Abstract
Purpose: The purpose of this study is to evaluate the safety and efficacy of stereotactic radiofrequency ablation (SRFA) for the treatment of subcardiac hepatocellular carcinoma (HCC).
Material and methods: From 2003 to 2018, 79 consecutive patients underwent 104 multi-probe SRFA sessions for the treatment of 114 subcardiac HCC with a median size of 2.5 cm (0.5–9.5 cm). The results were compared with a randomly selected control group of 79 patients with 242 HCC with a median size of 2.0 cm (0.5–12 cm) following SRFA in other (non-subcardiac) locations with propensity score matching.
Results: The 95.6% of the tumors were successfully treated by the first ablation session (primary technical efficacy rate) and 99.1% after the second session (secondary technical efficacy rate). Local tumor recurrence developed in 8 nodules (7.0%). Major complication and perioperative mortality rates were 7.7% (8/104) and 1% (1/104), respectively. The overall survival (OS) rates from the date of the first SRFA with single subcardiac HCCs were 92%, 77% and 65% at 1, 3 and 5 years, respectively, with a median OS of 90.6 months. The disease-free survival (DFS) after SRFA was 75, 34 and 34%, at 1, 3 and 5 years, respectively, with a median DFS of 19.1 months. There was no statistically significant difference with the control group in terms of local tumor control, safety, OS and DFS.
Conclusion: SRFA of subcardiac tumors is as safe and efficacious as when treating tumors remote from the heart.
Introduction
Hepatocellular carcinoma (HCC) arises in the context of background cirrhosis in 90% of cases [Citation1] and confers a poor prognosis even in liver-confined disease (18% 5-year survival) [Citation2]. Whilst the healthy liver is resilient to insult, the cirrhotic liver has a poor regenerative capacity, which very often precludes patients from surgical resection (SR) [Citation3]. Therefore, the management of HCC is a delicate balance between tumor and background liver destruction which has brought about an increasing demand for focal ablative treatments, including radiofrequency ablation (RFA) [Citation4–6]. To achieve low rates of residual or recurrent disease, the lethal ablation zone must cover the tumor with a sufficient safety margin [Citation7] which may be challenging where the tumor is located in the vicinity of critical structures such as the heart.
Since percutaneous treatment is more challenging in tumors which are located close to the liver capsule, early studies showed significantly higher local tumor recurrence compared to non-subcapsular tumors (65 vs. 19%) [Citation8]. However, more recent studies suggest that whilst RFA for subcardiac locations is technically feasible and not significantly different to non-subcardiac locations [Citation9,Citation10], recurrence rates reported to be relatively high (19.1–20%). Three-dimensional (3D) navigation systems have been implemented to allow for a more sophisticated 3D planning of multiple overlapping ablation zones, precise probe placement and intraoperative assessment of the result by means of image fusion [Citation11,Citation12].
However, the role of this technique for the treatment of subcardiac tumors remains undefined. The aim of this study is to evaluate the safety and efficacy of stereotactic radiofrequency ablation (SRFA) for subcardiac tumors in terms of primary and secondary efficacy and local recurrence rate (LR). We also consider overall survival (OS) and disease-free survival (DFS) which, to the best of our knowledge have never previously been reported. Our hypothesis was that there is no difference in LR, DFS and OS for subcardiac vs. non-subcardiac HCC.
Materials and methods
Patient cohort and inclusion criteria
This study was approved by our Institutional Review Board and written informend consent was obtained from all patients. In every patient, the treatment plan was established by a multidisciplinary tumor board consisting of hepatologists, oncologists, transplant surgeons and interventional radiologists. Decisions for therapy were based on tumor characteristics, Child-Pugh classification, anatomical considerations and performance status. Due to the multiprobe approach there were no limitations in the size or number of tumors. Thus, BCLC B and BCLC C patients were not excluded from SRFA despite BCLC guidelines recommending such patients are treated with transarterial chemoembolization or medical therapy. Patient selection was carried out retrospectively as part of a single-center, single-arm study.
