Abstract
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer death; percutaneous thermal ablation (TA) has proven feasibility, good local control and good tolerance in stage I tumors for patients with medical comorbidities and who are ineligible for surgery. In this context, stereotactic body radiotherapy (SBRT) has demonstrated high efficacy in treating T1 NSCLC and will need to be compared with percutaneous ablation. TA is also indicated in oligoprogressive disease; and can be proposed as a salvage treatment option for tumor recurrence after radiotherapy. Besides more advanced NSCLC could be also an indication of TA in combination with systemic treatments. A large majority of diagnosed NSCLC do not exhibit specific targetable genetic aberration. Those tumors present poorer prognosis and have been treated with standard chemotherapy regimen until the recent development of immune checkpoint inhibitors (ICIs) based immunotherapy. Combining TA with immunotherapy is promising and still needs to be explored.
Introduction
Non-small cell lung cancer (NSCLC) represents 85% of lung cancers [Citation1]. Adenocarcinoma is the most common histologic subtype, accounting for almost half of all lung cancers, and it is a leading cause of cancer death worldwide presenting significant therapeutic challenges. The incidence and mortality rates are higher in developed regions like Europe, than in developing countries [Citation2].
A majority of lung cancer patients are diagnosed with advanced disease because they frequently remain asymptomatic for a long time leading to late discoveries. For stage I NSCLC, lobectomy and lymph node resection is the recommended first-line treatment and best option [Citation3]. Despite the development of sub-lobar resection [Citation4] to limit functional damage of lobectomy, approximately 20% of patients are ineligible for surgery, mostly due to medical comorbidities, poor performance status and/or poor pulmonary function. The 5-year overall survival (OS) rate without treatment ranges from 6 to 14% for these patients [Citation5], and a treatment whenever possible is beneficial.
Jeppesen et al. [Citation6] retrospectively compared stereotactic body radiotherapy (SBRT) to no treatment in patients with medically inoperable T1-2N0 NSCLC. The median OS from the date of diagnosis was 40 months following SBRT, vs. 9.9 months after no treatment. The 5-year OS was 37% and 6%, respectively. Among the patients in the untreated group, 77% died from lung cancer compared to 39% in the SBRT treatment group.
Until recently, these ‘non-surgical’ patients were treated with conventional radiotherapy but local failure rates exceeded 50%, leading to long-term survival rates of only 15–30% [Citation7].
SBRT has replaced conventional radiotherapy, enabling a 3-year disease-free survival and OS of 48.3% and 55.8%, respectively [Citation8].
Percutaneous thermal ablation (TA) [Citation9–13] has been increasingly used for treatment of early staged NSCLC demonstrating favorable results. Radiofrequency ablation (RFA) is the most commonly used and evaluated image-guided TA technique and has been shown to be feasible and safe in highly selected patients [Citation9–13]. Other TA techniques such as microwave ablation (MWA) or cryoablation are also used to heat or freeze a tumor. One of the advantages of these TA techniques is that it is a stand-alone therapy that can be repeated in case of local failure, while causing minimal damage to the surrounding normal lung tissue.
In this article, we review the different percutaneous TA options available for non-surgical treatment of early-stage NSCLC and in more advanced stages like oligometastatic disease and oligoprogressive disease; how TA can be combined with systemic treatment in advanced stages and how these techniques can be proposed and indicated in salvaging radiotherapy failures. We describe the results (local efficacy and survival) and complications, and we lay out future perspectives for TA use in NSCLC.
Thermal ablation techniques and technical indications
Three different TA techniques are mainly used on lung tumors: RFA, MWA and cryoablation. Electroporation is a non-TA process using electric pulses of high voltage (1500 V/cm) of short duration. However, it is not effective on lung malignancies, reported a recurrence rate of 61% [Citation14].
