Abstract
Purpose: Intraperitoneal (IP) chemotherapy has several benefits but also can have severe hematologic side effects. We compared the effects of hyperthermic intraperitoneal chemotherapy (HIPEC) and conventional IP chemotherapy on bone marrow suppression and evaluated whether HIPEC increased neutrophil recovery.
Methods: HIPEC or IP chemotherapy was administered to ovarian cancer–bearing mice. Bone marrow progenitor cell colony-forming unit (CFU) count, serum cytokine levels, and peripheral leukocyte count after HIPEC and IP chemotherapy were compared.
Results: Peripheral neutrophil count, cytokine (G-CSF and CXCL1/KC) levels, and bone marrow progenitor cell CFU count were significantly higher after HIPEC than after IP chemotherapy.
Conclusions: Hyperthermia increased the serum neutrophil-recruiting cytokine levels and reduced the magnitude of chemotherapy-induced neutropenia. Thus, HIPEC improved neutrophil and bone marrow recovery compared with conventional IP chemotherapy.
Introduction
Ovarian cancer is a fatal gynecologic malignancy that causes 152,000 deaths annually worldwide [Citation1]. In 2015, population-based studies have reported a 5-year age-standardized relative survival rate of only 30–40% globally [Citation2,Citation3]. The treatment for advanced ovarian cancer generally involves cytoreductive surgery (CRS), followed by chemotherapy, including intravenous and intraperitoneal (IP) chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC). In theory, IP chemotherapy enables direct drug into peritoneal cavity at a 20–1000 times greater concentration than does intravenous chemotherapy. An IP chemotherapy regimen with cisplatin and paclitaxel was introduced in the Gynecologic Oncology Group (GOG) 172 trial [Citation4]. This phase 3 randomized trial demonstrated that progression-free survival (PFS) and overall survival (OS) were longer in the IP chemotherapy group than in the intravenous chemotherapy group [Citation5]. Jaaback et al. recently demonstrated that using IP chemotherapy in adjuvant chemotherapy prolonged the PFS and OS in patients with advanced ovarian cancer [Citation6].
The survival benefits of conventional IP chemotherapy for ovarian cancer have necessitated the use of HIPEC, which uses heat. Several advantageous effects of heat, including enhancing direct cytotoxic effect, enabling certain chemotherapeutic agents (e.g., cisplatin), increasing drug penetration into tumor tissue (especially heat-sensitive tumor cells) and therefore inducing tumor cell death [Citation7]. Since 1970s, Spratt et al. designed a system for adding hyperthermic treatment [Citation8] to IP chemotherapy, and HIPEC has now become a widely used therapy. During the procedure, the chemotherapeutic solution circulates throughout the abdomen, with IP temperature maintained at 41–43 °C for approximately 60–90 min. HIPEC enables the peritoneum-to-plasma ratios of active drug concentration higher than intravenous chemotherapy, to range from 20-fold (e.g., cisplatin and carboplatin) to (e.g., taxanes) [Citation9]. It has been estimated in an experimental model that cisplatin diffuses into peritoneal tissue at around 0.48 mm. The rate of microvascular uptake determines the distance of drug penetration [Citation10]. Since the penetration distance of drug into tissue is short, it reaches tissues mainly through circulation over this distance rather than direct penetration. The drug distribution during HIPEC is a complex process and there are several parameters to affect transport of the drug intraperitoneally [Citation11]. However, heat is the key factor. Because of the penetration of heat resulting in increases in toxicity of drug and cellular uptake up to 1 cm distance into the tissues, tumor cell killing may be increased locally [Citation10].
HIPEC has become a useful therapeutic strategy through hyperthermia and peritoneal lavage, debulking achieved by eliminating the residual microscopic component intraperitoneally [Citation12,Citation13]. It can be effectively used for treating peritoneal mesothelioma, pseudomyxoma peritonei, and peritoneal carcinomatosis due to colorectal, gastric, or ovarian cancer. HIPEC with CRS results in higher survival benefit than CRS alone for patients with epithelial ovarian carcinoma, primary advanced ovarian cancer [Citation12,Citation14–16], and recurrent ovarian cancer [Citation14,Citation17]. In 2018, a randomized phase 3 trial compared the efficacy and safety of interval CRS with HIPEC with those of interval CRS without HIPEC [Citation18]. The results revealed longer recurrence-free survival (14.2 vs. 10.7 months) and longer median OS (45.7 vs 33.9 months) in the surgery-plus-HIPEC group. Furthermore, a meta-analysis reported that disease-free survival and OS in both primary and recurrent ovarian cancers was higher after CRS with HIPEC than after CRS with intravenous chemotherapy [Citation19].
