Abstract
Objective: To investigate the feasibility and efficacy of localized, subtotal, cortical-sparing microwave thermal ablation (MTA) as a potential curative management for primary aldosteronism. The study investigated with equal importance the selected ablation of small volumes of adrenal cortex while sparing adjacent cortex.
Method: An in-vivo study was carried out in swine (n = 8) where MTA was applied under direct visualization, to the adrenal glands at 45 W or 70 W for 60 s, using a lateral, side-firing probe and a non-penetrative approach. Animals were survived for 48 h post-procedurally. Animals were investigated for markers of histological, immunohistochemical and biochemical evidence of adrenal function and adrenal damage by assessing samples drawn intra-operatively and at the time of euthanasia.
Results: Selected MTA (70 W for 60 s) successfully ablated small adrenocortical volumes (∼0.8 cm3) characterized by coagulative necrosis and abnormal expression of functional markers (CYP11B1 and CYP17). Non-ablated, adjacent cortex was not affected and preserved normal expression of functional markers, without increased expression of markers of heat damage (HSP-70 and HMGB-1). Limited adrenal medullary damage was demonstrated histologically, clinically and biochemically.
Conclusion: MTA offers potential as an efficient methodology for delivering targeted subtotal cortical-sparing adrenal ablation. Image-guided targeted MTA may also represent a safe future modality for curative management of PA, in the setting of both unilateral and bilateral disease.
Introduction
Primary aldosteronism (PA) accounts for 5–12% of all hypertension and confers a higher risk for cardiovascular and cerebrovascular complications compared to age and blood pressure (BP) matched essential hypertension [Citation1–4]. However, PA remains under-diagnosed and under-treated, largely due to the lack of definitive management options for the majority of affected individuals [Citation5–7].
PA is driven by unregulated aldosterone secretion from unilateral (40%) or bilateral (60%) aldosterone-producing adenomas (APA) (0.5–2 cm3) or aldosterone-producing cell clusters (APCC) (<0.5 cm3) and characterized biochemically by raised aldosterone and suppressed renin [Citation8,Citation9]. The current definitive (curative) approach to unilateral PA is surgical adrenalectomy. Bilateral disease is not currently definitively managed but rather treated pharmacologically using mineralocorticoid receptor antagonists (MRA) [Citation7]. Adrenalectomy offers the best option for complete cure, while pharmacological management effectively normalizes BP but improves cardiovascular outcomes only when renin is successfully de-suppressed [Citation2,Citation4,Citation10]. Pharmacological therapy is often also poorly tolerated due to off target anti-androgen effects of MRAs [Citation7].
Currently, collateral resection of normal cortex limits the option of surgery to 30% of individuals with unilateral disease, given the inevitable risk for adrenocortical insufficiency with bilateral adrenalectomy [Citation6,Citation11]. Therefore, there exists a need for improved definitive options for management of bilateral and unilateral PA which selectively eliminates pathological aldosterone secretion, while simultaneously preserving normal ipsilateral adrenocortical function. In this study, we aimed to investigate the feasibility and efficacy of localized, subtotal, cortical-sparing microwave thermal ablation (MTA) as a potential curative option for PA.
Image-guided thermal adrenal ablation presents a plausible approach to definitively manage PA. MTA offers the potential to minimally invasively and precisely target APA/APCC while simultaneously sparing adjacent normal adrenal cortex. This presents the additional potential for managing bilateral disease without inducing post-procedural adrenal insufficiency [Citation12–14]. Current radiofrequency ablation (RFA) electrodes are optimized for large volume ablation of tumors in vascular organs, such as the liver, and thus, were not well suited to localized ablation of small (∼1 cm3) targets in the adrenal gland [Citation13]. Hence, we used MTA as our preferred ablation modality.
Our previous computer-simulated and benchtop studies in ex-vivo tissue tested the feasibility of localizing thermal damage to ∼1 cm3 volumes using a side firing MTA probe design [Citation15]. We demonstrated using these models that MTA can precisely ablate adrenal volumes equivalent in size and shape to APAs while preserving adjacent normal cortex. In this study, we use this approach in a preclinical animal model and demonstrate that MTA is a safe and efficacious modality for selective cortical-sparing adrenal ablation.
Methodology
Study outline
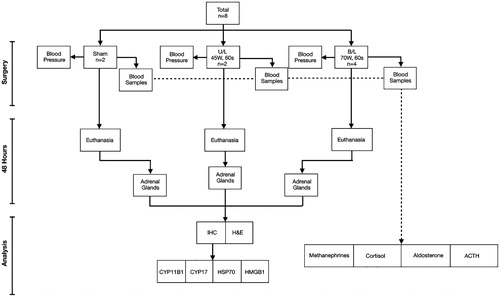
The study outline is illustrated in .
