Abstract
Background: Tertiary hyperparathyroidism (THPT) is very common in hemodialysis patients with secondary hyperparathyroidism. However, a medical treatment is not indicated for THPT.
Purpose: To investigate the feasibility, safety and efficacy of microwave ablation (MWA) in treating THPT.
Materials and methods: Twenty-three patients with THPT were enrolled and treated with MWA. Clinical characteristics, serum levels of intact parathyroid hormone (iPTH), calcium, phosphorus and alkaline phosphatase (ALP), as well as treatment outcomes, were evaluated pre- and post-MWA. All patients were followed for >36 months for all assessable clinical data.
Results: All patients successfully completed MWA. The mean follow-up was 47.0 ± 8.4 months. Immediately 1-day post-MWA, iPTH, calcium, phosphorus and ALP levels significantly decreased (all p < 0.001). During the long-term follow-up, iPTH levels increased gradually until 24 months and then remained at stable levels. After MWA, serum calcium reached stable levels at 24 months, while phosphorus and ALP reached stable levels at 6 months, and the levels were in the normal range or slightly higher than the upper normal limit. No obvious blood flow signals or significant recurrence was observed in the surgical nodules during follow-up. In the last follow-up, all nodules were persistent, but their maximum diameter and average volume were significantly lower after MWA (both p < 0.001), with an average reduction of 75.9 ± 11.3%. All patients had no major complications during MWA and follow-up.
Conclusions: MWA is feasible, safe, effective and minimally invasive in treating THPT. Thus, MWA can be a nonsurgical alternative for treating THPT patients who are ineligible for surgery.
Introduction
Secondary hyperparathyroidism (SHPT) is the commonest and serious complication of chronic renal failure and the most frequently encountered problem in the treatment of patients with end-stage renal disease (ESRD). SHPT is characterized by persistently elevated serum parathyroid hormone (PTH) levels, parathyroid gland hyperplasia and disturbances in the metabolism of minerals such as calcium and phosphorus [Citation1–3]. In contrast, tertiary hyperparathyroidism (THPT) is most commonly observed in patients with SHPT who have been on hemodialysis for years or after kidney transplantation and is characterized by excessive PTH secretion after a long-standing SHPT [Citation4–6]. The clinical manifestations of THPT are attributed to the high PTH levels, hypercalcemia and hyperphosphatemia and include symptoms of bone pain, decreased bone mineral density, fractures, pruritus, nephrolithiasis, peptic ulcer disease, pancreatitis, soft tissue or vascular calcifications, muscle weakness and mental status changes [Citation1,Citation2,Citation4,Citation7]. Such manifestations significantly affect the social and economic burden and quality of life of patients with THPT [Citation8].
Patients with SHPT are predominantly and sufficiently managed by medications that reduce PTH, such as vitamin D analogs and/or the calcimimetic cinacalcet, and those that reduce circulating levels of phosphorus, such as oral binders [Citation2,Citation9,Citation10]. In patients with THPT, however, medical treatment is generally not indicated and not curative, and the mainstay of treatment for these patients is surgery [Citation6,Citation10,Citation11]. Although a successful parathyroidectomy in THPT patients can lead to a significant reduction in PTH and serum calcium levels and therefore improved clinical symptoms and survival, the procedure has several disadvantages [Citation6,Citation7]. First, invasive parathyroidectomy for both SHPT and THPT is associated with significantly higher morbidity and mortality rates [Citation12]. Second, for THPT, there are no consistent evidence-based guidelines and criteria for selecting approaches to parathyroidectomy, which include subtotal, total and total with autotransplantation [Citation6,Citation13–15]. Lastly, the patients may be ineligible for surgery or unwilling to undergo surgery. Thus, the quest for effective and safe alternatives for the treatment of THPT is crucial.
