Abstract
Background and objectives: The incidence of incisional hernia (IH) after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is largely unknown.
Methods: We conducted a retrospective study to identify patients who underwent CRS/HIPEC from 2001 to 2016. Patients were followed postoperatively for a minimum of two years. The primary outcome was the occurrence of an IH identified either on CT scan or physical examination. Univariate and multivariable logistic regression models were used to test associations with IH.
Results: We identified 155 patients who underwent CRS/HIPEC; 26 patients (17%) were diagnosed with an IH at a median time of 245 days (Interquartile range [IQR] 175 – 331 days). On multivariable analysis, older age [50–64 vs. 18–49 years: hazard ratio (HR) = 0.08; 95% confidence interval (CI), 0.01 to 0.64)], female gender (HR = 0.09; 95% CI, 0.01 to 0.75), and increased BMI (>30 vs. <25; HR = 0.03; 95% CI, 0.01 to 0.37) were significant independent predictors of IH.
Conclusions: The incidence of IH in this high-risk patient population treated with CRS/HIPEC is similar to that after other abdominal cancer operations. Nevertheless, the occurrence of IH is an important patient outcome, so alternative closure techniques for reducing IH should be studied in this patient population.
In a single-institutional study, the incidence of incisional hernia was 17% after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Independent risk factors of incisional hernia were older age, female gender and obesity.
Synopsis
Introduction
Incisional hernia (IH) after intra-abdominal cancer surgery occurs frequently after a vertical midline incision. In one single-center study of 491 patients who underwent resection for intra-abdominal cancer, IH occurred in 43% of patients [Citation1]. Risk factors for IH can be either patient- or treatment-related and include obesity, previous surgery, ascites, male gender, decreased suture to wound length ratio, site-specific infections, long surgery time, long incision length and use of interrupted sutures [Citation2–7]. The occurrence of IH can cause substantial pain and discomfort, decreased physical functioning and lower body image outcomes [Citation8]. Occasionally, IH can lead to obstruction and strangulation necessitating emergent surgery. Furthermore, the long-term recurrence rate after IH repair is about 30% [Citation9]. Thus, avoidance of an IH is a meaningful patient outcome after oncologic surgery.
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is increasingly used to treat peritoneal metastases from intra-abdominal malignancies, especially appendiceal neoplasms, colorectal cancer, ovarian cancer, and peritoneal mesothelioma. CRS/HIPEC is a potentially ‘hernia-genic’operation because patients frequently have undergone previous abdominal surgeries; CRS/HIPEC is a long procedure; the incisions are generally long; many patients have ascites; and patients are immunosuppressed by the delivery of intraperitoneal chemotherapy. Since most studies only report short-term morbidity and/or long-term oncologic outcomes after CRS/HIPEC, the incidence of IH is largely unknown. The opportunity to evaluate IH is well-suited in this population because most patients undergo routine cancer surveillance with computed tomography (CT), so IH can be detected by physical examination and CT.
The purpose of this single-center study was to evaluate the rate of IH after CRS/HIPEC and to assess the impact of specific patient and treatment factors on the risk of IH in this unique patient population.
Methods
We conducted a retrospective single-center study to identify patients who underwent CRS plus HIPEC at a university referral hospital between April 2001 and June 2016. All included patients underwent a laparotomy via midline incision. Patients who underwent laparoscopy only or had mesh reconstruction for abdominal wall resection were excluded. Data extracted from the medical record included patient age, race, gender, body mass index (BMI), histology, presence of diabetes mellitus, prior hernia, peritoneal cancer index, presence of ascites, tobacco use, preoperative serum albumin, use of perioperative systemic chemotherapy, and CT scan results. Patients were followed postoperatively for a minimum of two years. This study was approved by the Institutional Review Board at the University of Minnesota.
