Abstract
Purpose: The objective of this study was to investigate the bactericidal effects of high intensity focused ultrasound (HIFU) on Bacillus Calmette–Guerin (BCG, a substitute for Mycobacterium tuberculosis) in vitro and in vivo, and to explore the underlying mechanisms.
Materials and methods: HIFU, at a fixed frequency of 1 MHz, was applied to both BCG culture suspensions and subcutaneous BCG abscesses in rats.
Results: HIFU irradiation significantly reduced the bacterial survival rate and caused temperature elevations both in vitro and in vivo. Furthermore, BCG suspensions irradiated for 15 s at 3185 and 6369 W/cm2 had increased cell wall damage, which resulted in morphological changes compared to the untreated control group. Additionally, we observed histological changes in the rat subcutaneous abscesses after HIFU ablation at 6369 W/cm2. H&E staining of infected lesions showed coagulative necrosis with central nucleus dissolution and increased infiltration of inflammatory cells, as well as nuclear pyknosis and nuclear fragmentation in the periphery. The volumes of the subcutaneous abscesses in the HIFU-treated group were significantly lower than those in the sham-treated group.
Conclusion: HIFU has the therapeutic potential to treat BCG-infected tissues in rats. We theorize that a combination of mechanical, cavitation, and thermal effects most efficiently inactivate BCG bacteria via HIFU. This study is expected to provide a bio-plausible basis for a noninvasive and effective treatment for tuberculosis.
Introduction
Tuberculosis (TB) – a disease that has existed for millennia – remains a major global health concern. Approximately 10 million people develop TB each year, and this disease is one of the top ten causes of death worldwide. For the past 5 years, TB has been the leading cause of death from a single infectious agent, ranking above HIV/AIDS [Citation1]. This recent intensification of the global TB epidemic is due to increased population migration, social aging, and the emergence of multi-drug resistant strains of Mycobacterium tuberculosis (MTB) [Citation2]. In 2017, an estimated 10 million people were newly infected with TB and 1.3 million died from the disease [Citation1]. Although the greatest burden of disease occurs in developing countries, developed countries are not spared from this disease [Citation3]. TB infection control remains a serious challenge.
TB is a chronic bacterial disease caused by slightly-curved, non-motile, aerobic, non-capsulated, and non-spore forming strains of MTB [Citation4]. MTB has an unusual waxy coating on its surface due to the presence of mycolic acid, which confers a level of natural antibiotic resistance to this bacterium [Citation5,Citation6]. TB infections may occur in any part of the body, but they are most commonly found in the lungs. Currently, TB is primarily treated with drug chemotherapy and surgery [Citation7]. However, because the TB treatment period is much longer than those of other antibiotics, poor therapy compliance among patients has been increasing, resulting in the evolution of antibiotic resistant strains [Citation8]. The widespread emergence of tuberculosis-resistant strains is a significant factor in the failure of tuberculosis treatment [Citation9]. Resectional surgery is utilized as adjuvant therapy only in multi-drug resistant tuberculosis (MDR-TB) cases, as TB patients are slow to recover from surgery-induced trauma [Citation7]. The interventional therapy for cavitary pulmonary MDR-TB is extremely expensive and complex, necessitating high-priced equipment [Citation10].
Currently, several research institutions are focused on the development of new drugs for the treatment of TB [Citation11]. Although the prospect of anti-TB drugs for the treatment of drug-resistant TB strains is promising, many challenges regarding drug delivery and drug toxicity remain [Citation12,Citation13]. Thus, TB treatment protocols would benefit from the development of a rapid, noninvasive therapeutic method.
High intensity focused ultrasound (HIFU), developed in the last decade, is a promising and noninvasive local tumor treatment technology that can be used in many organs [Citation14,Citation15]. HIFU has been widely used in several medical fields, including surgical oncology, obstetrics, and gynecology. HIFU techniques are being expanded for the treatment of benign and malignant tumors. Commercially available HIFU devices are used as replacements for, or as adjuncts to current standard treatments, including surgery, radiation, gene therapy, immunotherapy, and chemotherapy [Citation16]. In the past, lung tumors have not been treated with HIFU due to the air content of ventilated lungs. However, in the recent years, HIFU has been the focus of increasing clinical interest as a noninvasive method for the local therapy of lung tumors. Indeed, recent studies have reported the successful performance of ultrasound-guided HIFU ablations of lung tumors after lung flooding [Citation17–20]. Flooded lungs generate suitable acoustic pathways for the transthoracic application of HIFU, and enable sonography-guided HIFU for lung tumor ablation in ex vivo human and in vivo animal models. Based on these previous studies, HIFU technology may be feasible approach for lung disease therapy.
An HIFU clinical trial including 1038 cancer patients (e.g., hepatocellular carcinoma, osteosarcoma, and breast cancer) had an extremely low major complication rate [Citation21]. Although not severe, several complications commonly arise as a result of HIFU treatment, including a low-grade fever (up to 38.5°C), HIFU-induced skin burns, and mild local pain within 1 week after HIFU ablation. This clinical trail indicated that HIFU ablation was a safe, effective, and feasible modality for the ablation of carcinomas. We speculate that the incidence of complications of HIFU technology is also applicable to lung lesions/TB.