In recent years, various percutaneous ablation techniques have been developed to treat patients with hyperparathyroidism, such as ethanol or acetic acid injection [Citation16–18], laser ablation [Citation19], high-intensity focused ultrasound treatment [Citation20], radiofrequency ablation [Citation21] and microwave ablation (MWA) [Citation22]. More recently, studies have reported MWA as a successful effective nonsurgical alternative for treating SHPT [Citation23,Citation24]. However, these studies lacked long-term follow-up, and there has been no report on the use of MWA to treat THPT. Therefore, this study aimed to investigate the feasibility, safety, efficacy and complications of ultrasound-guided MWA in treating patients with THPT and the impact of the procedure on the patients’ quality of life in the long term.
Materials and methods
Patient population
This retrospective pilot study protocol was approved by the Ethics Committee of Huangshi Central Hospital (Number: 2012-CSK-011), which is certificated and in compliance with Joint Commission International accreditation, and written informed consent was obtained from every patient. Among 26 ESRD patients with THPT who underwent MWA in Huangshi Central Hospital between January 2013 and July 2015, 23 patients with >3 years of follow-up were enrolled in this study. The other three patients were excluded as one died from metastatic lung cancer 13 months post-MWA (female, age 54 years), one died from respiratory failure due to uncontrolled influenza infection 5-month post-MWA (male, age 67 years), and one died from septic shock due to abdominal cavity infection resulting from change to peritoneal dialysis 16-month post-MWA.
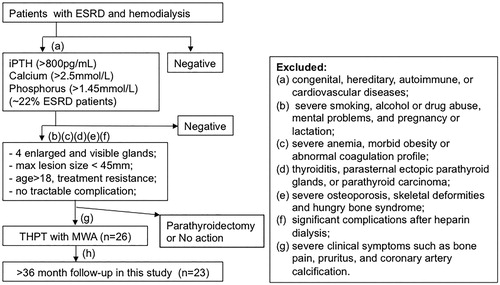
Patients were diagnosed as THPT based on their clinical and laboratory investigation as described previously [Citation4,Citation6]. The inclusion and exclusion criteria are shown in . All included patients for this study must meet all inclusion and exclusion. The inclusion criteria were (a) diagnosis of THPT with intact parathyroid hormone (iPTH) levels >800 pg/mL, serum calcium levels >2.5 mmol/L (10 mg/dL) and serum phosphorus levels >1.45 mmol/L (4.5 mg/dL) and significant disturbances in mineral metabolism; (b) all four parathyroid glands on both sides of the neck were enlarged and clearly visible on ultrasound; (c) the maximum lesion diameter was less than 45 mm based on ultrasound-guided fine-needle aspiration biopsy; (d) age larger than 18 years and having good compliance with systematic treatment and observational study; (e) resistance to current medication; (f) no intractable complication such as cardiac insufficiency or hypertension that could not be controlled with drugs; (g) ineligibility for surgery or unwillingness to undergo surgery and (h) the last follow-up after MWA being >3 years. Exclusion criteria were (a) congenital, hereditary, autoimmune, or cardiovascular disease; (b) severe smoking, alcohol abuse, history of drug abuse and mental problems, and pregnancy or lactation; (c) severe anemia, hemorrhage, morbid obesity or abnormal coagulation profile; (d) larger thyroid nodules, thyroiditis, parasternal ectopic parathyroid glands, or parathyroid carcinoma; (e) severe osteoporosis, skeletal deformities and hungry bone syndrome; (f) significant complications after heparin dialysis; and (g) severe clinical symptoms such as bone pain, pruritus and coronary artery calcification.
Figure 1. Flow chart shows patient selection criteria. iPTH: intact PTH. The indicated inclusion criteria (a)–(h) were described in the main text.
Technetium (99mTc) sestamibi imaging was used to exclude patients with ectopic parathyroid glands when the iPTH level did not decrease significantly after MWA. All patients did not undergo kidney transplantation.