Patients with abdominal malignancy underwent midline incision after the induction of general anesthesia. Complete cytoreduction was performed to remove all visible disease. Next, intraperitoneal chemotherapy was administered via a closed technique at 39–41 °C for 30 to 90 min. The most commonly used drug for HIPEC was mitomycin C (12.5 to 50 mg/m2 for 90 min; n = 115 patients), followed by oxaliplatin (300 to 400 mg/m2 for 30 min; n = 30 patients). After HIPEC, surgeons performed any necessary anastomoses and closed the fascia. The most common type of fascial closure utilized #1 looped polydioxanone (PDS II, Ethicon). After treatment, patients underwent physical examination every 4 months to assess for recurrent disease. CT scans were generally performed every 4 months for 2 years, then every 6 months for 3 years, then yearly for 5 years.
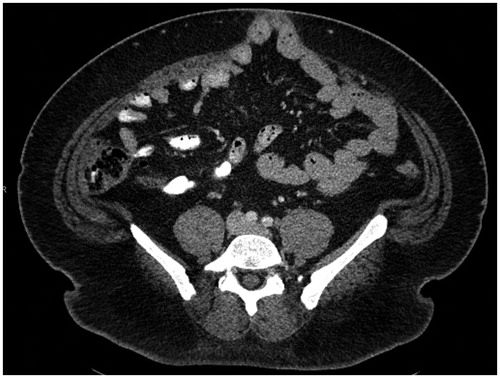
The primary outcome of this study was the occurrence of an IH postoperatively identified either on CT scan or physical examination. IH was defined as an abdominal wall defect with or without bulge in the area of a postoperative scar perceptible or palpable by clinical examination or imaging [Citation10] (). Parastomal and inguinal hernias were not included. Univariate analysis was performed to determine the association between patient and treatment characteristics and the occurrence of IH. Multivariable logistic regression models were used to test adjusted associations with IH. A p value of <.05 was considered statistically significant. All data analysis was performed in SAS.
Results
We identified 155 patients who underwent CRS/HIPEC and had a minimum of 2 years of follow-up after surgery (range: 2 to 13 years). Patient characteristics are included in . Most patients were female and had a history of prior abdominal surgery. The median patient age was 52 years. The most common primary tumors were appendiceal (n = 84, 54.2%), colorectal carcinoma (n = 44, 28.4%), ovarian cancer (n = 9, 5.8%), peritoneal mesothelioma (n = 7, 4.5%), gastric (n = 4, 2.6%), and other (n = 7, 4.5%). Most patients had ascites at the time of CRS/HIPEC.
Table 1. Patient, tumor, and treatment characteristics (n = 155 patients).
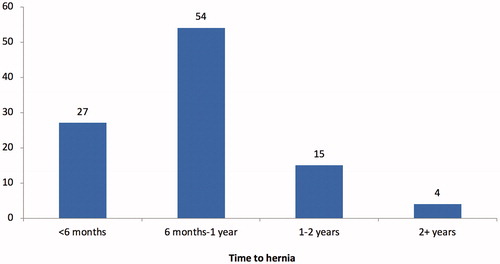
Twenty-six patients (17%) were diagnosed with an IH. The median time from surgery to diagnosis of an IH was 245 days (Interquartile range [IQR] 175 – 331 days). Among hernia patients, 27% of patients developed a hernia within the first 6 months and 54% developed a hernia 6 to 12 months after surgery (). Among patients with an IH, 42% were symptomatic. Ten patients (38%) with IH underwent surgical repair.
Figure 2. Development of incisional hernias after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy over time.
On univariate analysis (), obesity, previous history of an IH, use of preoperative chemotherapy (within 1 year of surgery), and use of postoperative chemotherapy (within 2 years after surgery) were significantly associated the development of an IH. The occurrence of wound infection was not significantly associated with IH; however, the wound infection rate was only 4% in this series, so lack of statistical significance may be secondary to low events. Tumor recurrence was associated with a lower risk of IH (data not shown); this finding is probably secondary to shorter survival and less time to develop IH among patients with tumor recurrence. Predictors of IH-free survival on listed in ; so lower odds ratio is associated with higher hernia risk. On multivariable analysis, older age, female gender, and BMI > 30 were significant independent predictors of IH. The use of preoperative and postoperative chemotherapy were combined into one variable for the multivariable analysis due to small numbers. On multivariable analysis, diabetes, tobacco use, serum albumin, prior hernia and PCI score were not significant predictors of IH.
Table 2. Univariate analysis of factors associated with incisional hernia.