Ablation probe
All ablations were performed with a custom 13 G (2.4 mm O.D.), 2.45 GHz water-cooled microwave applicator, with radiation confined to approximately one half of the angular expanse (i.e., 180°). The applicator incorporates a coaxial antenna augmented with a hemicylindrical reflector, which restricts radiation to the preferred direction. Circulating chilled water through the applicator provides cooling of the coaxial cable, thereby mitigating detrimental heating along the applicator length, and provides a suitable impedance match to the feeding transmission line. The applicator employed is detailed in Sebek et al. [Citation16]. This is a customized applicator design under development in our laboratory and is not available for clinical use.
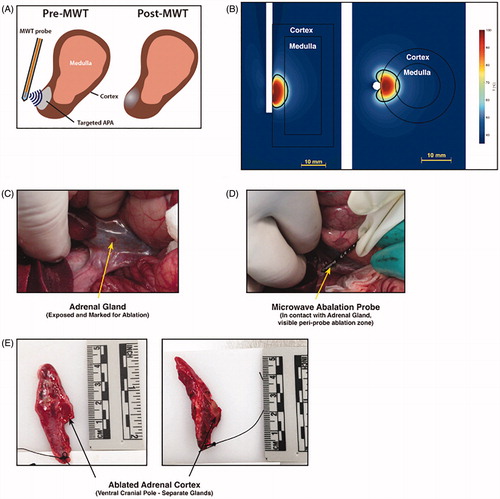
shows a bioheat transfer simulation to model microwave tissue heating of adrenal tissue, similar to the model described in Fallahi et al. [Citation15]. The directional microwave ablation device was positioned immediately adjacent to the adrenal cortex. Simulated applied power corresponded to 70 W (at the generator) applied for 60 s. These simulations indicate ablative damage extending ∼20 mm along the length of the applicator, and ∼8 mm radially into the adrenal gland from the surface of the applicator. This yields ablation of the 5-mm-thick adrenal cortex, as well as 1.35 mm of the adrenal medulla (.
Figure 2. (A) Schema for delivering microwave thermal therapy (MWT) to a benign adenoma in the adrenal target. Micro-wave power is delivered with a side-firing applicator positioned adjacent to the cortex. (B) Temperature profile and estimate of the ablation zone extent for the side-firing ablation probe. (C) Under open surgery in a porcine model, the adrenal gland was exposed by displacing the bowel. (D) The 2.45 GHz side-firing microwave applicator was positioned adjacent to the adrenal gland by direct visualization, and power delivered for 60 s to the cranial pole. (E) Adrenal glands were excised 48 h post-ablation; the region where thermal damage was delivered was identified by visible tissue discoloration, and sections were taken for histopathologic analysis.
Animal procedures
Animal studies were performed at Kansas State University (KSU) according to approved protocol (KSU Institutional Animal Care and Use Committee). Eight male castrated domestic swine, median weight 43.7 kg (40.2–45.7 kg) were used. Animals were fed liquid diet for 48 h pre-surgically to facilitate preparatory colonic emptying and access to the adrenal glands.
Animals were anesthetized with intramuscular (im) Telazol (4.4 mg/kg) and xylazine (2.2 mg/kg) and maintained on isoflourane (1–3%) for the duration of procedures [median = 75 min; (64–94 min)]. Intramuscular atropine (0.05 mg/kg) was given prior to intubation. Pigs were positioned in dorsal recumbency. Ventral midline celiotomy was performed and the adrenal glands located. The applicator probe was placed laterally, in direct contact with the ventral surface of the cranial pole of each adrenal for all procedures. Sham ablation procedures were carried out on two animals. Ablation on one gland only was performed on two animals and bilateral adrenal ablation was carried out on four animals ().
Continuous arterial BP monitoring was performed during surgery (Datex Ohmeda S-4 monitor and DTXBP transducer). Blood samples (∼20–30 ml) were drawn from the left jugular vein before, during and after each procedure. Lactated Ringer’s solution was administered (10 ml/kg/h) during surgery. Buprenorphine (0.005–0.1 mg/kg) and flunixin meglumine (1–2.2 mg/kg) analgesia was administered. Animals were recovered and survived for 48 h prior to euthanasia [iv pentobarbital (390 mg/ml), 65–100 mg/kg].
Light microscopy
Sections were cut (5 μm) from paraffin embedded adrenals and dried onto slides. After de-paraffination, antigen retrieval was performed using citrate (pH6; intracellular markers) or ethylenediaminetetraacetic acid (EDTA) buffer (pH8; cell-surface markers) in a pressure cooker at 110 °C for 15 min. Endogenous peroxidases were inactivated with 0.3% H2O2 in PBS for 30 min.