Percutaneous MWA
Percutaneous MWA was performed by two authors (Z.H. and E.H.) with >10 years of experience of performing MWA for hepatocellular carcinoma, thyroid nodules and hyperplastic parathyroid nodules. The characteristics of the parathyroid glands, including the size, number, shape, echo features, marginal and internal components, and internal blood flow, and the anatomy between nodules and adjacent tissues (especially the recurrent laryngeal nerve) were evaluated using two-dimensional ultrasound, color Doppler flow imaging (CDFI), and contrast-enhanced ultrasound (CEUS) (LOGIQ E9; GE Medical Systems, Waukesha, WI, USA) before MWA. The volume of a nodule was measured by ultrasound using the formula V = π×a × b × c/6 [Citation25], where V is the volume (in cm3 or mL) and a, b and c are the maximum diameters of three perpendicular sections of the nodule (in cm). The blood supply in the nodules based on the color Doppler image was visually evaluated, and the results were grouped into three categories based on the percentage of Doppler signal area in the total nodule area, as follows: less blood supply (<25%), medium blood supply (25%–50%) and rich blood supply (>50%). Before MWA, laboratory tests were routinely performed, including serum iPTH (normal range, 15–65 pg/mL), serum calcium (normal range, 2.11–2.52 mmol/L), serum phosphorus (normal range, 0.80–1.51 mmol/L), platelet count, coagulation test, calcitonin, hepatitis B virus antibody, hepatitis C virus antibody, syphilis antibody, AIDS antibody and electrocardiogram. All patients received two doses of 60,000 units of vitamin D per day for 7 days before MWA. Serum calcium levels and kidney function were monitored after surgery to assess the need for calcium infusion and renal calcinosis. Before MWA was performed, the antecubital vein was used for intravenous access. Antibiotic treatment was administered to prevent infection or abscess [Citation26]. Although painkillers and sedatives can be used to reduce pain during MWA [Citation27], these were not recommended in our study because a high sedation status might prevent the immediate discovery of intraoperative complications.
The patients were placed in the supine position with the neck extended. After disinfection of the operation site, local anesthesia with 1% lidocaine (Wuhu Kangqi Pharmaceutical Co., Wuhu, China) was injected in subcutaneous tissue around the site, the superficial tissue in front of the neck and the thyroid capsule. Under the guidance of ultrasound, normal saline solution was injected around the hyperplastic parathyroid gland capsule using a 21-gauge percutaneous transhepatic cholangiography needle to provide heat insulation and >5 mm thickness to protect adjacent tissue such as carotid, tracheal, esophageal, and thyroid tissue and nerves. The saline injection was performed either once or intermittently to ensure a stable insulation zone during the procedure. A microwave generator and a 16-gauge internally cooled antenna (KY-2450; Kangyou Microwave Energy Sources Institute, Nanjing, China) were used for the MWA procedures. The MWA device had a microwave frequency of 2450 MHz and an output power of 1–100 W. It was equipped with coaxial cables of low energy consumption and a peristaltic pump for water cooling. The ablation power was 20–25 W for each microwave application. The needle tip was held in a quiescent state for 2.5–15 min for two nodules in each side. Under ultrasound guidance, the microwave antenna was carefully inserted into the parathyroid gland to perform the ablation in a moving-shot technique [Citation28] to prevent heat injury to surrounding critical structures. The antenna tip moved from the deepest and farthest side initially and back to the surface and nearest side of the gland. The ablation zone was evaluated through the echo changes from the position of the antenna tip. The ablation power and time and the position of the antenna tip were also adjusted based on the echo changes. For cystic and mixed nodules, ablation was performed after fluid aspiration. Special attention was paid to protecting the posterior triangles to avoid damage to the recurrent laryngeal nerve, trachea and esophagus. For parathyroid nodules with rich blood supply (>50% colored nodule area by CDFI), the antenna tip was inserted into the area with rich blood vessels to ablate the vessels until the flow signals disappeared in the nodule whose ablation power and time were adjusted. When transient hyperechoic echotexture was observed throughout the gland but there was no flow signal on CDFI, ablation was terminated and the ablation necrosis was evaluated using CEUS by an additional intravenous injection of 2.4 mL SonoVue (Bracco, Milan, Italy) and a follow-up injection of 10 mL saline solution. CEUS was used to evaluate the extent of ablation of the parathyroid gland. The ablation was terminated when there was no enhancement of the entire nodule on CEUS. The ablation was terminated or the power was reduced when the patients could not tolerate the pain during ablation. After ablation, the patients were evaluated for complications immediately after the operation and for symptoms of nerve damage in the following 24 h.
