Abstract
Background: Isolated limb perfusion (ILP) is a treatment option for malignancies localized to an extremity and is performed by surgical isolation of the limb which is connected to an extracorporeal circulation system. A high concentration of a chemotherapeutic agent is perfused through the limb, while systemic toxicity is avoided. Currently, the use of packed red blood cells in the priming solution is the norm during ILP. The aim of this study was to investigate the possibility to replace an erythrocyte-based prime solution with a crystalloid-based prime solution while maintaining the regional metabolic oxygen demand during ILP.
Methods: In a single-center, randomized controlled, non-blinded, non-inferiority clinical trial, 21 patients scheduled for treatment with ILP were included and randomized 1:1 to either an erythrocyte-based prime solution (control) or a crystalloid-based prime solution (intervention).
Results: There was a significant difference in lactate level (mmol/L) during the perfusion between the intervention group and the control group (1.6 ± 0.4 vs. 3.6 ± 0.7, p = .001). No significant differences in oxygen extraction (%) (22 ± 11 vs. 14 ± 4, p = .06), oxygen delivery (ml/min) (90 ± 49 vs. 108 ± 38, p = .39), oxygen consumption (ml/min) (14 ± 2 vs. 14 ± 5, p = .85), regional central venous saturation (%) (83 ± 10 vs. 91 ± 4, p = .07) or INVOS (%) (76 ± 14 vs. 81 ± 11, p = .42) were found between the intervention group and the control group.
Conclusion: This study showed no significant improvement with the addition of packed red blood cells into the prime solution in ensuring the metabolic oxygen demand in the treated extremity during ILP, and we, therefore, recommend that a crystalloid-based prime solution should be used.
Background
Isolated limb perfusion (ILP) is a locoregional treatment option for malignancies of the extremities, including melanoma and sarcoma that was pioneered in the 1950s by Creech et al. [Citation1]. The method has since then been refined with current results showing an overall response rate (ORR) ranging between 65% and 100%, with a complete response (CR) rate between 25% and 76% [Citation2].
The technique consists of surgical isolation of the involved limb which is connected to an extracorporeal circulation (ECC). This allows delivery of high doses of a chemotherapeutic agent, usually melphalan (M-ILP) or the combination of melphalan and TNF-α (MT-ILP), to the affected limb, while systemic toxicity is avoided [Citation3]. An early pharmacokinetic study using melphalan in the ILP setting showed that the peak perfusate levels of melphalan could be 20–100 times higher than when given intravenously [Citation4].
ILP is dependent on ECC and oxygenation of the perfusate. Gibbon [Citation5] reported his pioneering work with perfusion of the entire body during the temporary functional exclusion of the heart and lungs from the rest of the circulation. Since then, perfusion of isolated organs has contributed to the development of various types of perfusions and oxygenation [Citation6,Citation7]. The primary goal of ECC is to provide the oxygen requirements and to facilitate oxygen distribution to all tissues under perfusion. Systemic DO2 (oxygen delivery) is the most important determinant of ‘optimal’ perfusion, and the oxygen delivery is foremost dependent on hemoglobin and its ability to carry oxygen [Citation8].
Currently, the use of a crystalloid priming solution is the norm during cardiopulmonary bypass (CPB), which gives rise to hemodilution which may compromise oxygen delivery at the tissue level. However, advantages of hemodilution include a lower blood viscosity and an improved microcirculatory flow. A determination of optimal hematocrit on CPB requires an assessment of the risks and benefits of both hemodilution anemia and transfusion of erythrocyte concentrate from donors [Citation9]. The use of erythrocyte concentrate from donors are known to be immunomodulatory and might have a negative effect on ILP response rate [Citation10]. Therefore, we hypothesize that a priming solution without addition of erythrocytes from donors would be as efficient concerning the metabolic needs of the perfused extremity.
Therefore, the overall aim of this study was to investigate the possibility to replace an erythrocyte-based prime solution with a crystalloid-based prime solution, while maintaining the same level of metabolic function in the perfused tissues. The primary endpoint was lactate levels during the perfusion and the secondary endpoints were regional central venous oxygen saturation, oxygen delivery, oxygen consumption, oxygen extraction, hemoglobin and hematocrit as well as response, toxicity, complications and survival.