RFA is the first technique used and reported for lung TA and as a result, numerous publications and a large volume of data are available. RFA is indicated for tumors less than 3 cm. MWA could be advantageous as it works quicker at a higher temperature, is less impacted by major arterial or bronchial heat sinks, and is not susceptible to escalating electrical resistance caused by charred or desiccated tissue. Theoretically, it allows for larger ablation volumes, which should reduce the chance of local recurrence, but such an outcome has not been completely demonstrated in clinical practice. However, the higher temperatures reached by MWA may lead to potential risks of persistent air leaks and increased bronchopleural fistula rates compared to RFA. An awareness of the asymmetric long ablation zone associated with certain MWA devices is important to avoid extension of the ablation zone into the chest wall and consequently pain, pleural fistula and skin burns. [Citation15].
When cryoablation is performed on lung tumors, a triple-freeze cycle is recommended [Citation16] with ablation times typically of the order of 25–30 min, which is much longer compared to RFA (10–15 min) or MWA (5–10 min). Nevertheless, the use of multiple probes provides a larger volume of ablation and allows to treat non-spherical tumors. The requirement of multiple probes carries however an increased risk of pneumothorax and a higher cost. Freezing is better tolerated than heating and therefore can be performed under local anesthesia or minor sedation. It is also interesting to note that cryoablation is potentially safer than heating ablative techniques with regards to thermal injury, because cryoablation preserves collagenous architecture and appears harmless for bronchial structures.
Across all techniques, there are however common limits. Central tumors may be treated, but tumors less than 1 cm from the hilum are generally considered contraindicated for TA due to the proximity of large vessels responsible for the heat sink effect and also due to the risk of blood vessel injury including pseudoaneurysm formation.
Since patients are not candidates for surgery, pulmonary function is generally poor in patients referred for TA. However, there is no lower threshold recommended for forced expiratory volume in 1 s (or FEV1) or diffusion capacity (or DLCO) before carrying out a TA.
CT or cone-beam CT is usually used for guidance, but a recent publication describes RFA under CT-imaging bronchoscopy, which may result in lower complication rates than transthoracic techniques [Citation17].
Clinical indications and results
Stage 1A NSCLC corresponding to T1 tumors (3 cm and smaller) N0 can be approached with TA techniques. NSCLC is an invasive tumor, so taking into account peripheral microscopic extension is a key point to obtain local control. In a pathology study after RFA on NSCLC [Citation18], even if the thermal lesion completely encompassed the tumor, margins assessed by the thickness of the surrounding area of ground glass opacity (GGO) from the neoplastic lesion appeared more narrow (<5 mm) in patients with incomplete ablation than in the patients with complete ablation (8 mm on average). As a result, an ablation zone ‘that includes the primary tumor plus at least an additional 8–10 mm of ablation beyond the visible tumor margin in all directions’ is recommended [Citation10]. For larger tumors (bigger than 3 cm), lower success rates of complete ablation with a drop of efficacy have been reported after RFA [Citation19].
Results of RFA
Two prospective studies with strong methodology have evaluated the efficacy of RFA in stage I NSCLC, with the American College of Surgeons oncology group Z4033 trial reporting a local recurrence-free rate of 68.9% and 59.8% at 1 and 2 years [Citation20], respectively. In the other study, the local control rate was 84.38% (95% CI [67.21–95.72]) at one year and 81.25% (95% CI [54.35–95.95]) at three years [Citation21]. In [Citation21], local recurrence refers to a recurrence at the RFA zone, whereas in the Z4033 trial it refers not only to a recurrence at the RFA site but also in the primary tumor lobe and the hilar lymph node.
Similar OS rates were found in the two studies. In [Citation20], the 1- and 3-year OS rates were 91.67% and 58.33%, respectively in [Citation21] and the 1- and 2-year OS rates were 86.3% and 69.8%, respectively. Furthermore, in these trials, the patient population was old, frail and contraindicated for surgery. In comparison with patients treated with sub-lobular resection and SBRT, patients for TA are often the frailest and the oldest [Citation22], and half of the patients died of causes other than their treated lung cancer [Citation21].