The concept using thermal pasteurization (60–82°C) for inactivating pathogenic microorganisms in certain foods and beverages is not new [Citation22]. In the HIFU process, low-energy ultrasound waves are focused on diseased tissue. The ultrasound radiation penetrates the tissue, generating energy accumulation in the multiple of thousands. This accumulation of energy typically heats the targeted tissue, possibly leading to thermally-induced cell death and/or coagulation necrosis [Citation16]. HIFU has been tested as a treatment for deep bacterial infections (e.g., Staphylococcus aureus) in a rat model because of its capability to induce a rise of temperature at a very precise location [Citation23–26]. Some studies have also demonstrated that cavitation-based HIFU histotripsy has shown potential for treating common bacterial pathogens, including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [Citation27–29]. Therefore, we hypothesized that the thermal and cavitational effects of HIFU might serve to treat and control MTB in vitro and in vivo.
As MTB is a highly infectious and pathogenic bacterium, only laboratories meeting National Biosafety Level 3 (BSL-3) requirements may experiment with live MTB. Bacillus Calmette–Guerin (BCG) is a live attenuated strain of M. bovis that is closely related to MTB, with a similar cell wall structure and slow growth rate, but that is nonpathogenic; as such, BCG is often used in research studies in place of MTB [Citation30]. Thus, we investigated the bactericidal effects of HIFU irradiation on BCG. We developed a model of subcutaneous BCG abscess formation to study the effects of HIFU ablation on BCG tissue infections. This study represents the first steps toward developing a noninvasive treatment for TB.
Materials and methods
Subcutaneous abscess model and wound observation
All experiments and procedures involving animals were approved by the Ethics Review Committee for Animal Experimentation at Chongqing Medical University (Chongqing, China). We obtained 24 healthy Sprague–Dawley rats (6–7 weeks; 150–180 g) from the Animal Center of Chongqing Medical University. All rats were housed in independent airflow-controlled cages, and allowed ad libitum access to the standard diet. For 3 days prior to the establishment of the abscess model, we added 1 mg/kg dexamethasone (1 mg/kg) to the rats' drinking water (250 mL) in order to suppress immunity. To establish the abscess model, 1 mL of the BCG bacterial suspension (2.85 × 108 CFU/mL; for details of the bacterial culture, see the Supplementary material) was subcutaneously injected into the shaved buttock tissue of each rat. Rats were observed for 14 days to confirm the development of a subcutaneous abscess. The long (a) and short diameters (b) of the subcutaneous nodules were measured with a vernier caliper (Guanglu Measuring Instrument Co., Ltd., Guilin, China), and the abscess volume (V) was calculated according to the following formula: V=(a×b2)/2. The abscess-bearing rats were randomly assigned to two groups: the control group (n = 12; sham exposure) and the HIFU-treated group (n = 12). On third day after HIFU irradiation, we killed six sham-treated and six HIFU-treated rats by cervical dislocation. The abscesses were excised; part of each abscess was examined histologically, and part was homogenized. The remaining six sham-treated and six HIFU-treated rats were observed for an additional 15 days in order to measure the volume change of the subcutaneous nodules. These 12 rats were euthanized as above after the completion of this experiment.
Ultrasonic irradiation and temperature monitoring
We used a JC200 focused ultrasound treatment system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) in this study. In this system, a B-mode ultrasound diagnostic probe, which provided real-time monitoring of the treatments, is incorporated within the therapeutic transducer. The ultrasound exposure parameters were as follows: 1 MHz transducer frequency, 145 mm focal length, and 9 mm × 2.7 mm × 2.7 mm focal region. The focal region of the JC200 focused ultrasound treatment system was measured using a hydrophone (ONDA, Sunnyvale, CA, USA). Passive cavitation detection technology was used to monitor cavitation during HIFU irradiation, as previously described [Citation31]. The acoustic intensity of the JC200 system (i.e., the spatial-peak temporal average) was reported by the associated software, and conformed to the standards of the National Institute Metrology Institute of China; values were derated to account for losses along the beam path.
For in vitro experiments, 3-mL aliquots of the BCG bacterial suspension were each placed into a separate homemade sterile plastic film apparatus, constructed by removing the bottom of a 15-mL eppendorf tube and covering the resulting opening with sterile plastic film. The modified tubes containing the bacterial suspensions were immersed in a 25°C degassed water bath, 3 cm under the horizontal plane. Under the guidance of a diagnostic ultrasound, the HIFU was focused at the center of the BCG suspension in each tube (. Each bacterial suspension was subjected to one of three treatments: sham exposure (control group; n = 6), exposure to 3185 W/cm2 for 15 s with continuous waves (low-dose group, n = 6), or exposure to 6369 W/cm2 for 15 s with continuous waves (high-dose group, n = 6). During exposure, we gently stirred each suspension with a sterile plastic stick to help spread temperature within the sample and may allow more of the solution to pass through the focus.
Figure 1. Schematic diagrams of the HIFU irradiation of (A) a BCG suspension and (B) a subcutaneous BCG abscess.