Patients and methods
Study design
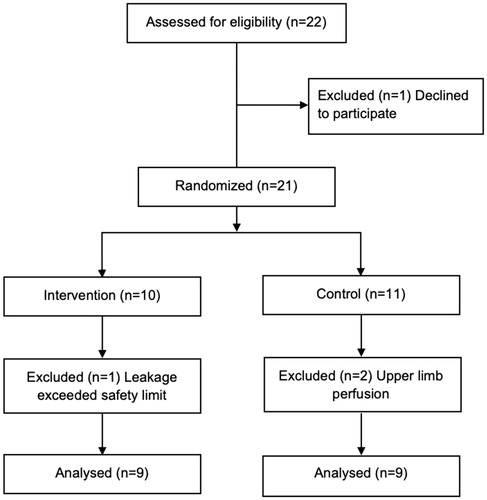
Twenty-one patients were included between 2017 and 2018 in a single-center non-blinded, randomized controlled clinical trial, and were randomized 1:1 to either an erythrocyte-based prime solution (control group) or a crystalloid-based prime solution (intervention group). Inclusion criteria were scheduled ILP, age ≥18 years and written informed consent. Two patients that underwent upper extremity ILP were included and randomized to the control group in the study but were excluded from the analysis due to anatomical differences between upper and lower extremities, especially concerning blood volume in relation to the circuit size, prime volume and expected hemodilution. One patient had a high systemic leakage and the decision not to proceed with ILP was made. This patient was also excluded from the analysis ().
Patients
There were 18 patients included in the final analysis. The median age was 74 years and 39% (n = 7) were females. There were no significant differences in baseline characteristics between the two groups, except in creatinine levels (). The study was approved by the Regional Ethical Review Board at the University of Gothenburg (Dnr 1145-16).
Table 1. Baseline characteristics in the intervention group and the control group.
Priming
The prime solution in the control group (erythrocyte-based, volume 700 ml) consisted of 250 ml Ringer’s acetate (Fresenius Kabi AB, Uppsala, Sweden), 100 ml Tribonat (Fresenius Kabi AB, Uppsala, Sweden), 2500 U heparin (LEO Pharma AB, Malmö, Sweden), 100 ml albumin (Baxalta Innovations GmBH, Vienna, Austria) and 1 unit of packed red cells (approximately 250 ml). The prime solution in the intervention group (crystalloid-based, volume 700 ml) consisted of 500 ml Ringer’s acetate, 100 ml Tribonat, 2500 U heparin and 100 ml albumin.
ECC
The ECC consists of a pediatric Affinity Pixie hollow fiber oxygenator and a 1.2 L hardshell reservoir coated with non-heparin Cortiva Balance Biosurface (Medtronic, Minneapolis, MN, USA) and a ¼-inch perfusion custom circuit (Sorin, Mirandola, Italy). The circuit was assembled on a Stöckert roller pump S5 ECC (Sorin, Munich, Germany) which provides temperature monitoring, line pressure monitoring, level sensor and bubble monitoring. The water heater for the extracorporeal circuit was an Heto HMT 200 (Heto-Holten A/S, Allerod, Denmark). After assembly of the circuit, the circuit was primed. The prime was recirculated and warmed with the heater set to 41–42 °C before starting the ECC.
ILP technique
All patients were operated under general endotracheal anesthesia using fentanyl, propofol, rocuronium and sevoflurane. Lower extremity ILP was performed via a femoral approach with dissection and cannulation of the corresponding artery and vein. The remaining collateral vessels were compressed using an Esmarch rubber band and the cannulas were then connected to the ECC. The perfusion was performed with a pump flow rate between 0.4–1.5 L/min to ensure a stable level in the reservoir. FiO2 was set to 0.40–0.60, with the aim to achieve a pO2 of 25–35 kPa. Sweep gas at 0.40–0.60 L/min and CO2 at 0.02–0.05 L/min to reach a pCO2 of 4.5–6.0 kPa and a pH of 7.35–7.45. The total perfusion time was approximately 90 min (warming during 30 min, infusion of melphalan during 20 min and circulation of the chemotherapy during an additional 40 min) and the highest perfused tissue temperature was kept at a maximum level of 40 °C (mild hyperthermia). The dose of melphalan was calculated as 10 mg/L perfused tissues for lower limb. At the end of the perfusion, the limb was irrigated with 3000–4000 ml of Ringer’s acetate (Fresenius Kabi AB, Uppsala, Sweden) [Citation11].