Comparison with non-thermal ablation techniques
Comparison between SBRT and surgery has been attempted but three randomized controlled trials in higher risk medically operable patients have failed to recruit (SABR: ROSEL (NCT00687986), STARS (NCT00840749), ASOSOG-RTOG (NCT01336894)). A retrospective analysis has shown that no difference in cancer-specific survival was observed between SBRT and surgery [Citation23].
In a retrospective study [Citation24] comparing 1102 patients treated with TA and 27,732 patients treated with SBRT, patients treated with TA had more comorbidities. This retrospective study also demonstrated that OS rates were comparable between TA and SBRT (1 year 85.4% vs. 86.3%, respectively, p = .76; 2 years 65.2% vs. 64.5%, respectively, p = .43; 3 years 47.8% vs. 45.9%, respectively, p = .32; 5 years 24.6% vs. 26.1%, respectively, p = .81). The absence of pathologic confirmation of cancer in a significant number of patients is a major weakness of numerous SBRT studies for suspected early-stage NSCLC, it may raise the question of whether some treated lung lesions may be benign. A systematic review [Citation25] and recent meta-analysis show a discrepancy in oncological outcomes for patients undergoing SBRT in whom there is a pathological confirmation of malignancy and those without (clinical diagnose only). The comparative studies show a lower 3-year OS and lower 2-year and 5-year cancer specific survival for biopsy-proven disease compared to clinical disease.
Results of cryoablation and microwave ablation
For cryoablation [Citation26], local control rates of 85% and a 5-year survival rate of up to 68% have been reported. As for RFA series, larger tumor size (>20 mm) and presence of a thick vessel (diameter >3 mm) close to the tumor were independent risk factors for local progression [Citation27].
In a series on 47 patients treated with MWA [Citation28], the local control rates at 1, 3 and 5 years were 96%, 64% and 48%, respectively. The OS rates at 1, 2, 3 and 5 years after MWA were 89%, 63%, 43% and 16%, respectively. Like for RFA [Citation13], tumors ≤3.5 cm were associated with better survival than tumors >3.5 cm.
A recent review [Citation29] covered 12 studies that included either primary or secondary lung malignancies, representing 985 patients and 1336 MWA treated lung nodules. Estimates of local recurrence varied from 9% to 37%. Tumor size was shown to increase the risk of local recurrence with the most frequent cutoff value being 3 cm. Among four studies that stratified results by tumor size, local recurrence among tumors smaller than 3–4 cm was 5–19%.
Recurrent disease
Oligo-recurrence has been defined as limited recurrent or metastatic disease appearing when a primary tumor is controlled. Recurrent or metastatic sites will be few and they will be treatable with local therapy [Citation30]. After complete treatment of a non-advanced NSCLC, the appearance of ipsilateral or contralateral NSCLC tumors with the same histology remains a staging challenge. To distinguish a new primary tumor from stage IVa disease is difficult. When lung RFA was performed for oligo-recurrent NSCLC after surgery RFA offered patients a chance of long-term survival [Citation31]. With a mean follow-up period of 29 months, the OS rates were 98% at 1 year, 73% at 2 years and 56% at 3 years. The recurrence-free survival rates were 77% at 1 year and 41% at 3 years. This study included 44 patients who were treated with RFA for single or multiple intrapulmonary recurrences.
In this setting, GGOs with a solid component, considered as malignant, are a safe and effective indication for RFA [Citation32] even though surgery remains the standard of care in this indication. However, patients with GGO are likely to develop new primary cancers and TA techniques should be considered for preservation of lung parenchyma. Furthermore, a single lung is not a contraindication for TA, when another cancer is depicted after pneumonectomy for a previous NSCLC [Citation33].
Oligometastatic disease is most commonly defined to be a state of local, low-volume disease with limited spread to other organs that can be treated locally and curatively whenever possible [Citation34]. However, since outcomes of metastatic NSCLC are worse than for other cancers, appropriate patient selection is warranted including PET CT and discussion in a tumor multidisciplinary meeting when considering an aggressive approach using ablative local therapies.