However, compared with patients who received intravenous chemotherapy, patients who received IP chemotherapy experienced higher toxicity and severer side effects (e.g., abdominal pain and bone marrow suppression) and complications (e.g., catheter obstruction and deteriorated renal functions) [Citation4–6]. Whether patients who received HIPEC suffer fewer or more complications and side effects than do patients who received intravenous chemotherapy remains unknown. Platinum-based HIPEC is often associated with severe hematologic toxicity, such as higher platelet and neutrophil toxicity, compared with to other HIPEC [Citation20]. Thus, patients may be unable to complete their six-course conventional IP chemotherapy [Citation4]. Incomplete courses and delayed schedules of IP chemotherapy can impede the benefits of HIPEC.
In this study, we used ovarian cancer–bearing mice to compare the effects of HIPEC and IP chemotherapy on neutropenia and bone marrow suppression. Neutrophil-recruiting cytokine levels in serum were also measured to evaluate the effect of hyperthermia on the homeostasis of blood cells after IP chemotherapy. The influence of hyperthermia on cytotoxic T cells and dendritic cells was explored to evaluate the effect of HIPEC on the cellular immune system.
Materials and methods
Mice
Sixty C57BL/6 female mice were purchased from BioLASCO, Taipei, Taiwan and maintained under pathogen-free conditions. An immunocompetent mouse model was established using ID8-luc cells derived from a mouse ovarian cancer cell line.
Ethics statement
All experiments in this study followed guidelines involving experimental animal welfare and were approved by IACUC of MacKay (MMH-A-S-100-44).
Grouping strategy and principles of IP chemotherapy and HIPEC
C57BL/6 mice were intraperitoneally injected with 1 × 106 ID8 cells transduced with luciferase gene (ID8-Luc) to establish ovarian cancer [Citation21]. After 5 days, laparotomy was performed after mice were anesthetized with Zoletil (VIRBAC, Carros, France) and Rompun (Bayer Vital GmbH, Leverkusen, Germany). In the control group, the peritoneal cavity was opened for 20 min through laparotomy. In the heat group, laparotomy and IP hyperthermia were administered. To administer IP hyperthermia, the peritoneal cavity of every mouse was continually filled with phosphate-buffered saline (PBS) heated to 45 °C. Heated PBS (6 ml) were added to replace the cooled PBS at a frequency of 20–30 s/cycle for 20 min to maintain the intraperitoneal temperature at 41 °C–43 °C. In HIPEC group, chemotherapeutic drugs (paclitaxel [10 mg/kg, 0.67 μg/mL in 1× PBS] and cisplatin [6 mg/kg, 0.4 μg/mL in 1× PBS]) were added into the peritoneal cavity immediately after the completion of hyperthermia. Mice were kept warm under heater lights until they recovered from anesthesia. Paclitaxel (Taxol) and cisplatin (Platinex) were purchased from Bristol-Myers Squibb, New York, NY, after approval from the Pharmaceutical Committee of MacKay, Taipei, Taiwan was obtained.
Leukocyte differential count
All hematologic components were analyzed through flow cytometry. Blood (200 µL) was collected from the tail vein of treated mice into EDTA blood collection tubes after treatment on Days 3 and 14. Hemograms of the mice were compiled using Hemavet HV950 Multispecies Hematologic Analyzer (Drew Scientific Inc, Oxford, CT). For flow cytometry analysis, blood samples were first treated with 10× ammonium–chloride–potassium (ACK) buffer to remove red blood cells. Mouse leukocytes were then stained with the following antibodies in designed panels: fluorescein isothiocyanate (FITC)-conjugated rat antimouse CD3 (AbD Serotec, Kidlington, UK; MCA 500 F, 1:20), allophycocyanin (APC)-conjugated rat antimouse CD8a (eBioscience, San Diego, USA; 17-0081, 1:100), FITC-conjugated rat antimouse CD11b (eBioscience, 12-0112, 1:100), and phycoerythrin (PE)-conjugated rat ant–mouse CD11c (eBioscience, 12-0114, 1:50). The staining panels for blood cell detection are listed in .