Leakage monitoring
Leakage was monitored with the injection of 10 MBq Technetium-labeled albumin (Vasculosis, Cis Bio, France) systemically and 100 MBq into the perfusion circuit. A precordial scintillation probe (MedicView, Sweden) was placed over the heart to detect and measure any leakage from the perfusion circuit to the systemic circulation [Citation11].
Temperature measurements
The temperature of the prime solution was adjusted (via a thermistor positioned in the arterial catheter) so that the highest tissue temperature, measured subcutaneously and intramuscular 20 cm above and below the lateral joint space in the knee, should reach a temperature of 40.0 °C.
Blood gas measurements
A CDI blood monitoring system 500 (Terumo, Elkton, MD, USA) was connected to the venous and arterial line. Blood gas samples were measured using the I-STAT system (Abbott, Princeton, NJ, USA).
INVOS rSO2 (regional oxygen saturation)
An INVOS™ Cerebral/Somatic Oximetry Adult Sensors (Medtronic, Minneapolis, MN, USA) was used, and this system provides real-time data about oxygen supply and demand. The INVOS sensors were placed on each leg (at the same level bilaterally), primarily on the anterolateral side of the tibialis muscle if possible, otherwise elsewhere on the tibia muscles. INVOS was continuously measured before perfusion, during the perfusion and up to 30 min after perfusion.
Response
Clinical responses were evaluated and reported as the response at 3 months using the WHO criteria [Citation12]. For a response to be considered as a complete one (CR), all lesions should have been clinically not detectable at the time of clinical examination. Partial response (PR) was defined as a clinical decrease of more than 50% of the total tumor burden both in terms of number of lesions or diameter. Progressive disease (PD) was defined as an increase of more than 25% in existing lesions or the appearance of new lesions not previously present. Stable disease (SD) was defined as a result where none of the abovementioned criteria for CR, PR or PD were met.
Local toxicity
Local toxicity was measured according to Wieberdink classification from I to V, where I is no reaction, II slight erythema and/or edema, III is considerable erythema and/or edema with some blistering, IV is extensive epidermolysis and/or obvious damage to deep tissues, and V induces a reaction that may necessitate amputation [Citation13].
Statistical evaluation
A power analysis was performed based on the lactate levels at perfusion start from a cohort consisting of all 28 patients treated with ILP at Sahlgrenska University Hospital during 2016. The mean value in this cohort was 3.0 with an SD of 0.7, and by defining non-inferiority as a lactate level of 4.0 or less using an alpha value at 0.05 and a power of 80% we included nine patients in each group. Statistical analyses were performed using SPSS (Statistical Package Social Sciences, IBM, version 22). Results are presented as mean and SD, median and range, or number and percentage (%). Student’s t-test, Mann–Whitney U test or Fisher’s exact test was used to compare groups. A probability level (p values) of less than .05 has been considered to indicate statistical significance.
Results
Response and survival
The ORR was higher in the intervention group (100% vs. 78%), but without statistical significance (p = .13), with a similar finding for CR rate (56% vs. 44%, p = .64) and PR rate (44% vs. 33%, p = .63). The 1-year survival was 78% in the intervention group and 67% in the control group with no statistically significant difference between the two groups ().
Table 2. Follow-up characteristics.
Toxicity
Local toxicity was measured 3 months postoperatively according to Wieberdink classification from I to V and showed no significant difference between the intervention group and control group (grade I–II, 67% vs. 56%, p = .63; grade III, 33% vs. 33%, p = 1.0; grade IV, 0% vs. 11%, p = .30) ().