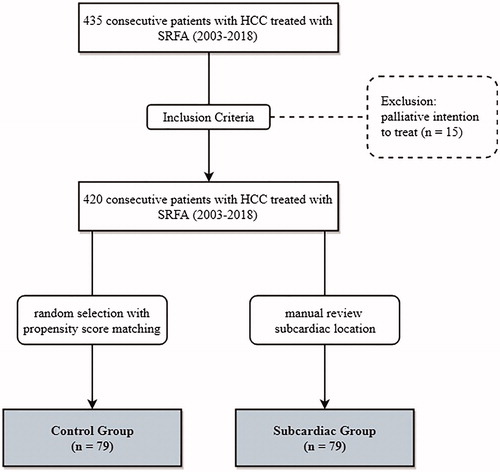
435 consecutive patients with HCC were treated with SRFA between 2003 and 2018 (). Arterial phase CT was reviewed by a board-certified radiologist with 10 years’ experience (PES), who assigned patients to the subcardiac group, defined as tumor location ≤ 1 cm from the pericardium in any direction. 79 patients met the inclusion criteria and 79 patients with non-subcardiac tumors were randomly selected using nearest neighbor propensity score matching by the R package ‘MatchIt’ with sex, age, tumor number and size and liver biochemistry as matching variables. The baseline characteristics of the two groups are shown in .
Table 1. Patient characteristics of 79 patients undergoing 104 SRFA sessions of 114 HCCs in the subcardiac group and of 79 patients undergoing 125 SRFA sessions of 242 HCCs in the control group.
Exclusion criteria for SRFA were a platelet count of <50,000/mm3 and prothrombin activity <50%.
Diagnosis of HCC was based on the identification of typical imaging hallmarks of HCC with detection of contrast hyperenhancement in the arterial phase (wash-in) and ‘wash-out’ in the portal venous and/or delayed phases on CT, MRI or CEUS for nodules ≥1 cm. In addition, thoracic CT was performed in all patients to exclude the presence of pulmonary metastases. All HCCs were pathologically confirmed by needle biopsy during the SRFA procedure.
Multi-probe SRFA
Our method of SRFA has been described in detail previously [Citation13,Citation14]. In brief, the procedure is carried out in the interventional CT-suite with anesthetized patients fixated on the CT-table by a single (Bluebag, Medical Intelligence Schwabmünchen, Germany) or double vacuum fixation technique (BodyFix, Medical Intelligence Schwabmünchen, Germany) using full muscle paralysis. For image-to-patient registration, 10–15 registration markers, (Beekley Spots, Beekley Corporation, Bristol, CT, USA) were broadly attached to the skin of the thorax and upper abdomen.
Subsequently, a contrast-enhanced planning CT (Siemens SOMATOM Sensation Open, sliding gantry with 82 cm diameter, Siemens AG, Erlangen, Germany) with 3 mm slice thickness was performed in the arterial and venous phase. The obtained CT-data were transferred to an optical based navigation system (Stealth Station Treon plus, Medtronic Inc., Louisville, KY, USA). One or multiple trajectories were planned using the navigation system with multiplanar and 3D reconstructed images. To eliminate respiratory motion, temporary disconnections of the endotracheal tube (ETT) were performed during the planning CT, during each stereotactic needle placement and the final CT. Multiple RF electrode positions were planned in order to cover the entire tumor volume and a sufficient safety margin with overlapping necrosis. Therefore, multiple coaxial needles (1–15, median 3) were placed in the entire tumor including the most peripheral parts with a maximum inter-probe distance of 2 cm. For all subcardiac lesions the planned distance between the tip of the probe and the diaphragm was >1 cm.