With systemic treatment
The use of local therapies including surgery, radiation and TA is frequently proposed for pulmonary metastases from different origins (colorectal cancer, kidney, sarcoma, thyroid) with good efficacy, having an impact on time without systemic treatment [Citation35]. Such a strategy alternating systemic and local treatment has become part of the standard of care. In advanced NSCLC, identifying subgroups of patients with favorable profiles who could benefit from local treatment is based on molecular biology, a complete imaging checkup including PET-CT and discussion in a tumor multidisciplinary meeting.
Regarding metastatic disease (stage IV patients), various other systemic treatments, from platinum-based doublets to single-agent chemotherapy, may be used. In advanced EGFR-mutated NSCLC, oral EGFR-tyrosine kinase inhibitors (TKIs) have become the standard of care as first-line therapy. A combination of RFA with chemotherapy was shown to improve OS compared with chemotherapy alone (42 vs. 29 months) in stages III to IV [Citation36]. A PFS of 16 weeks after chemotherapy treatment followed by RFA in patients with advanced NSCLC has also been reported [Citation37].
Percutaneous cryoablation of advanced NSCLC has been combined with molecular targeted therapy. In a small, randomized controlled trial, 36 nonsmoking female patients with stages IIIB and IV, EGFR-activating mutation-positive NSCLC received either percutaneous cryoablation before initiation of gefitinib therapy (n = 18) or gefitinib alone (n = 18) [Citation38]. ‘Neoadjuvant’ cryoablation followed by gefitinib was associated with greater rates of partial regression (55.6% vs. 27.8%), longer progression-free survival (8.4 months vs. 5.2 months) and a significantly better 1-year survival rate (66.7% vs. 33.3) than with gefitinib alone. However the mechanisms to understand the synergistic effects of this combined therapy were not explored.
With regard to metastatic disease in patients with partial response or stable disease, systemic treatment can be followed by a local treatment of the remaining metastatic sites.
Treating an oligoprogressive disease allows a continuation of the ongoing systemic treatment and to preserve further lines. A local treatment (SBRT or surgery) and continuation of the targeted agent, has been associated with more than 6 months of additional disease control [Citation39]. Likewise a consolidative local ablative therapy (radiotherapy) to all metastatic sites for patients with EGFR-mutant oligometastatic NSCLC during first-line EGFR-TKI treatment, offered significantly improved PFS and OS compared with consolidative local ablative therapy to partial sites or observation alone [Citation40]. In this setting, prospective studies evaluating effect of local treatments including TA would be of benefit.
When using a TA technique, before heating or freezing a biopsy of the progressive tumor is encouraged to identify and analyze mechanisms of acquired resistance.
Cancer immunotherapy aims at restoring the ability of the immune system to efficiently detect and eliminate tumor cells. The programmed-death-1 PD-1 receptor, which is expressed on activated T-cells, is engaged by ligands (programmed death-ligand 1 PD-L1 and PD-L2), which are expressed by tumor cells and tumor-infiltrating immune cells. Immune checkpoint inhibitors (ICIs) aim to disrupt PD-1/PD-L1 signaling and to restore antitumor immunity.
The introduction of ICI as a systemic treatment of metastatic NSCLC has demonstrated efficacy compared to chemotherapy. A phase III trial has demonstrated in patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells, pembrolizumab was associated with significantly longer progression-free and OSs, as well as resulting in fewer adverse events than after platinum-based chemotherapy [Citation41]. A current trend is to propose immunotherapy not only for metastatic disease or recurrent disease but also in early disease stages. Various combinations with immunotherapy are under evaluation such as double immunotherapy, immunotherapy and platinum-based chemotherapy. An interesting field of investigation is to combine immunotherapy and a local treatment like SBRT or TA techniques to enhance immunotherapy efficacy, given the incomplete response rates (45% on advanced NSCLC with PD-L1 expression on at least 50% of tumor cells [Citation41]). TA induces localized coagulation necrosis and leads to the local release of large amounts of cellular debris, which can serve as a source of tumor antigens to elicit host-adaptive immune responses against tumor similar to a vaccination [Citation42]. However, releasing tumor-derived self-antigens into circulation may not be sufficient enough to overcome the checkpoint escape mechanisms some cancers have evolved to avoid immune responses.