Hematoxylin and eosin (H&E) staining was performed to evaluate gland morphology. Sections were stained with hematoxylin for 30 s (Sigma Aldrich, Wicklow, Ireland) and stained with Eosin Y (Sigma Aldrich) for 2 min. All slides were scanned and imaged on an Olympus® virtual slide scanner (40×).
Immunohistochemistry
Monoclonal antibodies directed against the following markers were used: (i) for adrenocortical function the steroidogenic enzymes CYP17 and CYP11B1; (ii) for thermal-induced damage of non-ablated tissue HSP-70 and HMGB-1. Nonspecific antibody binding was ‘blocked’ using 2.5% horse (CYP17, HSP-70, HMGB-1) or goat serum (CYP11B1) (Vector Laboratories, Peterborough, UK) for 1 h.
Individual sections were incubated overnight at 4 °C with monoclonal antibodies: CYP17 (1/800), CYP11B1 (1/100); HSP-70 (1/200, Abcam, Cambridge, UK); HMGB1 (1/50, Abcam). Antibodies directed against steroidogenic enzymes were kindly donated by Professor Celso Gomez Sanchez, University of Mississippi [Citation17].
Each slide was then incubated with HRP-conjugated secondary antibody (Vector Laboratories) for 1 h. Slides were visualized using 3,3′-diaminobenzidine (DAB). Sections were counterstained with hematoxylin for 30 s before mounting.
Biochemistry
Cortisol and adrenocorticotropic hormone (ACTH)
Cortisol was measured using a competitive electrochemiluminescence immunoassay (ECLIA) on the Roche Diagnostics Cortisol II (Roche Cobas® EEE); reportable range 0.5–1750 nmol/L; between-run analytical precision at 112 nmol/L, 485 nmol/L and 1080 nmol/L is 3.4%, 2.5% and 3.2%, respectively. ACTH was measured on the Roche Cobas® e601 analyzer using a sandwich ECLIA. The assay measuring range is 1.0–2000 ng/L with inter-assay imprecision of less than 6% at a mean ACTH of 3.5 ng/L and 1450 ng/L, respectively.
Plasma metanephrine and normetanephrine
Off-line solid phase extraction was used to isolate metanephrines from 300-μL of EDTA plasma, followed by rapid separation with hydrophilic interaction chromatography using Waters® Acquity™ ultra performance liquid chromatography (Waters, Hertfordshire, UK). Mass spectrometry detection was performed in multiple-reaction monitoring mode using a Waters® Xevo TQ tandem quadrupole mass spectrometer with positive electrospray ionization. Lower limit of quantitation for MN is 38 pmol/L and 80 pmol/L. The linearity of the method is 30 000 pmol/L for all three analytes. Inter-assay imprecision is 12% at mean MN, NMN and 3MT concentrations of 200 pmol/L, 1000 pmol/L and 3000 pmol/, respectively.
Plasma aldosterone
Solid phase extraction was used to isolate aldosterone from 150-μL of EDTA plasma, followed by high-performance liquid chromatograph separation using the Shimadzu® system. Mass spectrometry detection was performed in MRM mode on the AB Sciex® (API5500) tandem quadrupole mass spectrometer with atmospheric pressure chemical ionization. The assay measuring range is 65–24000 pmol/L. Inter-assay imprecision is 7.4%, 6.1% and 5.6% at mean aldosterone concentrations of 369 pmol/L, 855 pmol/L and 1780 pmol/L, respectively.
Data analysis
H&E stained adrenal samples were evaluated by a single, blinded pathologist. Densitometry was carried out to evaluate DAB staining for immunohistochemistry using ImageJ® software. Hypothesis testing for normally distributed data was performed using the analysis of variance (ANOVA) for multiple group comparisons and Student’s t-test for two-group comparisons, where appropriate.
Results
Animal characteristics
Eight pigs (44.29 kg ± 2.14) underwent selective adrenal MTA as follows: two underwent sham procedures, two had MTA applied at a dose of 45 W for 60 s (hereafter referred to as the low MTA dose) and four had MTA applied to both adrenal glands at a dose of 70 W for 60 s (referred to as the high MTA dose) ().