Complications
No significant postoperative complications were found in the two groups. In 3 out of 18 patients, there was 1 Erysipelas, 1 advanced leg edema and 1 leg ulcer but no significant difference was found between the intervention group and the control group (11%, n = 1 vs. 22%, n = 2) and they did not prolong hospital stay ().
ECC
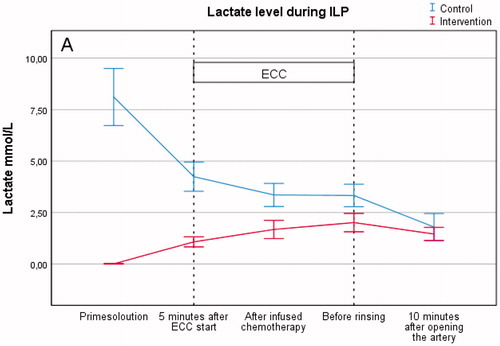
The mean ECC time was 97 min with no significant differences between the two groups. However, the control group had longer time of the femoral artery clamped compared to the intervention group (119 vs. 139 min). A slightly higher FiO2 (0.48% vs. 0.45%), arterial oxygen content (14 vs. 11 ml) and venous oxygen content (12 vs. 9). The major difference between the two groups was the lactate level in the prime solution (0.01 vs. 8.1 mmol/L) that also reflected on the lactate level on ECC (1.6 vs. 3.6). The reason for this was the addition of packed red blood cells, containing lactate, in the control group ().
Table 3. Treatment characteristics during the perfusion.
Lactate
The level of lactate (mmol/L) during the perfusion was significantly higher in the control group than in the intervention group (3.6 ± 0.7 vs. 1.6 ± 0.4, p = .001). The lactate level in the prime solution was also significantly higher in the control group (8.1 ± 1.8 vs. 0.01 ± 0.0, p = .001), however the baseline lactate level after induction showed no significant difference (1.2 ± 0.7 vs. 1.0 ± 0.3, p = .37) (, .
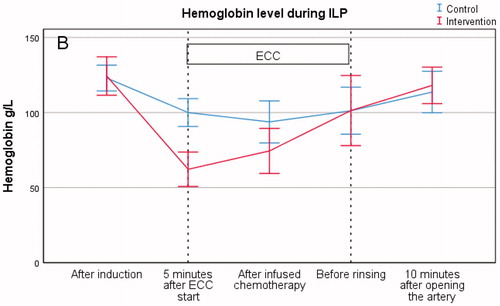
Hemoglobin
Hemoglobin level (g/L) showed a statistically significant difference initially after the 5-min measurement between the control group and the intervention group (100 ± 12 vs. 62 ± 15, p = .001). Measurements, following chemotherapy infusion, showed no statistical difference between the control group and the intervention group in hemoglobin level (94 ± 18 vs. 74 ± 20, p = .05). Furthermore, no difference was found between the two groups prior to extremity rinsing with Ringer’s solution (101 ± 20 vs. 101 ± 30, p = .99) (, .
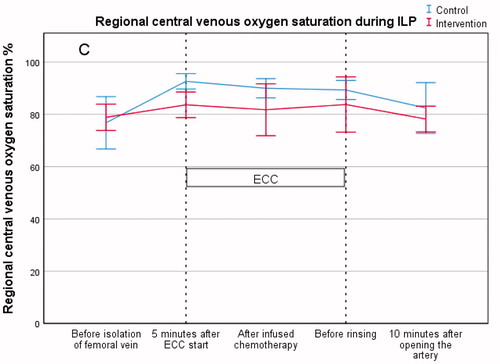
Regional ScvO2 (Central venous oxygen saturation)
Results showed a significant difference in ScvO2 (%) between the control group and the intervention group after 5 min of perfusion (93 ± 4 vs. 84 ± 6, p = .003). There was no significant difference in ScvO2 (%) between the groups following chemotherapy infusion (90 ± 5 vs. 82 ± 13, p = .10) nor before rinsing (89 ± 5 vs. 84 ± 14, p = .28) (, .