Table 3. Multivariable predictors of incisional hernia.
Discussion
Incisional hernia is one of the most common postoperative complications after abdominal surgery for cancer. In this retrospective single-center study, the incidence of IH after CRS/HIPEC was 17% with a minimum follow-up time of two years. Independent predictors of IH were older age, female gender, and obesity. To the authors’ knowledge, only one other study has reported IH rates after CRS/HIPEC. In a German study of patients undergoing CRS/HIPEC, Struller et al reported that the IH rate was 7%; factors associated with IH were older age, cardiopulmonary morbidity, pseudomyxoma peritonei, mesothelioma, and abdominal wall rupture [Citation11]. Notably, the use and frequency of surveillance CT were not described in this study from Germany, which may account for the lower incidence of IH compared to the current study.
The incidence of IH after CRS/HIPEC reported from these two studies is comparable to that after other abdominal cancer operations. In a study of 491 patients undergoing surgery for abdominal malignancy, Baucom et al reported an IH rate of 43% [Citation1]. Among patients with colorectal cancer, Claes et al reported an IH rate of 35% [Citation12]. Spencer et al reported that the IH rate was 17.7% after ovarian cancer surgery [Citation13]. In a study of patients undergoing liver resection for colorectal metastasis, the incidence of IH was 30.5% [Citation14]. Studies that do not utilize routine CT surveillance underestimate the incidence of IH. In a study of patients undergoing surgery for colorectal cancer, the surgeon-detected IH rate was 17.4%, while the CT-detected IH rate was 35% [Citation12].
The combination of CRS plus HIPEC seems to create a perfect storm for IH development. Patients with peritoneal surface malignancies often have many risk factors for IH including long surgery time, previous abdominal surgery, long incision length, and ascites. Many patients are immunocompromised from preoperative chemotherapy, a risk factor for IH in some studies [Citation14]. Additionally, the administration of intraperitoneal chemotherapy may further impair wound healing. In a rat model, Aarts et al reported that abdominal wall strength was significantly lower after CRS plus HIPEC compared to CRS alone [Citation15].
The occurrence of IH causes substantial patient morbidity. Patients can have pain and discomfort or develop obstruction or incarceration requiring emergency surgery. Patient quality of life is also impaired by the development of IH. van Ramshorts et al reported that IH was associated with significantly worse physical functioning, cosmetic, and body image scores [Citation8]. The recurrence rate after IH repair is about 30% [Citation9]. Surgical repair of symptomatic IH may be especially difficult and morbid after CRS/HIPEC due to the presence of adhesions. The occurrence of IH also results in substantial health care costs [Citation16], and additional indirect costs from lost wages, lost productivity and high disability insurance.
Thus, avoidance of IH after abdominal cancer surgery may markedly improve the health and quality of life of cancer patients. Many strategies have been evaluated to reduce the risk of IH after abdominal surgery. Fascial closure using slowly absorbable suture in small continuous stitches is well accepted. In the multicenter STITCH trial, patients were randomized to abdominal wall closure using large (1 cm every 1 cm) or small (5 mm every 5 mm) bites [Citation17]. The use of small bites was associated with a significantly lower IH rate (13% vs. 21%; p = .022) at one year. In a Swedish single-center randomized controlled trial, small stitch closure was associated with a significantly lower risk of IH compared to long stitch closure (5.6% vs. 18.0%; p < .001) [Citation18].
Prophylactic placement of mesh is another strategy that has been used to reduce IH formation. In the PRIMA randomized clinical trial, the use of an onlay mesh was associated with a significant reduction of IH formation in high-risk patients [Citation19]. In a systematic review that included 2114 patients, Borab et al reported that prophylactic mesh placement was associated with an 85% risk reduction in development of IH when compared to primary closure [Citation20]. However, the use and indications for prophylactic mesh placement are not well defined today.
The current study has important limitations to discuss. The study design is retrospective and the abdominal wall closure techniques were not standardized. The most common type of closure utilized in this study was fascial closure with a looped suture which has been associated with a higher IH rate [Citation21]. Other fascial closure techniques included large non-looped traditional bite suture. With this retrospective study spanning 13 years, we were unable to collect and analyze some factors (e.g., incision length, surgery time) that may be associated with IH. Further, since we included IH identified on CT alone, the clinical significance of these incidental IH is not clear. Finally, although all patients in this study were followed for at least 2 years after CRS/HIPEC, we may underestimate the rate of late IH development.