Collection of clinical data and follow-up
The clinical data of all patients were collected by all authors, and these included serum iPTH, calcium, phosphorus and alkaline phosphatase (ALP) levels and thyroid function before ablation and 1 day, 1 week, 1 month, 3 months, 6 months, 1 year, 2 years and 3 years after ablation. All data were obtained in the same laboratory at Huangshi Central Hospital. Ultrasound was performed and the results were evaluated by two of the authors (Z.H. and W.C.). The characteristics of the parathyroid glands post-MWA, including their maximum size and volume, echo features and blood flow, were evaluated using two-dimensional ultrasound, CDFI and CEUS. If the serum iPTH and calcium levels were >800 pg/mL and >2.75 mmol/L, respectively, a second MWA procedure was performed to address the incomplete ablation and the follow-up time was reset to start from the last procedure. According to the guidelines of the Kidney Disease: Improving Global Outcomes (KDIGO), if the serum iPTH, calcium and phosphorus levels drop to 124–558 pg/mL, 2–2.5 mmol/L and 0.97–1.62 mmol/L, respectively, MWA is considered effective regardless of the additional supplementation of calcitriol. It is considered ineffective if the serum iPTH level is >558 pg/mL [Citation29]. Major and minor complications were defined according to the criteria of the Society of Interventional Radiology.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Continuous data were presented as mean ± SD and analyzed using the paired t-test. p-values <0.05 were considered to indicate a significant difference.
Results
Patient demographics and clinical characteristics
shows the clinical characteristics of the enrolled patients. In total, 23 patients with THPT (9 males and 14 females; median age, 45.6 years; range, 27–63 years) underwent ultrasound-guided MWA from January 2013 to July 2015. Before MWA, all patients had THPT with four complete parathyroid glands in normal locations and received hemodialysis for a mean duration of 8.26 years. All patients did not undergo kidney transplantation. These patients showed high serum levels of iPTH (2899–5106 pg/mL; normal range, 15–65 pg/mL), calcium (2.54–3.53 mmol/L; normal range, 2.11–2.52 mmol/L) and phosphorus (1.99–2.78 mmol/L; normal range, 0.80–1.51 mmol/L). The average diameter and volume of nodules were 22.37 ± 0.67 mm and 2.68 ± 1.90 cm3, respectively.
Table 1. Clinical characteristics of patients with THPT.
Treatment outcomes after MWA
All 92 glands in the 23 patients were initially treated with MWA in two sequential sessions. The first session consisted of MWA of the upper nodule and lower nodule on one side. The second session was performed on the other two nodules on the other side 2 weeks to 1 month after the first session if there was no significant side effect or major complication after the first session. The ablation time was 2.5–15 min (mean, 7.26 ± 2.6 min) and the ablation power was 20–30 W (mean, 23.9 ± 2.28 W) for the two nodules on one side. Based on the iPHT levels post-MWA, 13 of the 23 patients received an additional MWA procedure, 2 received two additional MWA procedures, and 2 received three additional MWA procedures. Patients with general recurrence of hyperparathyroidism received the second session 6 months after the first session. shows a percutaneous MWA procedure.
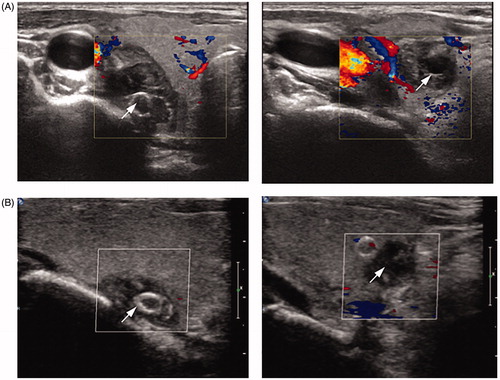
Figure 2. Ultrasound characteristics of a patient (female, 27 years old, on hemodialysis for 8 years) before and after microwave ablation (MWA). (A–C) Images of the upper and lower nodules (arrows) on the right side by two-dimensional ultrasound (A) and contrast-enhanced ultrasound (CEUS) (B) and the blood flow by color Doppler flow imaging (CDFI) (C) before MWA. (D) Saline injection into the parathyroid capsule for heat insulation. Arrow shows the separating saline interface. (E) Image of the MWA of the upper nodule. The antenna and ablating tip are shown by arrow and star, respectively. The ablation zone was evaluated through echo changes from the position of the antenna tip. (F–G) Images of the upper nodule after MWA by CDFI (F) and CEUS (G).