With radiotherapy
TA can be proposed as a salvage therapy when a local recurrence occurs at a previously radiated site (). However, patients who have had prior radiation may be at increased risk of larger ablation zones and possible vascular injury due to radiation-associated vasculopathy. Larger ablation zones may be at risk for acute lung insufficiency. Indications must be carefully discussed. TA has been used in conjunction with radiotherapy in patients with stage I or II NSCLC; some data suggest that the outcome of combined treatment may be more favorable than with either modality alone [Citation43].
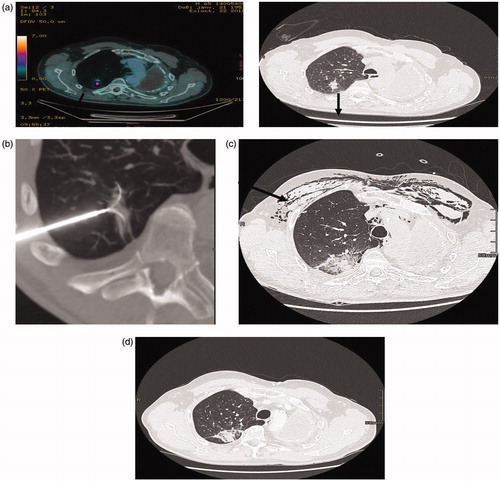
Figure 1. (a) (PET-CT, CT): patient 72 year old, previously treated for an NSCLC by pneumonectomy, 4 years later an adenocarcinoma of the upper right lobe was treated with SBRT recurrence 16 months after SBRT (black arrow). (b) RFA treatment under general anesthesia with a 3.5 cm expandable electrode. (c) CT two days after RFA: the ablation zone encompasses the tumoral volume, subcutaneous emphysema, pneumothorax drained (black arrow). (d) CT 3 months after RFA showing a good result, the ablation zone encompassing the tumor.
Complications
Across all TA techniques and indications, pneumothorax is the most frequent complication with a chest tube drainage required after 10–50% RFA procedures. Delayed pneumothorax is rare and often means that a broncho-pleural fistula has occurred. The use of multiple devices (e.g., cryotherapy, MWA, multipolar RFA), increases the theoretical risk of pulmonary complications. Complications after RF have been described in large series, one of the largest assessed complications for each RFA session for 420 consecutive patients who underwent 1000 RFA sessions in a single center [Citation44]. Overall, 9.8% of grades 3 and 4 complications were noted including aseptic pleuritis (2.3%), pneumonia (1.8%), lung abscess (1.6%) and pneumothorax requiring pleural sclerosis (1.6%), followed by bronchopleural fistula (0.4%). Puncture number (p < .02) and previous systemic chemotherapy (p < .05) were significant risk factors for aseptic pleuritis. Previous external beam radiotherapy (p < .001), emphysema (p < .02) and age (p < .02) were significant risk factors for septic complications. Emphysema (p < .02) was a significant risk factor for pneumothorax requiring pleural sclerosis. Reported mortality was 0.4% per session. The risk factors for septic complications and pneumothorax (previous external beam radiotherapy and emphysema) are more frequent in patients with primary lung cancer, and explain why they have a greater risk of complications and death when compared with metastatic patients. With these fragile patients, MWA may be used cautiously given the higher temperatures, to avoid potential risks of persistent air leak and bronchopleural fistula.
Exacerbation of interstitial pneumonia has been mentioned as a major cause of death in previous lung TA studies [Citation44]. Cryotherapy that is more respectful of the cellular architecture in frozen tissues could be an option in these fragile patients, but a disadvantage to this technique is that additional probes are often needed. In a study including 193 cryoablation sessions for 396 lung tumors in 117 consecutive patients [Citation45], a greater number of cryoprobes was a significant predictor of pneumothorax (p = .001), pleural effusion (p = .001) and hemoptysis (p < .001). In addition, higher rates of hemorrhage have been reported in lung cryoablation literature, exposing patients to increased risk for hemoptysis [Citation46].