Table 1. Antibody panels for mouse leukocytes flow cytometry analysis.
Bone marrow cell colony-forming unit assay
The femurs of five mice from each group were removed on Days 5, 10, and 15 after the designated treatment was completed. The bone marrow was flushed out of the shaft of the bone by using the RPMI medium (Gibco, Waltham, MA, USA), and the erythrocytes were lysed using the ACK buffer. Bone marrow cells (2 × 104) were then resuspended in 1 ml of RPMI 1640 complete medium, with 0.6% methyl cellulose (Sigma-Aldrich, St. Louis, MO, USA) and 100 ng/mL rmSCF, 20 ng/mL rmIL-3, and 20 ng/mL rmIL-6 (PeproTech, Rocky Hill, NJ, USA), and then seeded in 24-well plates. The cells were kept in culture until colony formation, and the number of CFU was counted using an inverted microscope.
Flow cytometry analysis of progenitor cells in bone marrow
All bone marrow cell components were analyzed through flow cytometry. The hematopoietic stem cells (HSCs), multipotent progenitor cells (MPPs), pre-granulocyte macrophage progenitors (pre-GMPs) and GMPs in the flushed bone marrow cells were enriched using a Mouse Hematopoietic Stem and Progenitor Cell Isolation Kit (BD Biosciences PharMingen [San Jose, CA, USA]: 560492) and stained with the antibodies described in the designed panels: APC mouse lineage antibody cocktail (BD Biosciences PharMingen; 558074), APC-conjugated rat anti-mouse Sca-1 (eBioscience; 17-5981, 1:50), PE-Cy7-conjugated rat antimouse Sca-1 (Biolegend, San Diego, CA, USA; 122513, 1:50), PE-conjugated rat anti-mouse CD117 (c-kit; Biolegend; 135105, 1:50), APC-conjugated rat anti-mouse CD150 (Biolegend; 115909, 1:50), Alexa Fluor 488-conjugated rat anti-mouse CD105 (Biolegend; 120405, 1:50), PerCP/Cy5.5-conjugated rat anti-mouse CD64 (FcγRI; Biolegend, 139307; 1:50), and APC-conjugated rat anti-mouse CD64 (FcγRI; Biolegend, 139305, 1:50) antibodies. The staining panels for HSCs and progenitor cells are presented in .
Table 2. Antibody panels for mouse stem and progenitor cells flow cytometry analysis.
Determination of cytokine concentrations
Peripheral blood was collected from mice at 16 and 24 h after the treatment. The blood was allowed to clot for 30 min at room temperature and then centrifuged at 1500×g for 10 min for serum separation. Kits for detecting mouse neutrophil-recruiting cytokines (G-CSF, 560152, BD Biosciences PharMingen; TGF-β, BMS8608FF, eBioscience; and CXCL1/KC, BMS86019FF, eBioscience) were used to determine their levels, according to the manufacturers’ instructions.
Statistical analysis
Student t test was used to compare the intergroup differences between two mouse groups. A p value of <.05 was considered statistically significant.
Results
Hyperthermia compensated for neutropenia caused by IP chemotherapy
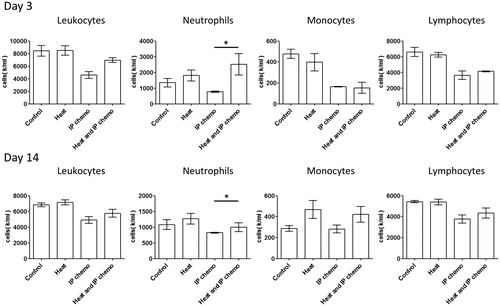
ID8 tumor-bearing mice were established and administered with IP chemotherapy or HIPEC. Because it was only a one-time treatment, the antitumor effect between IP chemotherapy and HIPEC did not differ significantly (Supplementary Figure S1). However, the hemogram analysis on Days 3 and 14 after treatment indicated significantly higher neutrophil counts in the HIPEC group than in the IP chemotherapy group (), but the differences in monocytes and lymphocyte counts were nonsignificant. These results demonstrated that hyperthermia alone did not affect hemogram results, whereas HIPEC reduced the magnitude of neutropenia caused by IP chemotherapy.