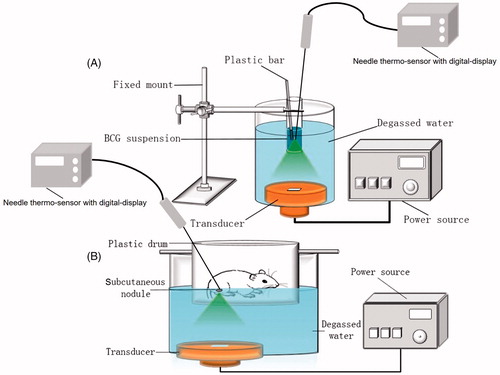
For the in vivo experiments, each abscess-bearing rat was anesthetized with an intraperitoneal injection of 1% pentobarbital sodium and restrained in a custom-made plastic drum with round hole (diameter: 1.5 cm) at the bottom, through which the abscess was accessed; the hole was located directly above the ultrasonic transducer (. The abscess of each rat was dipped in 25°C degassed water through the hole. The ultrasonic diagnostic probe was then used to scan the real-time ultrasound image of the subcutaneous abscess to determine the approximate therapeutic range and to ensure that the abscess was positioned at the center of the transducer focal zone. Because preliminary in vitro results suggested that the bactericidal effects of 6369 W/cm2 exposure were greater than those of 3185 W/cm2 exposure, abscesses were spot-scanned three times at an ultrasonic intensity of 6369 W/cm2. Each exposure lasted 15 s and was followed by a 30 s pause. After each exposure, the focus was moved 2 mm horizontally (moving the HIFU transducer under the supervision of B-mode ultrasound), such that the horizontal distance between two adjacent irradiation foci was about 2 mm. In the sham treatment, the ultrasonic device turned off.
Room temperature was maintained at 25°C during all ultrasonic treatments using air-conditioning. All bacterial suspensions were adjusted to room temperature before ultrasonic irradiation. The temperatures of the bacterial suspensions and the subcutaneous rat abscesses were monitored using a needle thermo-sensor with digital-display (batch 119, No. 02810232; Yuyao Temperature Instrument Factory Co, Ltd, Yuyao, Zhejiang, China). In the in vitro experiment, the needle thermo-sensor was placed at the HIFU focus under the guidance of the B-mode ultrasound; the temperature in each experimental tube was recorded before and immediately after irradiation. In the in vivo experiment, the needle thermo-sensor was carefully inserted in the center of the subcutaneous abscess of each rat; the temperature of each abscess was recorded before and immediately after irradiation.
Bacterial colony numbers following HIFU irradiation
After bacterial suspensions were exposed to HIFU irradiation, 1 mL of each bacterial suspension was serially diluted, and the number of surviving bacterial colonies were counted. To count the bacteria in the rat abscesses, 1 g of abscess tissue from each rat was homogenized in 1 mL of physiological saline on ice using a tissue grinder. Tissue homogenates were centrifuged at 3500g for 10 min. The sediment from each tube was plated on 7H10 media in ten-fold serial dilutions and incubated for 4 weeks at 37°C.
Effects of temperature on BCG activity levels
Based on the temperatures recorded during ultrasonic treatments, we exposed bacteria to temperatures of 25, 55, and 70°C. We then placed 3-mL aliquots of the BCG bacterial suspension into separate centrifuge tubes, and incubated the tubes in water baths at 25, 55, or 70°C. Once the temperature of the suspension in each tube stabilized at the temperature of the water bath for 15 or 45 s, the tube was immediately removed from the water bath and cooled to room temperature (25°C). Bacterial viability was determined using a confocal micrograph.
Measurement of bacterial viability via confocal laser scanning microscopy
After HIFU irradiation or heat treatment, BCG cells were collected and resuspended in 1 mL of sterile phosphate buffered saline buffer. We added 50 μL of the fluorescent dye SYTO9/PI (Live/Dead Baclight Bacterial Viability Kit; Thermo Fisher Scientific, Waltham, MA, USA) to each suspension (final concentration: 2 mM). The bacterial suspensions were then incubated in the dark at 37°C for 15 min. Suspensions were observed and photographed under a confocal laser scanning microscope (CLSM; A1 + R; Nikon, Tokyo, Japan), using red (Ex = 488 nm/Em = 515 nm) and green (Ex = 568 nm/Em = 600 nm) lasers. Bacterial viability was determined based on fluorescence intensity, using Image J (National Institutes of Health, USA) to analyze the confocal micrographs.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
We centrifuged 1-mL aliquots of each irradiated BCG suspension at 9600g for 10 min. The precipitate from each sample was gradually poured along the tube wall with 4% (v/v) precooled glutaraldehyde, and fixed for 2 h at 4°C. After dehydration, samples were embedded in paraffin, and ultrathin sections were prepared for TEM observation (JEM-1400PLUS; Hitachi High-Technologies, Tokyo, Japan). Additional aliquots of each BCG bacterial suspension were placed on coverslips in a six-well plate and fixed with 4% (v/v) precooled glutaraldehyde for SEM (SU8010; Hitachi High-Technologies, Tokyo, Japan).
Histological examination
Abscessed tissues were fixed with 10% buffered formalin for at least 24 h, and then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Histopathological changes were observed under a light microscope. In addition, acid-fast staining was performed on each specimen in order to detect BCG bacilli in the tissue.
Statistical analysis
Results were analyzed using one-way ANOVAs in SPSS 17 statistical software (IBM, Chicago, USA). The data were expressed as mean ± standard deviation. p< .05 was considered statistically significant.