Concerning lung function, patients with FEV1 less than 1 L/second have been treated with no post RFA complications. In a prospective study, there were no deleterious effects on pulmonary function on a population of patients with stage I NSCLC after RFA [Citation10]. Mild impairments of lung function at 3 months are correlated with severe post thermal-ablation pleuritis and an ablated parenchymal volume >20 cm3 [Citation20].
A positive impact on QoL [Citation21] also highlights the good level of tolerance of RFA. A majority of patients considered that RFA did not modify either their life status perception or their main vital function, including lung function.
Perspectives
Using TA in association with systemic treatments in NSCLC has previously been discussed; however, the perspectives are wide in this field. One of the most challenging approaches is to associate TA with immunotherapy, in order to boost immunotherapy. A relationship between TA and the immune system has been recognized, and of the existing TA techniques, the two modalities with the most established immune interactions are RFA and cryoablation [Citation47,Citation48]. Evaluating the possible local immunological effects of TA in lung cancer and how they might work warrants further investigation. Following TA, one study [Citation49] identified an intense infiltration of lymphocytes in the peritumoral environment. In this study, NSCLC biopsies before RFA treatment were performed in four patients. Eight days after RFA, NSCLC were resected by video-assisted thoracoscopic lobectomy. On surgically resected tumors, central areas were necrotic and devoid of lymphocytes, but intense peritumoral involvement of CD4-positive and CD8-positive T-cells were observed. These results suggest that necrotic tumor tissue after RFA can serve as an in-situ antigen source, inducing a persistent T-cell activation.
As such, TA could stimulate the production and activation of antitumor-specific T-cells. However, this is insufficient to result in a synergistic effect of local tumor reduction and distant metastases regression, otherwise known as an abscopal effect.
One mechanism to explain a lack of response to ICI is that NSCLC with insufficient tumor infiltration of tumor-specific T-cells may be refractory to treatment. Stimulating and increasing tumor-specific T-cells in the tumor microenvironment is a strategy to increase responses to ICI. The effects of TA on lung tumor PD-L1 expression have not yet been explored, and the efficacy of combining an immune check point inhibitor and TA in advanced NSCLC is unknown. There are two ongoing phase II clinical trials investigating the combination of percutaneous cryoablation and an ICI for treatment of advanced NSCLC (ClinicalTrials.gov identifiers NCT03290677 and NCT02469701).
Conclusions
Among the various TA techniques covered here, RFA remains the most robust with the highest level of evidence of efficacy (local control rate up to 81% at three years for T1 tumors).
In the future, TA should be compared with stereotaxic body radiation therapy, which also demonstrated high local control rate. A retrospective study demonstrated that OS rates were comparable between TA and SBRT for patients with stage I NSCLC.
Considering the challenge of viewing some cancers as a chronic disease, future strategies should extend the indications in combining TA with systemic therapies. The low invasiveness, the repeatability of TA and the ability of performing a biopsy before retreating are major advantages. Combining TA and immunotherapy for a synergistic effect should be promising and deserves further investigations.
Disclosure statement
The authors report no conflicts of interest.
References
- Meza R, Meernik C, Jeon J, et al. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One. 2015;10:e0121323.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602.
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33:426–435.
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007;132:193–199.
- Jeppesen SS, Schytte T, Brink C, et al. A comparison of stereotactic body radiation therapy (SBRT) versus no treatment in medically inoperable patients with early-stage non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2014;90:S642.
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076.
- Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol. 2011;6:2044–2051.
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology. 2010;254:301–307.
- Palussiere J, Lagarde P, Aupérin A, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years' experience in 87 patients from two centers. Cardiovasc Intervent Radiol. 2015;38:160–166.
- Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2007;134:857–864.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275.
- Ricke J, Jurgens JH, Deschamps F, et al. Irreversible electroporation (IRE) fails to demonstrate efficacy in a prospective multicenter phase II trial on lung malignancies: the ALICE trial. Cardiovasc Intervent Radiol. 2015;38:401–408.