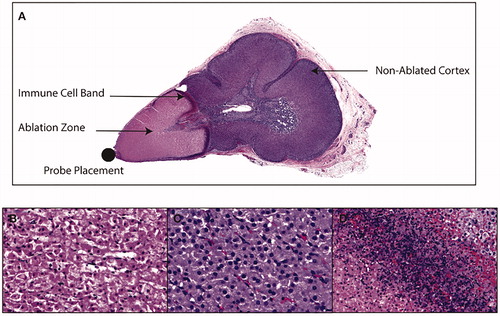
Selected, subtotal adrenal MTA selectively ablated adrenocortical tissue within selected zones while preserving normal tissue architecture outside of the ablation zone. H&E stained sections were compared across sham (n = 2), low MTA dose (n = 2) and the high MTA dose animals (n = 4). Visible evidence of adrenal ablation was apparent during ablation procedures for the high MTA dose (. Adrenals harvested at 48 h post-MTA demonstrated evidence of gross damage at the cranial adrenal pole () and histological evidence of coagulative necrosis adjacent to probe placement for the high MTA dose, indicating successful ablation (). A mean ablation volume 0.83 cm3 ± 0.07 was demonstrated for adrenal glands receiving the high MTA dose (). Non-ablated cortex, distal to probe placement demonstrated normal adrenocortical morphology (). Sham or lower MTA dose animals did not demonstrate tissue damage or coagulative necrosis at 48 h. Ablated and non-ablated adrenal cortex was clearly separated by a delineating immune infiltrate (. Therefore, there was clear evidence of both tissue destruction and tissue preservation, respectively, within the ablated and non-ablated zones. We next evaluated markers of adrenal function and steroidogenesis within these adrenocortical regions.
Figure 3. (A) A cross-section of ablated adrenal gland, stained with H&E, demonstrating subtotal ablation of the adrenal gland, with cortical sparing and a delineating transitional zone containing an immune cell infiltrate. (B) Typical features of coagulative necrosis in the adrenal cortex within the so-called ablation zone adjacent to microwave applicator probe placement. (C) Non-targeted adrenal cortex distal to probe placement, which contains zona fasciculata cells of normal morphology, and which are morphologically identical to sham adrenal cortex. The transitional zone between ablated and non-ablated tissue was characterized by an immune infiltrate (D).
Adrenal ablation reduced steroidogenic enzyme expression within the selected zone. Immunohistochemical evidence of steroidogenic enzyme expression was used to evaluate adrenocortical function [Citation17,Citation18]. The two chosen steroidogenic enzymes for evaluation, CYP17 and CYP11B1, respectively, modulate early steroidogenesis and cortisol production, and are both highly expressed within normal adrenal cortex. Normal expression of CYP17 and CYP11B1 indicates normal adrenocortical function, whereas the converse indicates functional disruption [Citation17]. An important consideration for this study was to develop a therapeutic approach to disrupt steroidogenesis in the area selected for ablation and to preserve cortisol steroidogenesis in non-ablated adrenal cortex, thereby avoiding post-procedural adrenocortical insufficiency.
CYP17 expression was present, across all adrenocortical zones within non-ablated and sham adrenal cortex. Normal cytoplasmic expression of CYP17 was significantly reduced in the ablation zone of high MTA dose adrenals (n = 4) as compared to non-ablated and sham cortex (). Similarly, CYP11B1 expression differed in pattern and intensity between ablated and non-ablated adrenal cortex (n = 4) (). Cytoplasmic expression of CYP11B1 expression was also reduced in ablated compared with non-ablated cortex (. As expected, neither steroidogenic enzyme was expressed in adrenal medulla () or in negative control tissue (Figures S2 and S3 in Supplementary Material).
Figure 4. (A) Immunohistochemistry images of expression of the steroidogenic enzyme CYP17 in adrenal gland exposed to high MTA dose. There is an absence of normal cytoplasmic expression of CYP17 in ablated [Ab] tissue as compared to non-ablated tissue [N], and this was sharply delineated by the transitional zone [Tr]. (B) Staining density for CYP17 was markedly reduced within the ablation zone of tissue exposed to high MTA dose (70 W damaged) as compared to sham, low MTA dose (45 W) and non-ablated tissue (70 W undamaged). (C) Immunohistochemistry images of expression of the steroidogenic enzyme CYP11B1 similarly showed disruption of normal cytoplasmic expression in [Ab] tissue as compared to non-ablated tissue [N], and this was also sharply delineated by the transitional zone [Tr]. (D) Staining density tor CYP11B1 was also reduced within the ablation zone of tissue exposed to high MTA dose (70 W damaged) as compared to sham. Low MTA dose (45 W) and non-ablated tissue (70 W undamaged). The immunohistochemical findings are consistent with blood biochemistry which demonstrated appropriate levels of aldosterone (E), cortisol (F) and stimulatory ACTH (G) at 48 h post-operatively. Staining density expressed as percentage (%); ACTH, adrenocorticotrophin; *p < .01 compared with damaged tissue (ANOVA with Tukey's post-hoc test).