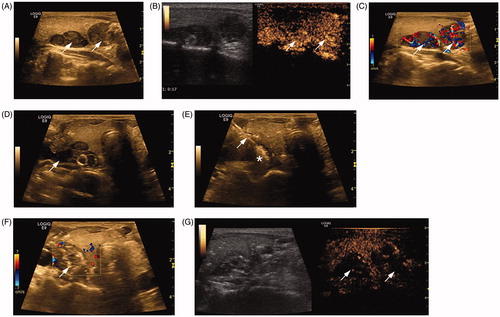
The mean follow-up period was 47 ± 8.4 months. No significant recurrence or regeneration was observed at the last follow-up of all patients, and no obvious blood flow signal was observed in the postoperative nodules. The maximum diameter and average volume of the nodule at the last follow-up were significantly lower after MWA (9.4 ± 3.2 vs. 22.37 ± 0.67 mm and 0.33 ± 0.23 vs. 2.68 ± 1.90 cm3, respectively; both p < 0.001). The average decrease in nodule volume was 75.9 ± 11.3% (58.6%–93%). shows the follow-up ultrasound characteristics of the patient in after MWA. All patients had no major complications such as esophageal perforation, tracheal injury, hoarseness, or skin burn. The majority of clinical manifestations of THPT, such as bone pain and pruritus, significantly improved or disappeared. The dietary behavior and sleep quality also improved significantly, except for three patients with skeletal malformations.
Laboratory analysis
The follow-up time for all patients was >3 years (47 ± 8.4 months; range 36–57 months). One day after MWA, the iPTH levels decreased significantly compared to levels before MWA (155.3 ± 33.8 vs. 3940 ± 484 pg/mL, p = 2.2 × 10−21). The iPTH levels were also significantly lower at 1 week (379.7 ± 66.1 pg/mL, p = 2.5 × 10−20), 1 month (395.1 ± 61.7 pg/mL, p = 2.8 × 10−20), 3 months (557.2 ± 48.6 pg/mL, p = 8.2 × 10−20), 6 months (725.3 ± 65.8 pg/mL, p = 7.8 × 10−20), 12 months (946.2 ± 19.2 pg/mL, p = 6.8 × 10−20), 24 months (1123.6 ± 91.7 pg/mL, p = 6.5 × 10−19) and 36 months (1159.3 ± 81.3 pg/mL, p = 4.8 × 10−19) after MWA than they were before MWA, although the levels increased gradually until 24 months (; ). After 24 months, the iPTH levels remained at stable levels until the end of follow-up ().
Figure 4. Time course of laboratory clinical data before and after microwave ablation (MWA). Serum levels of intact parathyroid hormone (iPTH) (A), calcium (B), phosphorus (C), and alkaline phosphatase (ALP) (D) before MWA and at 1 day, 1 week, 1 month, 3 months, 6 months, 12 months, 24 months, and 36 months after MWA. Statistics by paired t-test. p < 0.001.
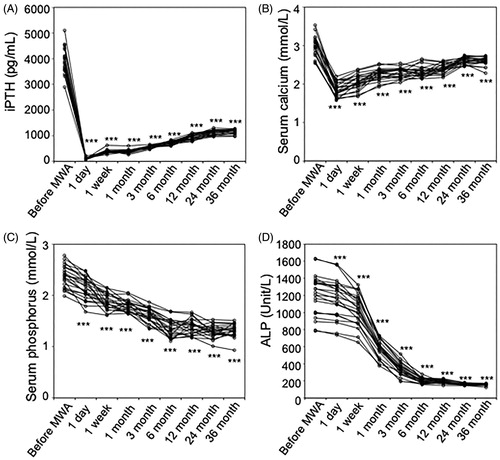
Table 2. Laboratory clinic data before and after MWA.