After registration, an accuracy check and sterile draping, the ATLAS aiming device (Elekta PSC Medical Intelligence Inc., Schwabmuenchen, Germany) was used for navigated trajectory alignment. 15 G/17.2 cm coaxial needles (Bard Inc., Covington, GA, USA) were subsequently placed through the aiming device during temporary ETT disconnection without the need for real-time imaging, using needle trajectories as defined by the coordinates on the planning CT. The depth from the aiming device to the target was automatically calculated by navigation software. The coaxial needles served as guides for the radiofrequency electrodes. For verification of correct needle placement, an unenhanced CT was performed following ETT disconnection and fused with the planning CT using the navigation system’s image 3D registration algorithm.
A 16 G biopsy sample was obtained via one of the co-axial needles in all cases. Thereafter, RF-electrodes (Cool-tip, Medtronic, Mansfield, MA, USA) were introduced through the coaxial needles for serial tumor ablation. A post-ablation completion contrast-enhanced CT was finally carried out in the arterial and portal venous phase during ETT disconnection and fused with the planning CT for verification of ablation zone coverage and for assessment of complications.
RFA was performed using a unipolar Cool-tip_RFsystem (Cool-tip, Medtronic, Mansfield, MA, USA), a Cool-tip_RF switching controller and a 17 G Cool-tip electrode with a length of 25 cm and an exposure of 3 cm. The standard ablation time for three electrodes (switching control) was 16 min. Needle track ablation was performed during repositioning and during final removal of the RFA electrodes to reduce the risk of bleeding and tumor seeding.
Example images from subcardiac multiprobe SRFA are shown in and .
Figure 2. Case of a 54-year old female with a 6-cm subcardiac HCC in segment II. (A) Arterial phase initial CT with a hypervascular hepatocellular carcinoma (HCC) in segment II (red dashed circle). (B) Maximum intensity projection (MIP) image of the control CT with 6 coaxial needles in place (red arrowhead). (C and D) Screenshots of the Treon navigation system – (C) showing fused images of the arterial phase planning CT with the arterial phase control CT (D). The red dashed circle delineates the HCC before and after ablation. (D) shows a completely overlapping ablation zone with no evidence of residual tumor and a sufficient safety margin. Follow up CT and after 3 (E) and MRI after 24 months (F) showing no evidence of local tumor recurrence. (E and F) with the red dashed circle delineating ablation zones. Note a pancreatic pseudocyst (red arrowhead) as an incidental finding.
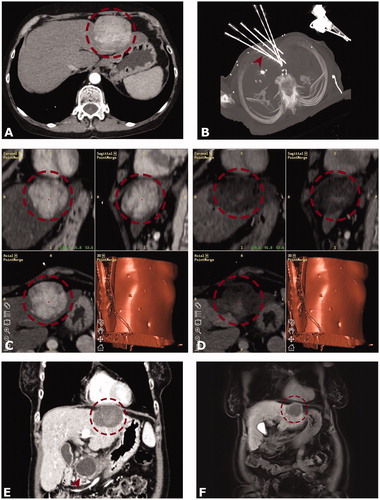
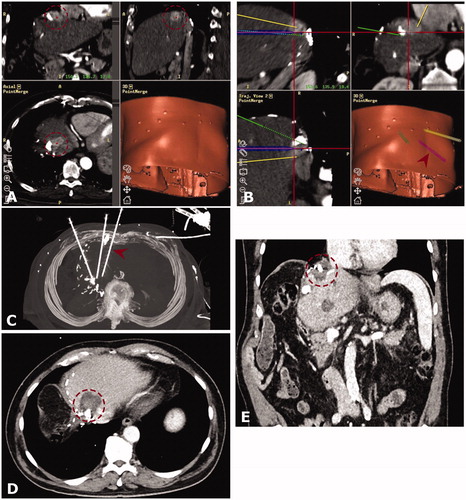
Figure 3. Case of a 75-year old male with a 4 cm hepatocellular carcinoma (HCC) between the heart, remaining left hepatic vein and inferior vena cava. Note the lipiodol deposition indicating a prior transcatheter arterial chemoembolization (TACE) and the surgical clips indicating a prior extended right hemihepatectomy. (A and B) Arterial phase CT mages from the navigation system in three planes (A, HCC marked by red dashed circle) and images of the 3-dimensional needle path planning (B). The red arrowhead shows the position of a placed co-axial needle. (C) Maximum intensity projection (MIP) of control CT with three coaxial needles in place. The needle from image (B) is marked by a red arrowhead. (D and E) Follow-up CT after 6 months (D) and 24 months. (E) showing no evidence of local tumor recurrence (red dashed circle is marking the coagulation zone).