Results
BCG activity after HIFU treatment
Colony counts were performed on BCG suspensions in vitro and in vivo after HIFU treatment. In the in vitro study, we recovered (2.75 ± 0.16)×108 CFU/mL in the control suspensions, (1.70 ± 0.24)×108 CFU/mL in the low-dose suspensions, and (0.81 ± 0.16)×108 CFU/mL in the high-dose suspensions (. In the in vivo study, we recovered 219.33 ± 34.39 CFU/gram of abscess in the sham-treated group and 39.33 ± 15.31 CFU/gram of abscess in the HIFU-treated group (.
Figure 2. The effect of HIFU irradiation on BCG viability. After HIFU treatment, colony counts were performed on (A) BCG bacterial culture suspensions in vitro and (B) BCG abscess tissue homogenates in vivo. (C) Bacterial viability was observed after HIFU irradiation. Cell were SYTO 9 (green) and PI (red) double-stained and viewed under a laser scanning confocal microscope (×100). (D) The percentage of viable cells (green) was calculated as the intensity of the green fluorescence divided by that of the red fluorescence. The intensities of 10 randomly-selected fields of view per sample were determined.
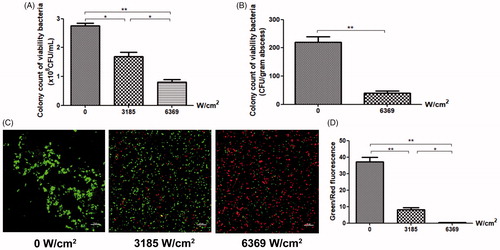
CLSM was also used to assess the activity of BCG in solution after HIFU irradiation (, where viable BCG cells are green and non-viable BCG cells are red). The control group predominately contained green fluorescent bacteria, closely aligned and grouped. Bacteria in the low-dose group formed small colonies; the red signal was significantly less intense than the green signal. The red signal in the high-dose group predominated, with only low amounts of green signal; bacteria in this group were evenly distributed. There was a significant decrease in the ratio of green-fluorescent intensity to red-fluorescent intensity in the low-dose and high-dose groups, as compared to the control group (; p< .01).
The survival rate of the bacteria in the HIFU-treated group was significantly lower than that of the control group (p< .01), both in vitro and in vivo. Furthermore, the number of viable bacteria decreased significantly as the irradiation intensity increased.
Changes in BCG structure and mycelial morphology after HIFU irradiation
Structural changes in the BCG bacteria after HIFU irradiation were observed with TEM (. The cellular structures of the bacteria in the control group were intact. In the 3185 W/cm2 group, the cytoplasm inside the BCG cells was partially clumped, and the capsule was incomplete. In the 6369 W/cm2 group, the capsule was incomplete, the cytoplasm was clumped, the organelles were swollen and dissolved, and the cell wall structure had been destroyed. This suggested that HIFU irradiation physically damaged, and sometimes ruptured, the BCG cell wall and membrane. The degree of bacterial cell damage increased with the ultrasound radiation dose.
Figure 3. BCG structures and mycelial morphologies were observed under TEM and SEM, respectively, after irradiation with different HIFU intensities. The cell wall was intact in the control group. The BCG cytoplasm was partially coagulated into blocks (red arrow) in the 3185 W/cm2 group. The cell wall was broken, with leaked cytoplasm, and the membrane organelles were swollen and lysed (red arrow) in the 6369 W/cm2 group. The BCG cell wall was shrunken and depressed in 3185 W/cm2 group (blue arrow); broken and bulging cells with spilled cytoplasmic contents were observed in the 6369 W/cm2 group (blue arrow).
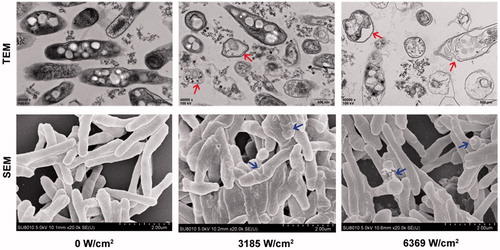
The mycelial morphologies of the BCG cells after HIFU irradiation were observed with SEM (. In the control group, the BCG cells had an obvious rod shape. In the 3185 W/cm2 group, the cells walls were shrunken, with many depressions. In the 6369 W/cm2 group, bacteria were irregularly arranged, with distorted morphologies and severely damaged cell structures that resulted in lysis.
Gray-scale changes in the subcutaneous nodules
The entire HIFU subcutaneous nodule ablation process was monitored by B-scan ultrasonography in real-time. Prior to HIFU ablation, the subcutaneous nodules were internally uniformly hypoechoic (; ). After ablation, the echo of the target tissue increased, characterized by a hyperechoic area (; ). The gray-value of the HIFU irradiation focus area increased to 38.67 ± 9.93 after HIFU ablation (). There were no significant changes in the echo values of the control group.
Figure 4. Sonogram showing subcutaneous nodules before and after HIFU ablation. (A) Before HIFU irradiation, the subcutaneous nodules in the rats were internally hypoechoic. (B) After HIFU irradiation, the target area was hyperechoic.