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation – what should you use and why? Radiographics. 2014;34:1344–1362.
- Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple-freeze protocol. Cardiovasc Intervent Radiol. 2010;33:1180–1185.
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-guided cooled radiofrequency ablation as a novel intervention therapy for peripheral lung cancer. Respiration. 2015;90:47–55.
- Ambrogi MC, Fontanini G, Cioni R, et al. Biologic effects of radiofrequency thermal ablation on non-small cell lung cancer: results of a pilot study. J Thorac Cardiovasc Surg. 2006;131:1002–1006.
- Gillams AR, Lees WR. Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol. 2008;18:672–677.
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121:3491–3498.
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018;13:91.
- Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg. 2013;145:692–699.
- Uhlig J, Ludwig JM, Goldberg SB, et al. Survival rates after thermal ablation versus stereotactic radiation therapy for stage 1 non-small cell lung cancer: a national cancer database study. Radiology. 2018;289:862–870.
- Spencer KL, Kennedy MPT, Lummis KL, et al. Surgery or radiotherapy for stage I lung cancer? An intention to treat analysis. Eur Respir J. 2019;53:1801568.
- IJsseldijk MA, Shoni M, Siegert C, et al. Survival after SBRT for clinically diagnosed or biopsy-proven early-stage non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2019;14:583–595.
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26:312–319.
- Inoue M, Nakatsuka S, Jinzaki M. Cryoablation of early-stage primary lung cancer. Biomed Res Int. 2014;2014:521691.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763.
- Nelson DB, Tam AL, Mitchell KG, et al. Local recurrence after microwave ablation of lung malignancies: a systematic review. Ann Thorac Surg. 2018;107:1876–1883.
- Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–111.
- Kodama H, Yamakado K, Takaki H, et al. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc Intervent Radiol. 2012;35:563–569.
- Iguchi T, Hiraki T, Gobara H, et al. Percutaneous radiofrequency ablation of lung cancer presenting as ground-glass opacity. Cardiovasc Intervent Radiol. 2015;38:409–415.
- Hess A, Palussiere J, Goyers JF, et al. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology. 2011;258:635–642.
- De Ruysscher D, Wanders R, Hendriks LE, et al. Progression-free survival and overall survival beyond 5 years of NSCLC patients with synchronous oligometastases treated in a prospective phase II trial (NCT 01282450). J Thorac Oncol. 2018;13:1958–1961.
- Fonck M, Perez JT, Catena V, et al. Pulmonary thermal ablation enables long chemotherapy-free survival in metastatic colorectal cancer patients. Cardiovasc Intervent Radiol. 2018;41:1727.
- Lee H, Jin GY, Han YM, et al. Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol. 2012;35:343–350.
- Li X, Zhao M, Wang J, et al. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancers. AJR Am J Roentgenol. 2013;201:1362–1367.
- Gu XY, Jiang Z, Fang W. Cryoablation combined with molecular target therapy improves the curative effect in patients with advanced non-small cell lung cancer. J Int Med Res. 2011;39:1736–1743.
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814.
- Xu Q, Zhou F, Liu H, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13:1383–1392.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833.
- den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029.
- Grieco CA, Simon CJ, Mayo-Smith WW, et al. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17:1117–1124.
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol. 2011;197:W576–W580.
- Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol. 2012;23:295–302.
- Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298.
- Mehta A, Oklu R, Sheth RA. Thermal ablative therapies and immune checkpoint modulation: can locoregional approaches effect a systemic response? Gastroenterol Res Pract. 2016;2016:1.
- Katzman D, Wu S, Sterman DH. Immunological aspects of cryoablation of non-small cell lung cancer: a comprehensive review. J Thorac Oncol. 2018;13:624–635.
- Schneider T, Hoffmann H, Dienemann H, et al. Immune response after radiofrequency ablation and surgical resection in nonsmall cell lung cancer. Semin Thorac Cardiovasc Surg. 2016;28:585–592.