Conclusions
In conclusion, we were surprised that the IH rate in this high-risk patient population treated with immunosuppression was comparable to the IH rate after other abdominal cancer operations. Perhaps, the contribution of intraperitoneal chemotherapy to impaired wound healing and hernia formation is small. Nevertheless, avoidance of any IH is a meaningful patient outcome for cancer survivors. To this end, the authors are now utilizing ‘small bite’stitches with single-strand suture to determine subsequent IH rates after CRS/HIPEC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baucom RB, Ousley J, Beveridge GB, et al. Cancer survivorship: defining the incidence of incisional hernia after resection for intra-abdominal malignancy. Ann Surg Oncol. 2016;23:764–771.
- Veljkovic R, Protic M, Gluhovic A, et al. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J Am Coll Surg. 2010;210:210–219.
- Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J (Clin Res Ed). 1982;284:931–933.
- Cobb WS, Carbonell AM, Snipes GM, et al. Incisional hernia risk after hand-assisted laparoscopic surgery. Am Surg. 2012;78:864–869.
- Llaguna OH, Avgerinos DV, Lugo JZ, et al. Incidence and risk factors for the development of incisional hernia following elective laparoscopic versus open colon resections. Am J Surg. 2010;200:265–269.
- Seiler CM, Deckert A, Diener MK, et al. Midline versus transverse incision in major abdominal surgery: a randomized, double-blind equivalence trial (POVATI: ISRCTN60734227). Ann Surg. 2009;249:913–920.
- Höer J, Stumpf M, Rosch R, et al. Prevention of incisional hernia. Chirurg. 2002;73:881–887.
- van Ramshorst GH, Eker HH, Hop WC, et al. Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg. 2012;204:144–150.
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398.
- Muysoms FE, Antoniou SA, Bury K, et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia. 2015;19:1–24.
- Struller F, Koenigsrainer I, Horvath P, et al. Abdominal wall morbidity following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Scand J Surg. 2017;106:294–298.
- Claes K, Beckers R, Heindryckx E, et al. Retrospective observational study on the incidence of incisional hernias after colorectal carcinoma resection with follow-up CT scan. Hernia. 2014;18:797–802.
- Spencer RJ, Hayes KD, Rose S, et al. Risk factors for early-occurring and late-occurring incisional hernias after primary laparotomy for ovarian cancer. Obstet Gynecol. 2015;125:407–413.
- Nilsson JH, Strandberg Holka P, Sturesson C. Incisional hernia after openresections for colorectal liver metastases - incidence and risk factors. HPB(Oxford). 2016;18:436–441.
- Aarts F, Bleichrodt RP, de Man B, et al. The effects of adjuvant experimental radioimmunotherapy and hyperthermic intraperitoneal chemotherapy on intestinal and abdominal healing after cytoreductive surgery for peritoneal carcinomatosis in the rat. Ann Surg Oncol. 2008;15:3299–3307.
- Alli VV, Zhang J, Telem DA. Impact of incisional hernia development following abdominal operations on total healthcare cost. Surg Endosc. 2018;32:2381–2386.
- Deerenberg EB, Harlaar JJ, Steyerberg EW, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015;386:1254–1260.
- Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg. 2009;144:1056–1059.
- Jairam AP, Timmermans L, Eker HH, et al. Prevention of incisional hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2-year follow-up of a multicentre, double-blind, randomised controlled trial. Lancet. 2017;390:567–576.
- Borab ZM, Shakir S, Lanni MA, et al. Does prophylactic mesh placement in elective, midline laparotomy reduce the incidence of incisional hernia? A systematic review and meta-analysis. Surgery. 2017;161:1149–1163.
- Guitarte C, Grant J, Zhao H, et al. Incisional hernia formation and associated risk factors on a gynecologic oncology service: an exploratory analysis. Arch Gynecol Obstet. 2016;294:805–811.