One day after MWA, the serum calcium levels decreased significantly, leading to hypocalcemia, compared to levels before MWA (1.87 ± 0.18 vs. 2.99 ± 0.25 mmol/L, p = 3.6 × 10−18). After immediate calcium supplementation, the calcium levels recovered to normal range (2.11–2.52 mmol/L) at 1 week (2.05 ± 0.18 mmol/L, p = 6.6 × 10−16), 1 month (2.26 ± 0.15 mmol/L, p = 5.6 × 10−13), 3 months (2.3 ± 0.13 mmol/L, p = 7.8 × 10−12), 6 months (2.32 ± 0.14 mmol/L, p = 3.2 × 10−12) and 12 months (2.41 ± 0.12 mmol/L, p = 2.7 × 10−10) up until 24 months. After 24 months, the calcium levels reached the upper normal limit and remained at stable levels but were still significantly lower than they were before MWA (36 months: 2.60 ± 0.10 vs. 2.99 ± 0.25 mmol/L, p = 1.4 × 10−7) (; ).
Both serum phosphorus and ALP levels decreased significantly 1 day after MWA (2.14 ± 0.21 vs. 2.37 ± 0.21 mmol/L, p = 3 × 10−8, and 1157.1 ± 219.3 vs. 1206.4 ± 230.5 U/L, p = 9 × 10−8, respectively). More importantly, serum phosphorus levels decreased gradually and reached the normal range (0.8–1.51 mmol/L) at 6-month post-MWA (1.37 ± 0.16 mmol/L, p = 2 × 10−15) until the end of follow-up (1.32 ± 0.12 mmol/L, p = 2 × 10−16) (; ). Serum ALP levels also decreased gradually and reached stable levels at 6-month post-MWA (201.2 ± 29.1 U/L, p = 1 × 10−16) until the end of follow-up (157.9 ± 10.0 U/L, p = 2 × 10−16), the levels being slightly higher than the upper normal limit (125 U/L) (; ).
Complications
No major complications were observed during MWA and at follow-up. All patients tolerated the procedure well. All ablation-related adverse events showed spontaneous remission without medical intervention. Adverse events during the ablation included the common mild to moderate neck pain; mild headache in two patients; mild backache, which disappeared after the termination of ablation, in one patient; and local mild distension in 14 patients, which disappeared in 3 weeks. One patient demonstrated transient vocal difficulty during the ablation, which disappeared after the procedure was terminated. No abnormalities were observed 3 min after the ablation was started, and the ablation was continued with the patients not showing any vocal difficulty. No vocal cord paralysis was observed on laryngoscopy, and no vocal abnormalities were observed during the follow-up. No patients required the termination of ablation during MWA. After the ablation, hypocalcemia occurred in all patients, but the calcium level recovered to normal range 1 week after calcium supplementation, and no patients developed refractory hypocalcemia during the follow-up. Moreover, there were no infections or burns in the puncture sites and no damage to surrounding structures such as carotid, tracheal, esophageal, and thyroid tissue and nerves.
Discussion
In this retrospective pilot study, MWA was successfully applied to ablate hyperplastic parathyroid nodules in 23 ESRD patients with THPT and followed the patients for >36 months. Although the long-term control of iPTH levels was not ideal, the iPTH levels after MWA were significantly lower than they were before MWA. Calcium and phosphorus levels were reduced to marginal and/or normal levels, and disturbances in mineral metabolism were well controlled. Meanwhile, the clinical symptoms and quality of life of patients were significantly improved. These results indicate that ultrasound-guided MWA is safe, effective and minimally invasive for patients with TPHT.