Endpoints and follow-up
Primary and secondary efficacy, as well as the local recurrence rate (LR) were the primary endpoints. The secondary endpoints included DFS and OS. Follow-up contrast-enhanced CT/MR scans were performed first at 1-month and then at 3-month intervals after the SRFA. The images were evaluated by two abdominal radiologists with more than 10 years of experience by consensus (PES and BR). Technical success was defined as accurate electrode placement according to protocol with immediate, complete coverage by the ablation zone. Primary technical efficacy rate was evaluated for every tumor as the absence of residual tumor on 1-month follow-up CT. Secondary technical efficacy rate included tumors with repeated ablation after identification of residual tumor. Evidence of newly detected tumors within or immediately adjacent to the ablation zone or initial tumor was deemed as LR. Complete ablation was defined as a circumscribed non-enhancing zone within and/or extending beyond the initial tumor borders with a well-defined margin. Newly detected nodules distant to the ablation zone and or to initial tumor location were defined as distant tumor recurrence. Complications were defined according to the Society of Interventional Radiology (SIR) Standards of Practice Committee classification [Citation15]. In patients with a single subcardiac HCC vs. those with a single HCC elsewhere in the control cohort, survival was calculated from the date of first stereotactic RF ablation to the date of death attributable to malignancy (HCC) or other causes (‘event’) vs. the most recent follow-up visit (i.e. censoring).
Statistical analysis
Statistical analysis was performed using IBM SPSS version 20 (IBM, Armonk, New York). Data were expressed as total numbers, median and range. Overall and DFS for subcardiac and other groups was evaluated using the Kaplan Meier method and compared with the log-rank test. The difference between categorical variables (primary and secondary efficacy, local recurrence, major complications) was compared with X2 test. For evaluation of independent continuous variables (hospital days), a Mann Whitney U test was used. For all statistical tests, statistical significance was defined as a p values < 0.05.
The software R (version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria) and the R package MatchIt (1:1 matching with the nearest neighbor) was used for the propensity matching process to select patients from the control group.
Results
Patient characteristics
Baseline characteristics of patients undergoing RFA are shown in . In the subcardiac group 79 Patients, 13 females and 66 males, with a median age of 65.5 years (45–85) had HCC in whom underlying liver cirrhosis was present in 65 cases (82.3%). Cirrhosis was caused by fatty liver disease in 35 (44.3%), hepatitis C (HCV) infection in 20 (25.3%), hepatitis B (HBV) infection in 5 (6.3%), haemochromatosis in 1 (1.3%), primary biliary cirrhosis in 1 (1.3%) and cryptogenic liver disease in 3 patients (3.8%). 54 (68.4%) belonged to Child-Pugh class A and 11 (13.9%) to class B. At initial therapy, none fell into class C. According to BCLC staging 3 (3.8%) patients were Stage 0, 24 (30.4%) Stage A, 50 (63.3%) Stage B and 2 (2.5%) were Stage C, respectively.
At initial SRFA therapy, 37 patients had a solitary liver nodule, 20 patients had two nodules, 11 had three nodules and 11 patients had more than three nodules (defined as multiple nodules), including nodules remote from the heart. Of the patients in the subcardiac group with >1 HCC at initial SRFA, 1 patient had 3 subcardiac HCCs, 4 patients 2 subcardiac HCCs and 37 patients 1 subcardiac HCC. 11 patients had SR (2 local recurrences), 24 patients underwent TACE (21 residual or recurrent tumors) and 5 patients (5 residual or recurrent tumors) conventional RF ablation prior to SRFA. Due to untreatable tumor progression with diffuse/disseminated HCC infiltration as detected in follow-up imaging 4 patients received TACE and 9 patients received systemic chemotherapy subsequently. Liver transplantation could be performed in 19 patients.