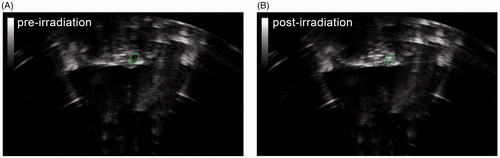
Table 1. Temperature changes at different intensities of HIFU irradiation in vitro and in vivo.
Histopathological observations
The size of each subcutaneous abscess was measured with a vernier caliper every 3 days, and the volume of the subcutaneous abscess was calculated (. On the day of HIFU ablation, the mean volume of the subcutaneous abscesses in the high-dose group was 934.77 ± 124.25 mm3. At 3 days after HIFU ablation, the mean volume of the subcutaneous abscesses in the high-dose group had decreased to 399.05 ± 131.05 mm3. We observed that the dissected abscesses from the irradiated groups were smaller than those from the sham group. The HIFU treatment area was round and a dull grayish-white color, indicating coagulative necrosis; there was no significant histological change in the abscesses of the sham group (.
Figure 5. The growth curves of the subcutaneous abscesses with and without HIFU treatment. (A) Representative images of the subcutaneous abscess and the dissected abscess. (B) Growth curves of the BCG abscesses in rats that did not undergo surgical resection after HIFU ablation. The abscess volume in the HIFU-ablation group was smaller than that of the sham group (*p<.05, **p<.01).
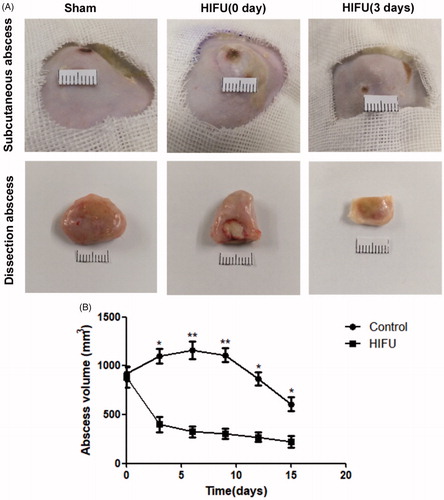
The subcutaneous abscesses of the rats that did not undergo surgical resection were observed for an additional 2 weeks after HIFU ablation. The mean volume of the subcutaneous abscesses in the sham-treated group slowly increased and then gradually decreased. In the HIFU-treated group, the mean abscess volume decreased significantly 3 days post-ablation, and continued to decrease over time. This indicated that abscess growth was more inhibited in the HIFU-treated group than in the sham-exposed group (.
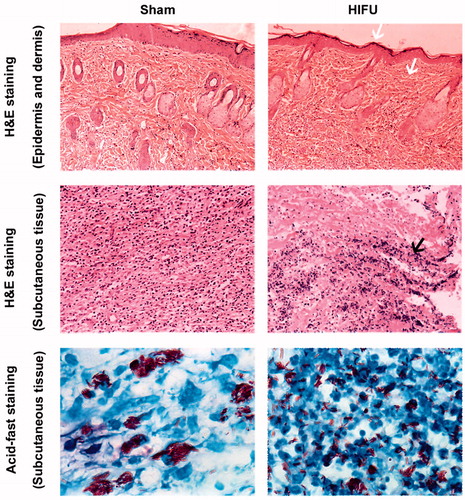
H&E staining in the HIFU treatment group demonstrated central nucleus dissolution in the center of the coagulative necrotic region, with nuclear pyknosis and fragmentation in the periphery and increased inflammatory cell infiltration. Importantly, HIFU ablation did not damage rat epidermal or dermal tissues. Acid-fast staining showed that BCG was aggregated in the tissues of the control group. In contrast, the BCG bacteria in the HIFU-treatment group were uniformly dispersed with a short rod shape (.
Figure 6. Subcutaneous nodules were sectioned, and stained with H&E and acid-fast. HIFU did not damage the epidermal or dermal tissues (white arrows). The black arrow indicates the central nucleus dissolution in the center of the coagulative necrotic region after HIFU ablation, with nuclear pyknosis and fragmentation in the periphery. H&E-stained images were taken at ×20 magnification, and the acid-fast staining images were taken at ×100 magnification.
Temperature change following HIFU
The temperatures of the BCG solutions and the subcutaneous nodules were measured immediately after ultrasound irradiation (). The temperature changed after ultrasonic irradiation, as expected. HIFU irradiation had a significant thermal effect (p<.01). Unfortunately, in vitro and in vivo experiments, the peak temperatures are likely underestimated because it is not possible to detect the peak temperature at the exact moment of HIFU irradiation with a needle thermo-sensor in this study.
Cavitation effects of HIFU
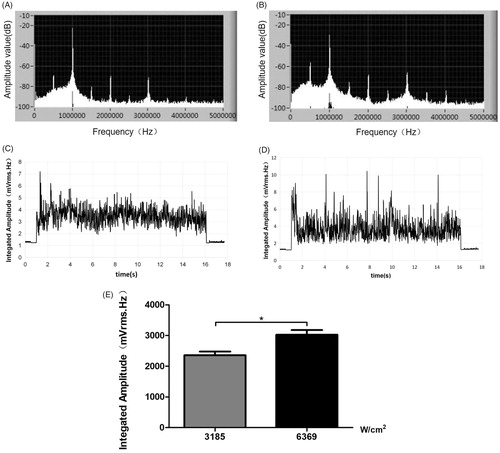
showed that subharmonics and broadband noise were generated during HIFU irradiation, which were the characteristic spectra of stable and inertial cavitation, respectively. Broadband emission signals were detected throughout the entire 15-s HIFU process under atmospheric pressure (at 3185 W/cm2, ; at 6369 W/cm2, ; cumulative, . The higher-dose HIFU broadband emissions were significantly stronger than the lower-dose HIFU broadband emissions (p<.05), indicating that HIFU caused cavitation.