Although parathyroidectomy is recommended by the KDIGO guidelines and is very effective in treating THPT, the procedure has been associated with increased rates of mortality and morbidity due to the perceived high risks of surgery [Citation12]. Patients with THPT who in general have a history of ineffective medical treatment, have surgical contraindications, and who experience weakness and illness may not be eligible or willing to undergo surgery. Nonsurgical methods are often preferable for the physical destruction of hyperplastic parathyroid glands. Particularly, MWA has unique advantages compared to other nonsurgical and minimally invasive treatments. For example, percutaneous ethanol injection therapy is more complicated than MWA because it requires multiple injections at different intervals for each gland and is associated with a high rate of recurrence and adverse effects [Citation16]. Laser ablation is not recommended due to the lower ablation volume of laser and the larger size of several nodules in patients with THPT [Citation30]. Furthermore, radiofrequency ablation is effective only for small nodules but not for nodules with a diameter >15 mm and rich blood flow [Citation31]. MWA was originally used to safely and effectively ablate liver and kidney tumors [Citation32] and as a subsequent ablation treatment for benign thyroid nodules [Citation22]. MWA devices create a uniform electromagnetic field surrounding the antenna that causes rapid volume tissue heating by means of the oscillation of polar water molecules. This mechanism can cause tissue temperatures to increase markedly, leading to cellular necrosis. Compared to radiofrequency ablative techniques, MWA has a higher heating efficiency and larger, faster and consistent ablation zones because it does not rely on the conduction of an electrical current and is not limited by charring and blood perfusion [Citation33]. MWA procedures have been reported to treat SHPT successfully and effectively [Citation23,Citation24], suggesting that MWA is also feasible for THPT.
Our pilot study of 23 patients with THPT showed that MWA was effective in the short-term and long-term improvement of the overall quality of life and the clinical symptoms of bones, joints, muscles, nerves, blood pressure, nutrition and the cardiovascular system in these patients. A marked decrease in serum iPTH level was observed after MWA, although the iPTH level in the long-term follow-up did not reach the target values in the KDIGO guidelines. Meanwhile, the serum calcium, phosphorus and ALP levels improved significantly toward or close to the normal ranges. Based on the comprehensive clinical characteristics of THPT patients with long-term follow-up, MWA achieved results comparable to those of parathyroidectomy. However, MWA was less invasive and safe, was more acceptable to the patients, and caused fewer complications with a faster recovery.
In the long-term follow-up post-MWA, we observed a gradual increase in iPTH levels, which might be due to the incomplete ablation of the parathyroid nodules. Incomplete ablation is also a common reason for recurrence in other treatments, including parathyroidectomy [Citation34–36]. The ablation efficacy is dependent on both the treatment technique and the technique of the operator. As ultrasound is limited in displaying the entire volume of large nodules in THPT, specially the marginal areas, and the parathyroid glands are in close proximity to the laryngeal nerve, operators tend to reduce the ablation volume to avoid injury to this nerve, resulting in incomplete ablation.
As mentioned, the patients in this study did not show any major complications during MWA or within the follow-up period. The commonest adverse event was hypocalcemia, which occurred in all patients 1 day to 1 week after MWA. The calcium level recovered to normal range 1 week after calcium supplementation, and the patients did not develop refractory hypocalcemia during the follow-up.
This study has certain limitations. It is a single-center and retrospective study and has a relatively smaller sample size of patients with >3 years of follow-up, so larger, multicenter and prospective studies are required to clarify the efficacy of MWA in patients with THPT, and studies with a follow-up period of >5 years are recommended to more accurately determine the recurrence rate of THPT after MWA.
In conclusion, this study showed that ultrasound-guided percutaneous MWA can be a feasible, safe and effective treatment for patients with THPT. MWA may represent a nonsurgical, minimally invasive alternative for treating THPT patients who are ineligible for surgery or unwilling to undergo surgery.
Disclosure statement
The authors declare no potential conflict of interest.
References
- Block GA, Martin KJ, de Francisco ALM, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–1525.
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98.
- Jamal SA, Miller PD. Secondary and tertiary hyperparathyroidism. J Clin Densitom. 2013;16(1):64–68.
- Lou I, Schneider DF, Leverson G, et al. Parathyroidectomy is underused in patients with tertiary hyperparathyroidism after renal transplantation. Surgery. 2016;159(1):172–180.
- Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–1239.