![Figure 4. (A) Immunohistochemistry images of expression of the steroidogenic enzyme CYP17 in adrenal gland exposed to high MTA dose. There is an absence of normal cytoplasmic expression of CYP17 in ablated [Ab] tissue as compared to non-ablated tissue [N], and this was sharply delineated by the transitional zone [Tr]. (B) Staining density for CYP17 was markedly reduced within the ablation zone of tissue exposed to high MTA dose (70 W damaged) as compared to sham, low MTA dose (45 W) and non-ablated tissue (70 W undamaged). (C) Immunohistochemistry images of expression of the steroidogenic enzyme CYP11B1 similarly showed disruption of normal cytoplasmic expression in [Ab] tissue as compared to non-ablated tissue [N], and this was also sharply delineated by the transitional zone [Tr]. (D) Staining density tor CYP11B1 was also reduced within the ablation zone of tissue exposed to high MTA dose (70 W damaged) as compared to sham. Low MTA dose (45 W) and non-ablated tissue (70 W undamaged). The immunohistochemical findings are consistent with blood biochemistry which demonstrated appropriate levels of aldosterone (E), cortisol (F) and stimulatory ACTH (G) at 48 h post-operatively. Staining density expressed as percentage (%); ACTH, adrenocorticotrophin; *p < .01 compared with damaged tissue (ANOVA with Tukey's post-hoc test).](/cms/asset/5fa7a075-5bc7-4202-bd0a-45d95c674660/ihyt_a_1650205_f0004_c.jpg)
These findings are consistent with disrupted steroidogenesis within the ablated zone and preserved adrenal function in the adrenal cortex which did not undergo ablation.
Measurement of circulating steroid hormones demonstrated that the animals remained adrenocortically sufficient post-procedurally following bilateral ablation. This was in agreement with immunohistochemical findings. Specifically, aldosterone release was low pre-operatively and during the procedure. This reflected a pre-operative high-salt diet. At 48 h, high aldosterone levels reflected appropriate physiological response to low postoperative salt intake (. Cortisol levels rose appropriately in response to intra-operative stress and returned to baseline postoperatively (. These correlated appropriately with ACTH (.
In tandem, these results have demonstrated a successful therapeutic outcome in animals undergoing bilateral ablation procedures whereby (i) selective disruption of steroidogenesis was achieved within the selected ablation zone, and (ii) normal adrenocortical function was preserved within the non-ablated adrenal, sufficient to maintain clinical adrenocortical sufficiency.
Markers of tissue damage were not upregulated in non-ablated tissue at 48 h post-adrenocortical MTA. We next evaluated the expression of HSP 70 and HMGB1 each of which acts as a damage-associated molecular pattern (DAMP) [Citation19,Citation20]. HSP 70 is a molecular chaperone, protecting against the unfolded protein response and constitutively expressed in normal adrenal, where it responds to ACTH stimulation. HSP-70 is upregulated in the acute response to heat, but is secreted as a humoral factor with prolonged heat exposure. Its expression is absent from necrotic tissue [Citation19,Citation20]. HMGB-1 is a conserved nuclear protein which regulates transcription. In response to cellular damage, HMGB-1 migrates to cytoplasm and acts as a DAMP, where it regulates autophagy and apoptosis. In response to necrosis, HMGB-1 is released extracellularly and acts as a pro-inflammatory cytokine. Expression patterns of HMGB-1 therefore also serve as an indicator of cellular damage [Citation21].
We investigated the expression of both markers in tissue which had not been selected for ablation to determine whether or not molecular damage had occurred outside of the ablation zone (i.e., damage not apparent from gross or basic histological inspection).
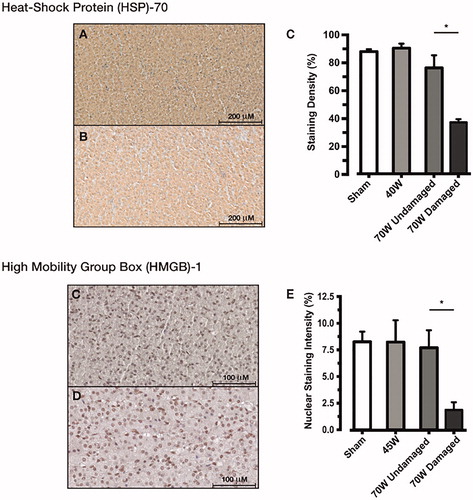
At 48 h postoperatively, there was no difference between non-ablated adrenal cortex (), exposed to the high MTA dose as compared to low MTA dose or sham adrenocortical tissue () indicating that non-ablated tissue remained undamaged at this timepoint. Expression of HSP-70 was downregulated in the necrotic, ablated tissue at 48 h as expected. Similarly, the pattern and density of HMGB-1 expression was no different in non-ablated to sham adrenal (). Importantly, the expression of HMGB-1 was normal within non-ablated tissue up to the ablation margin indicating no residual damage of non-ablated tissue at the 48 h timepoint (. Immunohistochemistry images for HSP70 and HMGB1 within the ablation zones are demonstrated in Figures 4 and 5 in Supplementary Material, respectively.