Figure 1. Alterations in hemogram after different treatments in mice. Hyperthermia affected the differential leukocyte count and reduced neutrophil loss caused by IP chemotherapy. Standard errors are represented in each chart. Results are presented as means ± standard error (n = 5 in each group). *p < .05
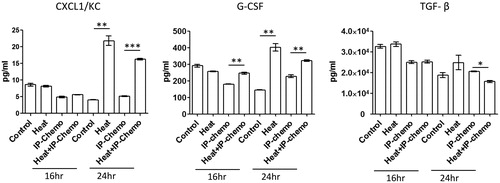
Hyperthermia facilitated increase in serum neutrophil-recruiting cytokine levels
Hyperthermia could prevent neutropenia resulting from conventional IP chemotherapy; however, the working mechanism was unclear. Therefore, the variations in serum neutrophil-recruiting cytokine levels after treatment were examined. Except for TGF-β levels, hyperthermia increased serum neutrophil-recruiting cytokine levels in 24 h of treatment [all p < .01 for CXCL1/KC and G-CSF (hyperthermia vs. control and HIPEC vs. IP chemotherapy); ]. These results demonstrated that hyperthermia may relieve the neutropenia caused by IP chemotherapy by increasing the serum neutrophil-recruiting cytokine levels.
Hyperthermia increased proportion of cellular immunity-related cells
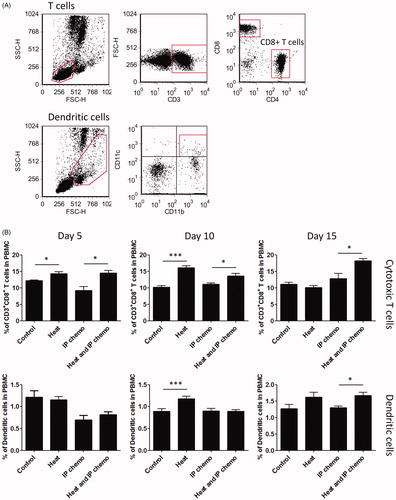
During cancer chemotherapy, host immunity, particularly cellular immunity, improves the outcome of cancer patients. Because hyperthermia can prevent neutrophil loss during IP chemotherapy, we investigated the effect of hyperthermia on cellular immunity-related cells during IP chemotherapy. Mouse blood was harvested at Days 5, 10, and 15 after treatment, and flow analysis was performed to detect the proportion of cytotoxic T cells and dendritic cells among the leukocytes (). Mice treated with hyperthermia had higher proportions of cytotoxic T cells (at Day 5: hyperthermia vs. control and HIPEC vs. IP chemotherapy, both p < .05; at Day 10: hyperthermia vs. control, p < .001 and HIPEC vs. IP chemotherapy, p < .05; at Day 15: HIPEC vs. IP chemotherapy, p < .05; ). Hyperthermia also increased dendritic cell proportion at Days 10 (hyperthermia vs. control, p < .001) and 15 (HIPEC vs. IP chemotherapy, p < .05) after treatment (). These results indicated that hyperthermia can increase the proportions of cytotoxic T cells and dendritic cells and thus may enhance cellular immunity after IP chemotherapy.