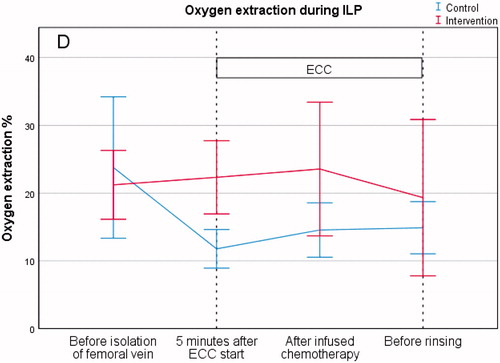
Oxygen extraction
The results from the measurements of oxygen extraction (%), before perfusion, showed no significant differences between the control group and the intervention group (24 ± 16 vs. 21 ± 7, p = .62). There was a significant difference between the control group and the intervention group at the 5-min reading (12 ± 4 vs. 22 ± 7, p = .002). However, there was no difference between the control group and the intervention group in oxygen extraction (%) after infused chemotherapy (15 ± 5 vs. 24 ± 13, p = .08) or before rinsing (15 ± 5 vs. 19 ± 15, p = .42) (, .
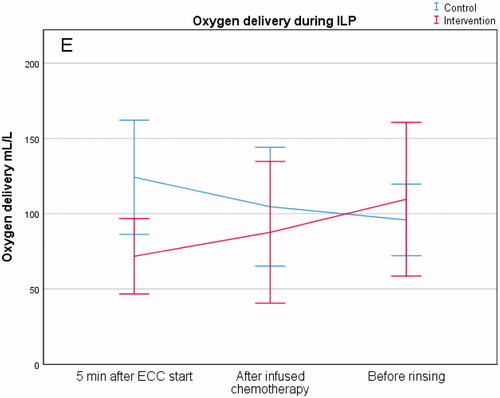
Oxygen delivery
The amount of oxygen delivery (ml/min), after 5 min of perfusion, showed a difference between the control group and the intervention group (124 ± 49 vs. 72 ± 33, p = .019). There was no difference between the two groups after the infusion of chemotherapy (105 ± 51 vs. 88 ± 61, p = .53) or before rinsing (96 ± 31 vs. 110 ± 66, p = .58) (, .
Discussion
This is the first randomized trial studying the value of adding packed red blood cells to the prime solution during ILP. The hypothesis was that a crystalloid-based prime solution would be just as efficient in ensuring adequate perfusion concerning metabolic oxygen demand as the blood-based solution and the lactate level, as a marker for perfusion quality, was chosen as the primary endpoint. The primary endpoint with a lactate level of ≤4.0 mmol/L was met for all included patients (highest lactate level measured during ECC in the intervention group was 2.9 mmol/L). The results during the perfusion showed no significant statistical difference between the two groups concerning oxygen delivery, oxygen consumption, oxygen extraction or regional central venous oxygen saturation. Based on this, we can conclude that ILP of the lower extremity with or without packed red blood cells results in equal quality perfusion in terms of metabolic oxygen demand.
The primary endpoint was based on the lactate levels from 28 patients treated with ILP during 2016 in our institution. The level was set to 3.0 mmol/L and the power analysis was based on this. However, we did not account for the fact that we used packed red blood cells with a variable range of lactate, which caused higher lactate levels in the control group, during the perfusion. The lactate levels decreased during the ECC in the control group, while slightly increased in the intervention group. However, the mean lactate level during ECC in the intervention group was still within the conventional normal range (<2.0–2.5 mmol/L) [Citation14].
Ranucci et al. [Citation15] concluded that a high degree of hemodilution during CPB is a risk factor for postoperative renal dysfunction; however, the detrimental effects may be reduced by increasing oxygen delivery. Our study show that the oxygen delivery in the control group and in the intervention group are well over the critical level of 272 ml/min/m2 that Ranucci et al. [Citation15] reported in the study for total body oxygen delivery, considering that there are no vital organs in the leg and that a femoral blood flow at rest is approximately 5–10% of total cardiac output (0.15–0.45 L/min) [Citation16].