The median size of the 114 nodules was 2.5 cm (0.5–9.5 cm). Median 2 tumors [Citation1–7] were treated per ablation session, including tumors remote from the heart.
From the control group, 79 patients, 11 females and 68 males, with a median age of 65 years (41–85) underwent SRFA for treatment of 242 HCCs in 125 ablation sessions. The median tumor size was 2.0 cm (0.5–12 cm).
Perioperative complications
Perioperative complications are shown in . One death occurred following ablation due to therapeutic induced liver failure (mortality rate of 1.0% (1/104)). The total major complicate rate was 7.7% (8 of 104). Three of these complications were clearly related to simultaneous thermal ablation of nodules in other locations, leading to a major complication rate of 4.8% (5 of 104) in subcardiac tumors. Thermal injury to the pericardium in one patient resulted in recurrent pericardial effusion and a prolonged intensive care admission with death 51 days after SRFA in one patient with a Child B cirrhosis. Two patients developed postoperative liver abscesses. Two patients were transferred to intensive care for temporary postoperative organ support and two patients developed perihepatic hemorrhage managed by angiographic coil embolization in the same general anesthetic session. Despite subcardiac location we did not observe any modifications in heart pace and/or hypo/hypertension.
Table 2. Details of major complications of the subcardiac group.
Fever (>37 °C, measured peripherally) developed in all patients but subsided in 1–2 days with symptomatic treatment. The median hospital stay was 5 days [Citation1–21].
There was no significant difference in major complication rate vs. the control group (8.8%, 11/125; p = 0.76) or in hospital stay (median 5 days, p = 0.805), respectively.
Technical success
SRFA was completed according to plan in all 114 nodules (technical success rate 100%). Tumor enhancement disappeared after the initial RF session in 109/114 cases resulting in a primary technical efficacy rate of 95.6%. Thereafter, four nodules were successfully retreated resulting in a secondary technical efficacy rate of 99.1%. One nodule was not retreated due to untreatable tumor progression involving multiple newly developed tumors ().
Table 3. Tumor-based therapy success rates in the subcardiac and control groups.
There was no significant difference of primary or secondary efficacy compared with the control group (95.1%, 230/242, p = 0.93; 97.1%, 235/242, p = 0.23).
Local recurrence rate and distant recurrence
Local tumor recurrence developed in 8 of 114 nodules (7.0%). In five nodules, pretreatment, including TACE, conventional RFA and resection, was performed (). There was no significant difference compared with non-pre-treated nodules (p = 0.320). Distant tumor recurrence in the liver was found in 38 patients (48.1%). Of the 38 patients who experienced intrahepatic recurrence, 28 (73.7%) patients received repeated SRFA of whom 12 patients (42.9%) developed untreatable tumor progression. The mean imaging follow-up was 25.2 months (0–162 months).
Table 4. Details of unsuccessful local tumor control of subcardiac tumors following SRFA.
The local recurrence rate in the control group was 4.1% (10/242) with no significant difference vs. the subcardiac group (p = 0.25).
OS and DFS
OS and DFS rates are depicted in and . The OS rates at 1, 3 and 5 years from the date of the first SRFA were 92%, 77% and 65% with a median OS of 90.6 months in patients with exclusively single subcardiac HCC (n = 37). The corresponding DFS at 1, 3 and 5 years was 75, 34 and 34% with a median DFS of 19.1 months.
Figure 4. Overall Survival from the date of initial SRFA for patients with exclusively singular HCCs.