Figure 3. Effect of hyperthermia on the proportional variation in cell-mediated immunity. (A) Gating strategies of flow cytometry analysis for cytotoxic T cells and dendritic cells in mice. (B) Proportion of cytotoxic T cells and dendritic cells in peripheral blood. Hyperthermia increased the proportion of cytotoxic T cells initially and to 15 days after HIPEC. Dendritic cell increase was delayed to 10 days after HIPEC. Results are presented as means ± standard error (n = 5 in each group). *p < .05, ***p < .001
Hyperthermia increased number of stem and progenitor cells
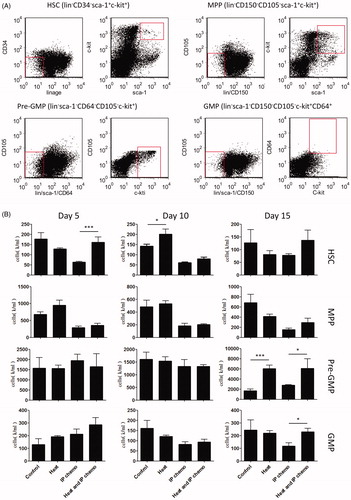
Bone marrow suppression, another adverse effect of chemotherapy, affects the hematopoietic function of patients. To investigate whether hyperthermia could relieve this side effect, the mouse bone marrow cells were collected at different timepoints after treatment. The proliferation and differentiation patterns of bone marrow progenitor cells were analyzed using colony-forming cell assay. Although hyperthermia and control groups demonstrated no differences, the number of bone marrow cell CFUs were higher in the HIPEC group than in the IP chemotherapy group on Days 5, 10, and 15 after treatment (p < .05; ). The HSC and progenitor cell counts were then determined through flow cytometry (). HSC count in the bone marrow was higher in the HIPEC group than in the IP chemotherapy group (p < .05), particularly during the initial days (e.g., Day 5). Similarly, there were more pre-GMPs and GMPs in the HIPEC group than in the IP chemotherapy group (p < .05), particularly in later days (e.g., Day 15; ). In general, the numbers of progenitor cells in the bone marrow were higher in mice that received HIPEC than in those that received IP chemotherapy.
Figure 4. Difference between CFUs of bone marrow cells after treatment in mice. Hyperthermia alone did not affect proliferation and differentiation patterns of bone marrow progenitor cells. However, it prevented bone marrow suppression caused by IP chemotherapy. Results are presented as means ± standard error (n = 5 in each group). *p < .05
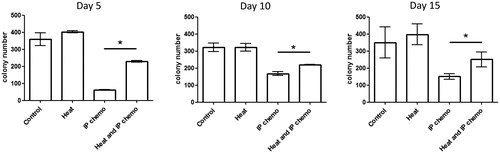
Figure 5. Variation of number of progenitor cells after treatment. The cell numbers of progenitor cell types in the bone marrow of mice were determined by multiplying the total cell counts with the proportion of each type of cell identified through flow cytometry analysis. (A) Gating criteria of HSCs and other progenitor cells. (B) Hyperthermia prevented HSC loss caused by IP chemotherapy at an early stage (Day 5). However, the effect of hyperthermia on preventing neutrophil progenitor loss, such as pre-GMP and GMP, could be detected only at a late stage (Day 15). Results are means ± standard error (n = 5 in each group). *p < .05, ***p < .001
Discussion
Principal findings
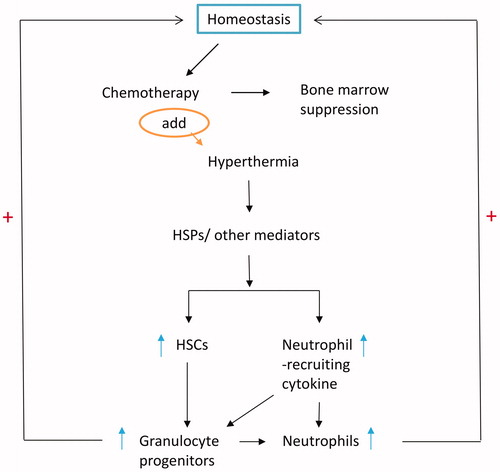
Our findings suggested that adding hyperthermia to conventional IP chemotherapy enhances neutrophil and bone marrow recovery. In this study, hyperthermia alone could affect the release of neutrophil-recruiting cytokines but could not influence peripheral neutrophil count and bone marrow cell CFU count. As a result, hyperthermia compensated for neutropenia and bone marrow suppression caused by conventional IP chemotherapy. Thus, the function of hyperthermia in chemotherapy may be to maintain blood cell homeostasis (). The precise mechanism for such regulation requires further exploration.
Hyperthermia was associated with inflammation and induced neutrophil-recruiting cytokine release
IP hyperthermia induced inflammation in peritoneal cells and the related inflammatory effects. Hyperthermia and heat shock proteins (HSP) levels are associated. Heat inducible protein, such as HSP70, is released during tissue necrosis and has potent immunostimulatory functions [Citation22,Citation23]. HSP expression is induced in bone marrow-derived mesenchymal stem cells (MSCs) through thermal stress [Citation24]. G-CSF responds to hyperthermia and regulates neutrophil recruitment, augments neutrophil delivery to inflammation sites, results in expansion of the circulating neutrophil pool [Citation25]. Moreover, CXC chemokines are considered HSPs [Citation26]. Exposure to hyperthermia increased generation of endogenous CXC chemokine family [Citation27,Citation28]. Hyperthermia directly enhances the expression of HSPs, G-CSF and CXC chemokines, thereby augmenting neutrophil delivery and neutrophil amount.