Figure 5. HSP-70 expression did not differ between non-ablated adrenal cortex exposed to the high MTA dose (A) and sham adrenal cortex (B) at 48 h. A significantly reduced expression was found in ablated cortex as compared to non-ablated, sham and low MTA dose exposed cortex (C). HMGB-1 demonstrated normal nuclear expression, and no difference between non-ablated adrenal cortex was exposed to the high MTA dose (D) and sham adrenal cortex (E). This was confirmed by densitometry measurements of nuclear expression of HMGB-1 (F). Staining density expressed as percentage (%); HSP, heat shock protein; HMGB, high mobility group box. *p < .01 compared with damaged tissue (ANOVA with Tukey's post-hoc test).
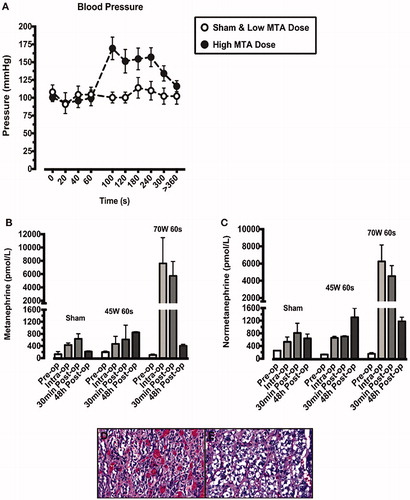
Selective adrenocortical ablation induced an unsustained BP rise with associated transiently with increased circulating metanephrines, consistent with minimal collateral medullary damage. There was an acute BP increase following 60 s of the high MTA dose, lasting 5 min (. The acute BP rise was accompanied by a significant increase in circulating plasma metanephrine () and normetanephrine () in animals exposed to the high MTA dose. This was not observed for low MTA dose exposed or sham animals (). H&E staining of adrenal medulla demonstrated a small area of coagulative necrosis adjacent to ablated adrenal cortex (), with preserved medulla adjacent to non-ablated cortex ().
Figure 6. Blood pressure readings were taken throughout each ablation procedure (A). Animals exposed to the higher microwave thermal ablation (MTA) dose (70W for 60s) are represented by closed circles. Sham animals and those exposed to the lower MTA dose (45W for 60s) are represented by open circles. The blood pressure rise demonstrated at 60s in the high MTA dose animals was accompanied by a rise in both metanephrine (B) and normetanephrine (C), indicative of adrenal medullary damage. This was confirmed by H&E staining which demonstrated coagulative necrosis and haemorrhage in adrenal medulla, adjacent to ablated cortex (D). Distal medulla, adjacent to non-targeted cortex retained normal morphology (E).
Discussion
We took a novel approach of selected subtotal adrenal ablation in a preclinical model which (i) ablated the intended tissue volume within the desired zone to disrupt steroidogenesis but which (ii) preserved adjacent tissue sufficient to maintain normal post-procedural adrenocortical function. Our approach was conceived and modeled to address the clinical challenge of curative management of PA, particularly in the context of bilateral disease, for which no feasible definitive therapy currently exists [Citation6]. We achieved our objective of tissue sparing, bilateral adrenal ablation using a directional, side-firing ablation probe, which was placed adjacent to the area of desired destruction on both adrenal glands, specifically taking care to avoid glandular penetration.
Our findings demonstrate several clinically important outcomes. Firstly, we provide histological evidence of limited adrenocortical damage, which nonetheless achieved the desired ablation volume (0.8–1 cm3). Within the ablation zone, evidence of coagulative necrosis is accompanied by a loss of steroidogenic enzyme expression. Therefore, physical and functional adrenocortical tissue disruption of similar volume to pathological aldosterone producing lesions (APA/APCC) was achieved [Citation9].
It is an important clinical consideration when treating benign functioning endocrine lesions to preserve as much normal tissue adjacent to the pathological lesion as possible, in order to avoid post-therapy hormonal deficiency [Citation6,Citation22,Citation23]. The majority of systemic endocrinopathies including hyperparathyroidism, Cushing’s syndrome, etc., are driven by small benign, hyperfunctioning lesions located within an otherwise normal gland. Margins are not necessary when removing these lesions, yet current surgical and ablative approaches usually make selective destruction of the pathological lesion impossible without compromising the adjacent normal tissue [Citation10,Citation22]. In this study, we have specifically addressed this challenge within the adrenal gland.
We provide clinical, biochemical and histological evidence that we preserved normal adrenocortical tissue adjacent to the desired ablation zone. Importantly, we demonstrate that animals undergoing bilateral ablation had normal circulating adrenal hormones and normal clinical parameters of adrenal function up to euthanasia at 48 h following ablation. The clinical relevance of this finding is highly significant as it demonstrates the feasibility and safety bilateral MTA as a potential approach to definitive therapy for PA underpinned by bilateral disease. This is in fact the first time that bilateral subtotal destruction of adrenocortical tissue has been demonstrated without incurring long-term adrenocortical insufficiency as an unintended consequence of therapy.