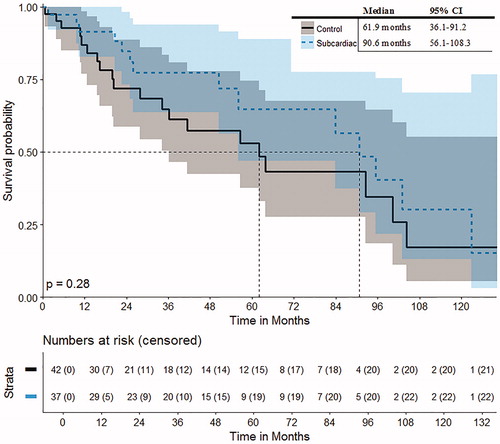
Figure 5. Disease-free survival from the date of initial SRFA for patients with exclusively singular HCCs.
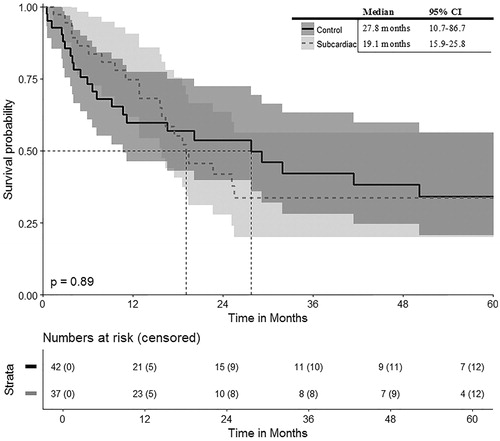
The OS rates of the control group at 1, 3 and 5 years were 87, 65 and 53% with a median OS of 61.9 months for the patient control group with single HCC in non-subcardiac location, respectively. The DFS rates at 1, 3 and 5 years were 60, 42 and 34% with a median DFS of 27.8 months.
There was no significant difference in OS or DFS of the subcardiac vs. control group (p = 0.28 and p = 0.89, respectively).
Discussion
The results of this study show that SRFA of subcardiac tumors is as safe and efficacious as when treating tumors remote from the heart. Specifically, we found no statistically significant difference between cases (i.e. patients with subcardiac tumors) vs. controls (those without subcardiac tumors) in terms of major complications (p = 0.76), primary and secondary efficacy (p = 0.93 and 0.23, respectively), local recurrence (p = 0.25), OS (p = 0.28, in terms of singular HCC) and DFS (p = 0.89, in terms of singular HCC). These results are important because they show that patients with subcardiac tumors should still be offered the procedure, that the consent process should be similar for tumors remote from the heart.
Historically, the literature suggested that percutaneous RFA of liver tumors in difficult locations results in more complications, insufficient ablation and poor tumor control [Citation16,Citation17]. More specifically, subcapsular tumors were associated with increased risk of complications [Citation8,Citation16,Citation18,Citation19] although more recent studies have been contradictory, reporting similar complication rates for subcardiac vs. non-subcardiac tumors (1–4%) [Citation7,Citation9]. Since the major complication rate in our study was 7.7% (8/104) and not significantly different from the control group (p = 0.56), our results are in keeping with these more recent reports. However, the higher complication rate found in our study may be explained by larger tumor size and the treatment of multiple tumors in one session. Indeed, three patients with major complications in our study were attributed to the treatment of other tumors, meaning the major complication rate specific to subcardiac tumors was 4.8% (5/104). However, by comparison, the surgical literature reports higher levels of postoperative morbidity ranging from 5 to 45% [Citation20].
Due to the stereotactic multiprobe approach, our treatment strategy deliberately challenges BCLC guidelines, whereby >60% of cases were BCLC B and C. We strongly believe that SRFA is preferable to TACE ± sorafenib in these patients, since ablation offers homogenous, lethal cell kill with the potential for curative treatment.
