complications included Horner’s syndrome (5%), hematoma (5%) and hypocalcemia. Kim et al. reported a lower incidence of Horner’s syndrome (0.1%, 1/875) in a retrospective study about RFA of benign thyroid nodules and recurrent thyroid cancer [Citation37]. Thermal injury or compression by hematoma of the sympathetic ganglion was considered to explain this complication. In the present study, the cause of Horner’s syndrome may be the direct thermal injury of the sympathetic nerve because of its proximity to the ectopic SHPT nodule as seen in the pre-ablation ultrasound. Sufficient hydrodissection between the nodule and nerve could have prevented thermal injury [Citation37]. The cause of hematoma is that the ectopic nodule in the suprasternal fossa is too close to artery, and antenna mispuncture of the artery may occur during ablation. These cases have been mentioned in a prior report [Citation26]. Hypocalcemia was present in eight patients one day after MWA, which should have been induced by the decrease in iPTH value and hungry bone syndrome; calcium supplementation proved effective in managing this condition.
Limitations of the study are related to the small sample size and the relatively short duration of follow-up. A definitive conclusion of the safety and efficacy of MWA in ectopic SHPT should be based on additional studies with larger sample sizes and longer follow-up periods. Another limitation relates to the lack of pathological biopsy in the present study. According to a prior study, MIBI scans exhibited a sensitivity of 85–89% and specificity of 100% for SHPT nodules [Citation28,Citation30]. Since all the nodules were identified simultaneously by MIBI scans and ultrasound, pre-ablation biopsy was not performed in the present study.
Among the treatments for parathyroid hyperplastic glands in chronic renal failure patients with HPT, RFA, LA and ethanol ablation could achieve similar clinical efficiency compared with that of MWA [Citation18,Citation25,Citation35,Citation38–40]. Although the prominent advantage of MWA is the higher thermal efficiency which could achieve larger ablation with less time used, it is with less manipulative for smaller lesions compared with that of RFA and LA. And the 16-gauge MWA antenna is too thick, it is difficult to puncture the parathyroid lesions with maximum diameter less than 1 cm, especially for the free nodules. A finer antenna may facilitate puncturing and improve the success rate.
In summary, MWA seems to be a technique that is potentially safe and effective in the treatment of ectopic SHPT nodules. Further prospective studies with a larger sample size should be carried out to allow for more definitive conclusions.
Disclosure statement
All the authors have no conflicts of interest or financial connections to disclose.
Additional information
Funding
References
- Cannata-Andía JB, Fernández-Martín JL, Zoccali C, et al. Current management of secondary hyperparathyroidism: a multicenter observational study (COSMOS). J Nephrol. 2008;21:290–298.
- He Q, Zhuang D, Zheng L, et al. Total parathyroidectomy with trace amounts of parathyroid tissue autotransplantation as the treatment of choice for secondary hyperparathyroidism: a single-center experience. BMC Surg. 2014;14:26.
- Sarfati E, Drueke TB. Surgical management of secondary hyperparathyroidism. In: Olgaard K, Silver J, Salusky IB, editors. The spectrum of mineral and bone disorders in chronic kidney disease. 2nd ed. Oxford (UK): Oxford University Press; 2010. p. 543–559.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–S130.
- Tublin ME, Yim JH, Carty SE. Recurrent hyperparathyroidism secondary to parathyromatosis: clinical and imaging findings. J Ultrasound Med. 2007;26:847–851.
- Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp Clin Endocrinol Diabetes. 2012;120:604–610.
- Lu HI, Chou FF, Chi SY, et al. Thoracoscopic removal of hypertrophic mediastinal parathyroid glands in recurrent secondary hyperparathyroidism. World J Surg. 2015;39:400–409.
- Alesina PF, Moka D, Mahlstedt J, et al. Thoracoscopic removal of mediastinal hyperfunctioning parathyroid glands: personal experience and review of the literature. World J Surg. 2008;32:224–231.
- Ravipati NB, McLemore EC, Schlinkert RT, et al. Anterior mediastinotomy for parathyroidectomy. Am J Surg. 2008;195:799–802.
- Randone B, Costi R, Scatton O, et al. Thoracoscopic removal of mediastinal parathyroid glands. A critical appraisal of an emerging technique. Ann Surg. 2010;251:717–721.
- Simental A, Ferris RL. Reoperative parathyroidectomy. Otolaryngol Clin North Am. 2008;41:1269–1274.
- Yen TW, Wang TS, Doffek KM, et al. Reoperative parathyroidectomy: an algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery. 2008;144:611–619.
- Torres PU, Prié D, Beck L, et al. New therapies for uremic secondary hyperparathyroidism. J Ren Nutr. 2006;16:87–99.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44:14–36.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:11–22.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38:25–36.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39:1023–1030.
- Solbiati L, Giangrande A, De Pra L, et al. Percutaneous ethanol injection of parathyroid tumors under US guidance: treatment for secondary hyperparathyroidism. Radiology. 1985;155:607–610.
- Xu SY, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54:e137–e140.
- Wang R, Jiang T, Chen Z, et al. Regression of calcinosis following treatment with radiofrequency thermoablation for severe secondary hyperparathyroidism in a hemodialysis patient. Intern Med. 2013;52:583–587.
- Singh Ospina N, Thompson GB, Lee RA, et al. Safety and efficacy of percutaneous parathyroid ethanol ablation in patients with recurrent primary hyperparathyroidism and multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2015;100:E87–E90.
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol. 2012;199:1164–1168.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. High-intensity focussed ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27:76–80.
- Machi J. Radiofrequency ablation for hyperparathyroidism: can it be a new treatment? Surg Laparosc Endosc Percutan Tech. 2006;16:116.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease—a pilot study. Radiology. 2017;282:576–584.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32:180–186.
- Juergensen PH, Cooper K, Kliger AS, et al. Hyperparathyroidism: a seven-year follow-up. Adv Perit Dial. 1998;14:188–190.
- Roy M, Mazeh H, Chen H, et al. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg. 2013;37:102–106.
- Kim HS, Choi BH, Park JR, et al. Delayed surgery for parathyroid adenoma misdiagnosed as a thyroid nodule and treated with radiofrequency ablation. Endocrinol Metab. 2013;28:231–235.
- Yang J, Hao R, Yuan L, et al. Value of dual-phase (99m)Tc-sestamibi scintigraphy with neck and thoracic SPECT/CT in secondary hyperparathyroidism. AJR Am J Roentgenol. 2014;202:180–184.
- Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244:296–304.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202.
- Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89:1227–1239.
- Rajagopalan MS, Narla VV, Kanderi T, et al. Para-hyoid ectopic parathyroid adenoma localized by Tc-99m MIBI SPECT. Clin Nucl Med. 2008;33:880–881.
- Chen HH, Lin CJ, Wu CJ, et al. Chemical ablation of recurrent and persistent secondary hyperparathyroidism after subtotal parathyroidectomy. Ann Surg. 2011;253:786–790.
- Said SM, Cassivi SD, Allen MS, et al. Minimally invasive resection for mediastinal ectopic parathyroid glands. Ann Thorac Surg. 2013;96:1229–1233.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Peng C, Zhang Z, Liu J, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation of hyperplastic parathyroid gland for secondary hyperparathyroidism associated with chronic kidney disease. Head Neck. 2017;39:564–571.
- Jiang T, Chen F, Zhou X, et al. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J Endocrinol. 2015;2015:1.
- Li X, An C, Yu M, et al. US-guided microwave ablation for secondary hyperparathyroidism in patients after renal transplantation: a pilot study. Int J Hyperthermia. 2019;36:322–327.