Our probe design and choice of MTA was specifically chosen to address the challenges and limitations of prior clinical studies which have used adrenal RFA to treat benign functioning adenomas [Citation13]. Previous investigators ablated visible APAs by applying pulsed RFA, applied over 15–20 min, at doses sufficient to induce global adrenal necrosis without specific regard for preserving normally functioning tissue [Citation13,Citation24–26]. When designing our approach to adrenal ablation, we attributed equal importance to preservation of tissue which was not selected for ablation, in addition to subtotal destruction of selected tissue. Our MTA system was evaluated in simulated models and a side-firing applicator probe was chosen to provide a lateral non-penetrative approach, positioning the probe between the adrenal and the peri-adrenal fat [Citation15,Citation16,Citation27]. The focal spot size for the selected power/time combinations was well matched to the target and microwave energy was delivered at low doses (45 W and 70 W) for a shorter time period (60 s vs. 15 min) than described in RFA studies [Citation12–14]. The choice of a non-penetrative approach was important to avoid the risk of adrenal hemorrhage, given the fragility of the adrenal gland [Citation28]. Additionally, placing the probe on the surface of the gland, rather than penetrating the gland, facilitated preferential ablation of the subcapsular cortex, while minimizing damage to the medulla, deeper within the gland.
In line with our intended therapeutic design, we achieved minimal adrenal medullary damage. Moderate intraprocedural hypertension resolved quickly and was accompanied only by a transient increase in circulating metanephrines. There was also accompanied by minimal histological evidence of medullary damage (<5% of total medullary volume). These findings are encouraging, but nonetheless precaution the need for periprocedural, preparatory alpha adrenoreceptor blockade when undertaking adrenal ablation. Clinical studies of adrenal RFA in humans which did not take an adrenocortical sparing approach have been complicated by intra-procedural hypertensive crises in up to 67% of recipients consistent with widespread medullary damage [Citation12,Citation13,Citation29].
The implications of this study for future therapy of PA are significant. There is a need to improve the therapeutic approach to definitive management of PA. PA represents a common endocrinopathy and is recognized as the most common secondary and potentially reversible cause of hypertension [Citation1]. Greater understanding of the pathogenesis of PA as well as refined screening approaches have increased the diagnostic rate for this condition in recent years. Yet, surgical adrenalectomy is the only definitive option for unilateral disease and non-curative pharmacological therapy with MRAs remains the preferred option for the 60% of individuals with bilateral disease [Citation6,Citation10]. There is therefore clear purpose to shift the current treatment paradigm toward delivering curative therapy to the increasing number of individuals diagnosed with bilateral PA, as well as providing less invasive therapeutic approaches for unilateral disease.
Recent advances in molecular diagnostic imaging allow us to localize specific areas of aldosterone hypersecretion within the adrenal gland. The landscape is therefore ripe to develop more refined therapies to specifically target regional areas of aldosterone hypersecretion within the adrenal cortex and deliver a minimally invasive approach to cure [Citation30–32]. Our study clearly demonstrates in a preclinical context, the feasibility of using subtotal, tissue-sparing, targeted adrenocortical ablation as a therapeutic approach. We acknowledge that we have taken a direct surgically visualized approach to probe placement in this study. However, we will now advance our current technology to minimally invasive, image-guided probe placement which is informed by preclinical diagnostic molecular imaging.
A preclinical, swine model was chosen to reflect mean human adrenal volumes. It is noteworthy that we chose regional subtotal ablation of normal adrenal cortex rather than pathological nodules. There is no current animal model of benign adrenocortical adenomas of sufficient size to investigate the effects of MTA. Preparatory dielectric measurements validated our approach. APAs are homogenous, benign lesions, histologically similar to normal adrenal cortex and with dielectric properties that are no different to normal cortex [Citation33]. We harvested the adrenals at 48 h post-ablation for two reasons. Firstly, this timepoint reliably allows coagulative necrosis to manifest. Secondly, this timepoint allowed a reasonable period following acute insult for clinical and biochemical evaluation of adrenocortical function in animals undergoing bilateral ablation.
In conclusion, we present a set of experiments which demonstrate MTA as a suitable methodology for delivering targeted subtotal cortical-sparing bilateral adrenal ablation. We point toward the safety of MTA for potential definitive management of PA, in the setting of both unilateral and bilateral disease. We now propose translating developing our model as a minimally invasive therapeutic modality and translating it to a form suitable for future human use.
Supplemental Material
Download TIFF Image (37.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820.