In terms of technical success, we were able to treat tumors with complete disappearance of tumor enhancement in the first session in 95.6% of cases and 99.1% after retreatment with a local recurrence rate of 7% and no significant difference compared with the control group. We recently published a study which used the same radiological methods to assess ablation zone coverage [Citation21] and found no viable tumor in 97.3% of cases as part of a histopathological validation study with whole explanted livers as the reference standard, meaning our data are likely to be histologically accurate. Whilst other groups have used iatrogenic pleural effusions [Citation22] and pneumothoraces [Citation23] to protect the thoracic structures, our reported high levels of complete treatment suggest such approaches may be unnecessary, whereby increased procedure time, radiation exposure and reduction of lung capacity in patients with co-existing respiratory disease are all considerations.
Early investigations [Citation8,Citation24] reported higher local recurrence rates for subcapsular tumors, which was thought to be due to undertreatment for fear of sustaining thermal damage to adjacent structures or causing liver capsular pain. However, more recent investigations which specifically focused on subcardiac tumors [Citation9,Citation10] reported no statistical difference in complete ablation (83–96%) or local recurrence rates between subcardiac and non-subcardiac tumors, although reported relatively high recurrence rates of 15–22%. Cha et al. compared the therapeutic outcomes of conventional US- guided radiofrequency (RF) ablation for subcardiac and non-subcardiac HCC in 73 patients with subcardiac HCC and the same number of patients with non-subcardiac HCC. Similar to our study there was no significant difference observed between both groups in terms of technical success rates and cumulative LR rates. However, despite their inclusion of BCLC A and B patients only, with a mean tumor size of 1.6 cm, their LR rates were > 15% in the subcardiac group. By contrast, we included tumors with a median size of 2.5 cm (maximum 9.5 cm) and found LR was 4–7%, which is similar to the surgical literature [Citation25]. We attribute the good local tumor control after SRFA to consistent achievement of a sufficient ablation margin of at least 5 mm. SRFA offers three-dimensional planning and very precise targeting to create multiple overlapping coagulation volumes. In case of poor tumor visibility, SRFA planning may include fusion with previously acquired MR images. Immediate post ablation contrast-enhanced CT fusion with the planning CT allows for rapid, reliable judgment of the ablation results with the option for re-ablation.
We have previously reported the OS for SRFA in intrahepatic cholangiocarcinomas [Citation25] and hepatic colorectal [Citation14], breast cancer [Citation26] and melanoma metastases [Citation27] and found a comparable survival advantage to liver resection. In the present study, we report OS rates of 92, 77 and 65% at 1, 3 and 5 years, respectively, with a median OS of 90.6 months in patients with exclusively single HCC. Another study of HCC ablation for tumors abutting the diaphragm [Citation28] showed OS rates of 91.7, 56.7 and 36.7% at 1, 3 and 5 years with a median OS of 44 months. SRFA achieved a higher survival rate despite unfavorable subcardiac location. We also found DFS of 75, 34 and 34%, at 1, 3 and 5 years respectively, with a median DFS of 19.1 months, whereas Kim et al. [Citation29] reported rates of 66.5, 20.4 and 17% at 1, 3 and 5 years, respectively, for HCC in all locations.
In comparison with the surgical literature, a meta-analysis showed OS of 72.3 and 55.5% and DFS of 35 and 25.7% at 3 and 5 years for non-anatomical liver resections for HCC, meaning our survival statistics are very similar [Citation30], even for large tumors as were ablated in the current study.
The relatively high rates of distant tumor recurrence (48.1% of patients) can be explained by the inclusion of BCLC B and BCLC C patients and the relatively long follow-up time (mean: 25.2 months and 0–162 months) in patients’ cirrhotic livers with a propensity to develop further HCCs.
The limitations of our study include its retrospective design which precluded a prospective power calculation. In addition, use of additional treatments such as liver transplantation (24 vs. 19%), TACE (5 vs. 6%) and chemotherapy (11 vs. 3%) differed slightly between each of the groups and can impact on OS. Whilst our results are not highly generalizable due to limited use of navigation systems as part of ablation procedures, their use is growing and we have previously demonstrated our technique is reproducible [Citation31]. In this way, we hope that the findings of this study will help generate further interest in our technique with a view to a prospective powered study, ideally in a multicenter setting. The lower complication rates and similar survival statistics vs. surgical alternatives suggests a comparison in the form of a randomized trial would be welcome.