Figure 7. The frequency spectrum of the emissions generated during HIFU irradiation at ultrasonic intensities of (A) 3185 W/cm2 and (B) 6369 W/cm2. Broadband emission signals were detected throughout the entire 15-s HIFU process under atmospheric pressure (C: at 3185 W/cm2; D: at 6369 W/cm2; E:cumulative).
BCG activity after heat treatment alone
To explore the mechanisms underlying the bactericidal effects of HIFU on BCG bacteria, we tested the effects of heat treatment alone (without HIFU) on bacterial activity using fluorescent analysis (. After a 15-s heat treatment, bacteria primarily fluoresced green, indicating viability (. In addition, there was no significant difference in the ratio of green-fluorescent intensity to red-fluorescent intensity between heat-treated bacteria (heated to 55 or 70°C for 15 s) compared with the control bacteria (kept at 25°C; p> .05; . Thus, the results indicated that a 15-s heat treatment (55 or 70°C) did not significantly reduce bacterial activity.
Figure 8. The effects of temperature on BCG viability. Bacterial viability was observed after treatment at different temperatures for (A) 15 s and (C) 45 s. Cells were SYTO 9 (green) and PI (red) double-stained and viewed under a laser scanning confocal microscope (×100). (B), (D) The percentage of viable cells (green) was calculated as the intensity of the green emissions divided by that of the red emissions. The intensities of 10 randomly-selected fields of view per sample were determined (*p<.05, **p<.01).
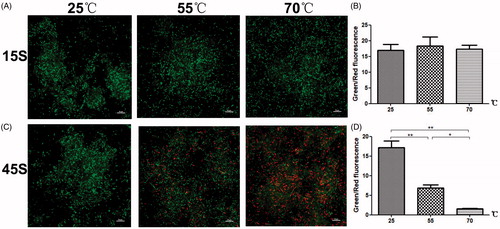
However, when bacteria were treated at 55 or 70°C for 45 s, there were significantly fewer green fluorescent bacteria as compared to the control bacteria (kept at 25°C). In addition, the ratio of green-fluorescent intensity to red-fluorescent intensity for the 45-s heat-treated bacteria (at 55 or 70°C) was significantly lower than that for the control bacteria (25°C; p<.05; . That is, heat treatment at 55 or 70°C for 45 s significantly reduced bacterial activity.
Discussion
HIFU is a modern, energy-based medical technology that is currently used in both research and clinical practice, particularly for the treatment of malignant and benign tumors [Citation32,Citation33]. HIFU treatment of lesion tissue does not depend on cell type [Citation16]. Although few studies have investigated the treatment of infectious diseases with HIFU, these studies have yielded promising results. For example, HIFU exhibits bactericidal activity against some bacterial abscesses, including Enterococcus faecalis, E. coli, and S. aureus [Citation23–25,Citation34]. Here, we hypothesized that HIFU might inhibit MTB growth as the basis of a noninvasive TB treatment.
To explore the bactericidal effects of HIFU on BCG, in vitro and in vivo experiments were performed. After HIFU treatment, we observed the survival rates of BCG suspensions. HIFU sound intensities of 3185 and 6369 W/cm2 inactivated significantly more BCG cells than the control treatment (p< .05). Furthermore, TEM images indicated that BCG cell membranes and cell walls were severely damaged following irradiation (. Ruptured cell membranes and walls were observed using SEM (.
In addition, the concentrations of DNA in the BCG supernatants and pellets were evaluated after HIFU irradiation (). We found that the concentrations of DNA in the BCG supernatants after ultrasonic irradiation at 3185 and 6369 W/cm2 were significantly higher than that of the control group. Thus, our results suggested that bacterial damage increased in severity with increasing ultrasound intensity. We found that HIFU treatment exerts bactericidal activity by damaging the bacterial cell, causing DNA and cytoplasm to leak, and eventually resulting in cell death.
In the rat model established herein, we observed gray-scale changes in the subcutaneous nodules after HIFU irradiation guided by B-ultrasound. Tissues were harvested from rat abscesses 3 days after ultrasound irradiation. We observed coagulation necrosis within the abscessed tissue. Postirradiation, the nodule volumes in the HIFU-treated group were smaller than those of in the sham-exposure group (p< .05), indicating that HIFU ablated the nodules.
The plate count method was used to calculate the number of viable BCG cells per gram of tissue. The survival rate of the irradiated group was significantly lower than that of the control group (p<.05). In rats, HIFU treatment decreased nodule size and the percentage of live bacteria in the abscess, but did not cause open wounds or further infection after HIFU treatment. In only one treatment case did our histological examination of the abscess slides identify a possible disruption of the abscess capsule (data not shown). However, it was also possible that this change in structure was caused by the histology processing itself. Our results were similar to those of Rieck et al. [Citation24], where no open wounds were observed after HIFU treatment, but the abscess capsules on some of the histological slides were disrupted. We have no evidence of other adverse effects related to HIFU-induced abscess sterilization in the rat model. These results suggested that HIFU had a therapeutic effect on BCG-infected rat tissues.