- Yang RL, Freeman K, Reinke CE, et al. Tertiary hyperparathyroidism in kidney transplant recipients: characteristics of patients selected for different treatment strategies. Transplantation. 2012;94(1):70–76.
- Lee B, Qiao L, Lu M, et al. C/EBPα regulates macrophage activation and systemic metabolism. Am J Physiol Endocrinol Metab. 2014;306(10):E1144–E1154.
- Eidman KE, Wetmore JB. Treatment of secondary hyperparathyroidism: how do cinacalcet and etelcalcetide differ? Semin Dial. 2018;31(5):440–444.
- Triponez F, Clark OH, Vanrenthergem Y, et al. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248(1):18–30.
- Kebebew E, Duh QY, Clark OH. Tertiary hyperparathyroidism: histologic patterns of disease and results of parathyroidectomy. Arch Surg. 2004;139(9):974–977.
- Tang JA, Salapatas AM, Bonzelaar LB, et al. Parathyroidectomy for the treatment of hyperparathyroidism: thirty-day morbidity and mortality. Laryngoscope. 2018;128(2):528–533.
- Drakopoulos S, Koukoulaki M, Apostolou T, et al. Total parathyroidectomy without autotransplantation in dialysis patients and renal transplant recipients, long-term follow-up evaluation. Am J Surg. 2009;198(2):178–183.
- Evenepoel P, Claes K, Kuypers D, et al. Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant. 2005;20(8):1714–1720.
- Gasparri G, Camandona M, Abbona GC, et al. Secondary and tertiary hyperparathyroidism: causes of recurrent disease after 446 parathyroidectomies. Ann Surg. 2001;233(1):65–69.
- Chen HH, Lin CJ, Wu CJ, et al. Chemical ablation of recurrent and persistent secondary hyperparathyroidism after subtotal parathyroidectomy. Ann Surg. 2011;253(4):786–790.
- Douthat WG, Cardozo G, Garay G, et al. Use of percutaneous ethanol injection therapy for recurrent secondary hyperparathyroidism after subtotal parathyroidectomy. Int J Nephrol. 2011;2011:1.
- Onoda N, Kurihara S, Sakurai Y, et al. A case of secondary hyperparathyroidism whose high turnover bone improved after the direct injection of acetic acid into the parathyroid glands. Clin Nephrol. 2004;61(1):68–73.
- Bennedbaek FN, Karstrup S, Hegedüs L. Ultrasound guided laser ablation of a parathyroid adenoma. Br J Radiol. 2001;74(886):905–907.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. High-intensity focussed ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(1):76–80.
- Carrafiello G, Laganà D, Mangini M, et al. Treatment of secondary hyperparathyroidism with ultrasonographically guided percutaneous radiofrequency thermoablation. Surg Laparosc Endosc Percutan Tech. 2006;16(2):112–116.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Zhao J, Qian L, Zu Y, et al. Efficacy of ablation therapy for secondary hyperparathyroidism by ultrasound guided percutaneous thermoablation. Ultrasound Med Biol. 2016;42(5):1058–1065.
- Zhuo L, Peng L-L, Zhang Y-M, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease – a pilot study. Radiology. 2017;282(2):576–584.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Wong KP, Lang BHH. Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol. 2013;2013:428363.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13(2):117–125.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. Am J Roentgenol. 2010;194(4):1137–1142.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Wang R, Jiang T, Chen Z, et al. Regression of calcinosis following treatment with radiofrequency thermoablation for severe secondary hyperparathyroidism in a hemodialysis patient. Intern Med. 2013;52(5):583–587.
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation – what should you use and why? Radiographics. 2014;34(5):1344–1362.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6):2967–2973.
- Diao Z, Wang L, Li D, et al. Efficacy of microwave ablation for severe secondary hyperparathyroidism in subjects undergoing hemodialysis. Ren Fail. 2017;39(1):140–145.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Li X, An C, Yu M, et al. US-guided microwave ablation for secondary hyperparathyroidism in patients after renal transplantation: a pilot study. Int J Hyperthermia. 2019;36(1):322–327.