In conclusion, our results suggest that stereotactic RFA is a safe and efficacious treatment strategy for subcardiac HCC with no statistically significant difference vs. tumors remote from the heart in terms of major complication, technical success and local recurrence rates and overall and DFS. These results are important because they show that patients with subcardiac tumors should still be offered stereotactic ablation and that the consent process should be the same as for tumors remote from the heart.
Disclosure statement
No potential conflict of interest was reported by the authors. RB is a consultant for CAScination and i-Sys Medizintechnik.
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109. DOI:10.1093/jnci/djx030.
- Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192–204.
- Higgins H, Berger DL. RFA for liver tumors: does it really work? Oncologist. 2006;11:801–808.
- Liver EAS, Liver EAS, Canc EORT. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma (vol 56, pg 908, 2012). J Hepatol. 2012;56(6):1430.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes-a 10-year experience at a single center. Radiology. 2016;278:601–611.
- Kim YS, Lee WJ, Rhim H, et al. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. Ajr. 2010;195:758–765.
- Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262.
- Cha DI, Kang TW, Song KD, et al. Radiofrequency ablation for subcardiac hepatocellular carcinoma: therapeutic outcomes and risk factors for technical failure. Eur Radiol. 2019;29(5):2706–2715.
- Kwan J, Appuhamy C, Lim GHT, et al. Safety and efficacy of percutaneous thermal ablation of juxta-cardiac hepatic tumours. Cardiovasc Intervent Radiol. 2018;41:920–927.
- Wallach D, Toporek G, Weber S, et al. Comparison of freehand-navigated and aiming device-navigated targeting of liver lesions. Int J Med Robotics Comput Assist Surg. 2014;10:35–43.
- Widmann G, Schullian P, Haidu M, et al. Targeting accuracy of CT-guided stereotaxy for radiofrequency ablation of liver tumours. Minim Invasive Ther Allied Technol. 2011;20:218–225.
- Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Intervent Radiol. 2011;34:852–856.
- Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2012;22:930–937.
- Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003;14:S293–S5.
- Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428–436.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108.
- Shibata T, Maetani Y, Kubo T, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15:1323–1327.
- Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983–7991.
- Bale R, Schullian P, Eberle G, et al. Stereotactic radiofrequency ablation of hepatocellular carcinoma – a histopathological study in explanted livers. Hepatology. 2018. DOI:10.1002/hep.30406
- Uehara T, Hirooka M, Ishida K, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007;42:306–311.
- de Baere T, Dromain C, Lapeyre M, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology. 2005;236:666–670.
- Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981.
- Bale R, Schullian P, Haidu M, et al. [Stereotactic radiofrequency ablation (SRFA) of intrahepatic cholangiocellular carcinomas: a minimal invasive alternative to liver resection]. Wien Med Wochenschr. 2013;163:128–131.
- Bale R, Richter M, Dunser M, et al. Stereotactic radiofrequency ablation for breast cancer liver metastases. J Vasc Interv Radiol. 2018;29:262–267.
- Bale R, Schullian P, Schmuth M, et al. Stereotactic radiofrequency ablation for metastatic melanoma to the liver. Cardiovasc Intervent Radiol. 2016;39:1128–1135.
- Ding H, Su M, Zhu C, et al. CT-guided versus laparoscopic radiofrequency ablation in recurrent small hepatocellular carcinoma against the diaphragmatic dome. Sci Rep. 2017;7:44583.
- Kim SS, Kang TW, Kim M, et al. Initial radiofrequency ablation failure for hepatocellular carcinoma: repeated radiofrequency ablation versus transarterial chemoembolisation. Clin Radiol. 2018;73:216 e1–e8.
- Zhou Y, Xu D, Wu L, et al. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396:1109–1117.
- Widmann G, Schullian P, Haidu M, et al. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35:570–580.