The biological effects of HIFU has thermal, cavitational, and mechanical effects [Citation30,Citation31]. Previous studies have suggested that these HIFU-induced biological effects might cause bacterial cell disruption and bacterial death [Citation27,Citation35]. In this study, we also explored the mechanisms by which HIFU damages BCG cells. After 15 s of exposure to the most intense irradiation in this study (6369 W/cm2), the mean temperatures of the bacterial solutions and rat tissues were 51.80 ± 3.31°C and 65.32 ± 3.94°C, respectively. Heat treatment for 15 s at these temperatures (i.e., 55 or 70°C) did not inactivate BCG cells (. However, when we heated BCG cells to 55 or 70°C for 45 s, the BCG inactivation rate was significantly higher than that when cells were heated to 25°C (. Our results were consistent with a previous studies about the thermal inactivation of pathogenic microorganisms such as M. bovis, M.tuberculosis and M. paratuberculosis during milk pasteurization [Citation22,Citation36]. These studies describe that a high-temperature, short-time (HTST) treatment of milk led to a significant reduction of the target organisms. Further, the results of Grant’s thermal inactivation experiments indicated that when large numbers of M. paratuberculosis cells were present in milk, the organisms may not be completely inactivated by a HTST method (71.7°C for 15 s) under laboratory conditions [Citation22]. Our ability to detect the temperature with a thin metal thermosensor at the HIFU irradiation target in real-time remains limited. The temperature of heat treatment on BCG solution in our study may be lower than the true peak HIFU temperature. HIFU-induced thermal effects play a very important role in HIFU-associated bacterial deaths.
It is known that we observed that an inertial cavitation effect was produced during HIFU (. Cavitation-associated effects include shear (during either bubble expansion or bubble collapse), jetting (i.e., a mechanical jet impacting the bacteria), or shock waves (from the inertial collapse of the bubble) [Citation37,Citation38]. Here, we speculated that cavitation disrupted the structures of BCG cell walls. HIFU most efficiently inactivates bacteria when focused on a very small area. This causes bubbles to rupture due to the vibrations generated by strong shock waves. Intense jet waves damage and destroy BCG cell walls due to the pores created by ultrasound cavitation.
Previous studies of HIFU ablation in a murine tumor model demonstrated that HIFU activated specific immune cells (e.g., cytotoxic T-lymphocytes) and elicited specific anti-tumor immunity [Citation39,Citation40]. Consistent with these studies, we observed an increased infiltration of inflammatory cells in the tissue sections from the treatment group. The recruited immune cells might also help to remove the abscess following sonication. In future work, we aim to test whether HIFU treatment induces an inflammatory response by evaluating the impact of HIFU on white blood cell count and local neutrophil recruitment.
Our in vitro and in vivo studies showed that HIFU irradiation caused irreversible damage to BCG cells. HIFU has been used in clinics with image monitoring, precision control, and treatment programs [Citation41]. While HIFU has been widely used in clinical applications to treat various tumors, we are unaware of any studies using HIFU as a therapeutic treatment for diseases associated with TB. Importantly, the lung is no longer considered a forbidden area for ultrasound diagnosis and treatment. This study represents the first steps towards the use of HIFU for the physical eradication of MTB infections, or, at a minimum, for the biologically significant reduction of bacterial burden. However, the development of HIFU for MTB treatment requires additional intensive study. Our goal is to develop this noninvasive and effective method into an efficient treatment option for TB.
Supplemental Material
Download PDF (662.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: WHO; 2018.
- Millen SJ. The global burden of tuberculosis – combating drug resistance in difficult times. N Engl J Med. 2009;360:2393–2395.
- Littleton J, Park J, Thornley C, et al. Migrants and tuberculosis: analysing epidemiological data with ethnography. Aust NZ J Public Health. 2010;32:142–149.
- Santha T, Renu G, Frieden TR, et al. Are community surveys to detect tuberculosis in high prevalence areas useful? Results of a comparative study from Tiruvallur District, South India. Int J Tuberc Lung Dis. 2003;7:258–265.
- Yang L, Sinha T, Carlson TK, et al. Changes in the major cell envelope components of Mycobacterium tuberculosis during in vitro growth. Glycobiology. 2013;23:926–934.
- Favrot L, Ronning DR. Targeting the mycobacterial envelope for tuberculosis drug development. Exp Rev Anti-infect Ther. 2012;10:1023.
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2004;128:523–528.
- Wang F, Sambandan D, Halder R, et al. Identification of a small molecule with activity against drug-resistant and persistent tuberculosis. Proc Natl Acad Sci USA. 2013;110:E2510–E2517.
- Yang C, Shen X, Peng Y, et al. Transmission of Mycobacterium tuberculosis in China: a population-based molecular epidemiology study. Clin Infect Dis. 2015;61:219–227.
- Saeed W. Cavitating pulmonary tuberculosis: a global challenge. Clin Med. 2012;12:40.