No significant difference was found in hemoglobin levels between the two groups during ECC before rinsing, even though there is a significant difference in hemoglobin levels between the two groups 5 min after ECC start. This finding could be due to leakage from the systemic circulation to the extremity and ECC circulation, however, there was no significant difference in the amount of leakage between the two groups. In the group of patients having no leakage at all (three patients in each group), the hemoglobin level in one patient was as low as 36 g/L at one point, however, this did not affect the level of delivered oxygen.
Measuring the INVOS oximetry on both legs during perfusion enabled us to compare the non-ECC perfused leg with the ECC perfused leg during the procedure. This could be used as a guide to establish a threshold for the INVOS level not to go below to ensure a perfusion comparable to the patients’ native oximetry values in the contralateral leg. The results showed that the INVOS oximetry values were higher in the ECC perfused leg compared to the non-ECC perfused leg in both groups.
We hypothesized that a prime solution without addition of erythrocytes from donors would be as efficient concerning the metabolic oxygen demand of the perfused extremity, but with less side effects, as a prime solution containing donor blood especially concerning immunogenic response. In accordance with our hypothesis, we were able to demonstrate that an ILP without donor blood in the prime solution was just as efficient in meeting the metabolic oxygen demand of the perfused extremity as a prime solution containing donor blood.
Interestingly, there was a non-significant trend in improved response between the two groups, favoring a prime solution without donor blood (ORR 100% vs. 78%). If such a relationship holds in a much larger series of patients it could serve as an area for further investigation in the potential mechanism including immunological effects caused by the donor blood [Citation17]. However, the results concerning any of the secondary endpoints in this study have to be analyzed with extreme caution due to the low number of patients, and any conclusions cannot be drawn.
In conclusion, our study could not detect any significant differences between the two groups in ensuring the metabolic oxygen demands during ILP, treatment response, toxicity or complications although no packed red blood cells were added in the prime solution in the intervention group. Based on this, we recommend not to add packed red blood cells in the perfusate during ILP.
Acknowledgements
Captain Seutkins and the crew at Stena Impeccable are acknowledged for giving ROB the possibility to experience the Stena Bulk research retreat award.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Creech O Jr., Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632.
- Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, et al. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 2010;15:416–427.
- Olofsson R, Mattsson J, Lindner P. Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hyperthermia. 2013;29:551–557.
- Minor DR, Allen RE, Alberts D, et al. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55:2638–2644.
- Gibbon JH. Artificial maintenance of circulation during experimental occlusion of pulmonary artery. Arch Surg. 1937;34:1105–1131.
- Kirklin JW, Donald DE, Harshbarger HG, et al. Studies in extracorporeal circulation. I. Applicability of Gibbon-type pump-oxygenator to human intracardiac surgery: 40 cases. Ann Surg. 1956;144:2–8.
- Miller BJ, Gibbon JH, Fineberg C. An improved mechanical heart and lung apparatus: its use during open cardiotomy in experimental animals. Med Clin North Am. 1953;1:1603–1624.
- Machin D, Allsager C. Principles of cardiopulmonary bypass. Contin Educ Anaesth Crit Care Pain. 2006;6:176–181.
- Murphy GS, Hessel EA 2nd, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–1417.
- Goubran H, Sheridan D, Radosevic J, et al. Transfusion-related immunomodulation and cancer. Transfus Apher Sci. 2017;56:336–340.
- Katsarelias D, Radbo E, Ben-Shabat I, et al. The effect of temperature and perfusion time on response, toxicity, and survival in patients with in-transit melanoma metastases treated with isolated limb perfusion. Ann Surg Oncol. 2018;25:1836.
- World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979. p. 45.
- Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910.
- Wacharasint P, Nakada TA, Boyd JH, et al. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38:4–10.
- Ranucci M, Romitti F, Isgro G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–2220.
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249.
- Johansson J, Kiffin R, Andersson A, et al. Isolated limb perfusion with Melphalan triggers immune activation in melanoma patients. Front Oncol. 2018;8:570.