- Hundemer GL, Curhan GC, Yozamp N, et al. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59.
- Hundemer GL, Curhan GC, Yozamp N, et al. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–774.
- Hundemer GL, Curhan GC, Yozamp N, et al. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658.
- Reincke M, Beuschlein F, Bidlingmaier M, et al. Progress in primary aldosteronism. Horm Metab Res. 2010;42:371–373.
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916.
- Mulatero P, Monticone S, Burrello J, et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34:2253–2257.
- Boulkroun S, Golib Dzib JF, Samson-Couterie B, et al. KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling. Mol Cell Endocrinol. 2013;371:221–227.
- Omata K, Anand SK, Hovelson DH, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1:787–799.
- Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699.
- Balla A, Ortenzi M, Palmieri L, et al. Laparoscopic bilateral anterior transperitoneal adrenalectomy: 24 years experience. Surg Endosc. 2019;33:1–351.
- Hasegawa T, Yamakado K, Nakatsuka A, et al. Unresectable adrenal metastases: clinical outcomes of radiofrequency ablation. Radiology. 2015;277:584–593.
- Sarwar A, Brook OR, Vaidya A, et al. Clinical outcomes following percutaneous radiofrequency ablation of unilateral aldosterone-producing adenoma: comparison with adrenalectomy. J Vasc Interv Radiol. 2016;27:961–967.
- Yang MH, Tyan YS, Huang YH, et al. Comparison of radiofrequency ablation versus laparoscopic adrenalectomy for benign aldosterone-producing adenoma. Radiol Med. 2016;121:811–819.
- Fallahi H, Clausing D, Shahzad A, et al. Microwave antennas for thermal ablation of benign adrenal adenomas. Biomed Phys Eng Express. 2019;5:025044.
- Sebek J, Curto S, Bortel R, et al. Analysis of minimally invasive directional antennas for microwave tissue ablation. Int J Hyperthermia. 2017;33:51–60.
- Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117.
- Meyer LS, Wang X, Susnik E, et al. Immunohistopathology and steroid profiles associated with biochemical outcomes after adrenalectomy for unilateral primary aldosteronism. Hypertension. 2018;72:650.
- Blake MJ, Udelsman R, Feulner GJ, et al. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci USA. 1991;88:9873–9877.
- Udelsman R, Blake MJ, Stagg CA, et al. Endocrine control of stress-induced heat shock protein 70 expression in vivo. Surgery. 1994;115:611–616.
- Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388.
- Singh Ospina N, Thompson GB, Lee RA, et al. Safety and efficacy of percutaneous parathyroid ethanol ablation in patients with recurrent primary hyperparathyroidism and multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2015;100:E87–90.
- Morhard R, Nief C, Barrero Castedo C, et al. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci Rep. 2017;7:8750.
- Liu SY, Ng EK, Lee PS, et al. Radiofrequency ablation for benign aldosterone-producing adenoma: a scarless technique to an old disease. Ann Surg. 2010;252:1058–1064.
- Liu SY, Chu CC, Tsui TK, et al. Aldosterone-producing adenoma in primary aldosteronism: CT-guided radiofrequency ablation-long-term results and recurrence rate. Radiology. 2016;281:625–634.
- Liu SY, Chu CM, Kong AP, et al. Radiofrequency ablation compared with laparoscopic adrenalectomy for aldosterone-producing adenoma. Br J Surg. 2016;103:1476–1486.
- Sebek J, Albin N, Bortel R, et al. Sensitivity of microwave ablation models to tissue biophysical properties: a first step toward probabilistic modeling and treatment planning. Med Phys. 2016;43:2649
- Monticone S, Satoh F, Dietz AS, et al. Clinical management and outcomes of adrenal hemorrhage following adrenal vein sampling in primary aldosteronism. Hypertension. 2016;67:146–152.
- Espinosa De Ycaza AE, Welch TL, Ospina NS, et al. Image-guided thermal ablation of adrenal metastases: hemodynamic and endocrine outcomes. Endocr Pract. 2017;23:132–140.
- Burton TJ, Mackenzie IS, Balan K, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn's adenomas. J Clin Endocrinol Metab. 2012;97:100–109.
- Ouyang J, Hardy R, Brown M, et al. (11)C-metomidate PET-CT scanning can identify aldosterone-producing adenomas after unsuccessful lateralisation with CT/MRI and adrenal venous sampling. J Hum Hypertens. 2017;31:483–484.
- O'Shea PM, O'Donoghue D, Bashari W, et al. (11) C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clin Endocrinol. 2019;90:670–679.
- Gioco F, Seccia TM, Gomez-Sanchez EP, et al. Adrenal histopathology in primary aldosteronism: Is it time for a change? Hypertension. 2015;66:724–730.