- Lienhardt C, Raviglione M, Spigelman M, et al. New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J Infect Dis. 2012;205:S241.
- Ginsberg D. Drugs in development for tuberculosis. Drugs. 2010;70:2201–2214.
- Parumasivam T, Leung SS, Quan DH, et al. Rifapentine-loaded PLGA microparticles for tuberculosis inhaled therapy: preparation and in vitro aerosol characterization. Eur J Pharm Sci. 2016;88:1–11.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195:W245.
- Kruse DE, Mackanos MA, O'Connellrodwell CE, et al. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys Med Biol. 2008;53:3641–3660.
- Dogra VS, Man Z, Bhatt S. High-intensity focused ultrasound (HIFU) therapy applications. Ultrasound Clin. 2009;4:307–321.
- Wolfram F, Boltze C, Schubert H, et al. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res. 2014;19:1–9.
- Wolfram F, Reichenbach JR, Lesser TG. An ex vivo human lung model for ultrasound-guided high-intensity focused ultrasound therapy using lung flooding. Ultrasound Med Biol. 2014;40:496–503.
- Wolfram F, Dietrich G, Boltze C, et al. Effects of HIFU induced cavitation on flooded lung parenchyma. J Ther Ultrasound. 2017;5:21.
- Wolfram F, Lesser TG. A simulation study of the HIFU ablation process on lung tumours, showing consequences of atypical acoustic properties in flooded lung. Zeitschrift für Medizinische Physik. 2019;29:49–58.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–154.
- Grant IR, Ball HJ, Neill SD, et al. Inactivation of Mycobacterium paratuberculosis in cows' milk at pasteurization temperatures. Appl Environ Microbiol. 1996;62:631–636.
- Brayman AA, Macconaghy BE, Wang YN, et al. Inactivation of planktonic Escherichia coli by focused 2-MHz ultrasound. Ultrasound Med Biol. 2017;43:1476.
- Rieck B, Bates D, Zhang K, et al. Focused ultrasound treatment of abscesses induced by methicillin resistant Staphylococcus aureus: feasibility study in a mouse model. Med Phys. 2014;41:063301.
- Curiel L, Mougenot C, Rieck B, et al. Focused ultrasound treatment of methicilin resistant Staphylococcus aureus induced abscesses: pre-clinical study. J Ther Ultrasound. 2015;3:P58.
- Rieck B, Curiel L, Mougenot C, et al. Treatment of localized abscesses induced by methicillin-resistant Staphylococcus aureus (MRSA) using MRgFUS: first in vivo results. 12th International Symposium on Therapeutic Ultrasound. AIP Conference Proceedings, Volume 1503. AIP Conference Proceedings, Volume 1503, Issue 1, p.173–178. 2012.
- Bigelow TA, Northagen T, Hill TM, et al. The destruction of Escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound Med Biol. 2009;35:1026–1031.
- Bigelow TA, Thomas CL, Wu H, et al. Histotripsy treatment of S. aureus biofilms on surgical mesh samples under varying pulse durations. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:1420–1428.
- Xu J, Bigelow TA, Halverson LJ, et al. Minimization of treatment time for in vitro 1.1 MHz destruction of Pseudomonas aeruginosa biofilms by high-intensity focused ultrasound. Ultrasonics. 2012;52:668–675.
- Alderton H, Smith D. Safety in the laboratory. School Sci Math. 2001;54:367.
- He M, Zhong Z, Li X, et al. Effects of different hydrostatic pressure on lesions in ex vivo bovine livers induced by high intensity focused ultrasound. Ultrason Sonochem. 2017;36:36–41.
- Cheung VYT. High-intensity focused ultrasound therapy. Best Pract Res Clin Obstet Gynaecol. 2017;46:74–83.
- Dababou S, Marrocchio C, Scipione R, et al. High-intensity focused ultrasound for pain management in patients with cancer. Radiographics. 2018;38:603–623.
- Wardlow R, Sahoo K, Dugat D, et al. High intensity focused ultrasound (hifu) heating improves perfusion and antimicrobial efficacy in mouse Staphylococcus abscess. Ultrasound Med Biol. 2018;44:909.
- Iqbal K, Ohl SW, Khoo BC, et al. Effect of high-intensity focused ultrasound on Enterococcus faecalis planktonic suspensions and biofilms. Ultrasound Med Biol. 2013;39:825–833.
- Hammer P, Richter E, Rüsch-Gerdes S, et al. Inactivation of Mycobacterium bovis ssp. caprae in high-temperature, short-term pasteurized pilot-plant milk. J Dairy Sci. 2015;98:1634–1639.
- Xu Z, Fowlkes JB, Rothman ED, et al. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117:424–435.
- Tempany CMC, Mcdannold NJ, Hynynen K, et al. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259:39–56.
- Ran LF, Xie XP, Xia JZ, et al. Specific antitumour immunity of HIFU-activated cytotoxic T lymphocytes after adoptive transfusion in tumour-bearing mice. Int J Hypertherm. 2015;32:1.
- Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34.
- Copelan A, Hartman J, Chehab M, et al. High-intensity focused ultrasound: current status for image-guided therapy. Semin Intervent Radiol. 2015;32:398–415.