We observed that the significantly increased serum cytokine levels may have led to recruitment of inflammatory cells and release of cytokines to promote neutrophil and bone marrow recovery. G-CSF, which accelerates the production or stimulation of cells from the bone marrow and is frequently used in patients with severe or febrile neutropenia who are undergoing chemotherapy, may shorten the neutrophil recovery period [Citation29]. The chemokine CXCL1/KC, released by neutrophils, mast cells, and macrophages, can initiate early-phase neutrophil recruitment from the bone marrow to the acute inflammatory tissue [Citation30,Citation31]. Although not well-studied, TGF-β could inhibit neutrophil activity (i.e., degranulation) [Citation32], and a study suggested that blocking the TGF-β pathway increases neutrophil recruitment in several chronic disease [Citation33]. Furthermore, TGF-β blockage increases the mRNA levels of neutrophil chemoattractants [Citation34], such as CXCL1/KC, G-CSF, CXCL2, and CXCL5. We observed significant increase in CXCL1/KC and G-CSF levels and decrease in TGF-β levels at 24 h after the mice received HIPEC. This was compatible with our observation that selected chemokines have established roles in neutrophil recruitment and chemoattraction.
Hyperthermia has potential benefits for preserving antitumor immune response
Dendritic cells and cytotoxic T cells play an important role in initiation and regulation of innate and adaptive immune responses for effective cancer killing. Dendritic cells present tumor-related antigen to T cells and activate cytotoxic T cells to kill malignant cells. Thus, maintenance in the amount and modulation of these immune effective cells after chemotherapy are essential issues to improve cancer immunotherapy [Citation35,Citation36]. Our study findings suggest that hyperthermia could effectively preserve the number of dendritic cells and cytotoxic T cells after IP chemotherapy. In addition, hyperthermia has been shown to induce cell surface expression of HSP 70 which is associated with antigen presentation [Citation37], which facilitate malignant cell killing. Hyperthermia added with immune modulators, such as α-galactosylceramide, has been demonstrated to enhance antitumor immune responses [Citation21]. All of these results indicate that hyperthermia has benefits in facilitating anti-tumor immune responses. Therefore, the combination of immune therapy after CRS plus HIPEC in facilitating tumor cell killing might be an interesting approach to be addressed.
Implications for future research
In malignant cells, hyperthermia induces increase in the number of lysosomes and enzyme activity, because of reversibly inhibiting RNA synthesis and mitosis arrest. Besides, hyperthermia decreases blood flow of microcirculation or causes vascular stasis in malignant tumor tissues [Citation38]. Hyperthermia also selectively increases sensitivity of mitochondrial membranes in malignant cells [Citation39]. These factors further decrease oxidative metabolism, leads to lactic acid accumulation, and lower pH in microenvironment of tumor tissue [Citation38]. Comparing to normal cells, malignant cells are more fragile to heat stress, which results in increased destructive capacity and further accelerated cell death. To sum up, chemotherapeutic cytotoxicity drug and hyperthermia synergistically augment cancer cell killing.
A study reported a steady and significant increase in interleukin (IL)-6 and procalcitonin levels in serum and peritoneum samples during HIPEC, mimicking sepsis [Citation40]. The heat-related systemic inflammatory response may evoke pathways related to reactive oxygen species, and G-CSF, interferon-γ, and IL-6 induce quiescent HSCs to differentiate into progenitor cells [Citation41], which is similar to the response caused by IP hyperthermia. We observed a significantly higher neutrophil count and increased differential counts of leukocyte subtypes (including cytotoxic T cells and dendritic cells) after HIPEC than after conventional IP chemotherapy at early and later timepoints. Thus, hyperthermia potentially enhances immunosurveillance to mediate the antitumor response.
As mentioned above, hyperthermia in HIPEC is not only beneficial in cancer cell killing but also helpful in improving bone marrow recovery and enhancing antitumor immune response. Clinically some factors might be important for the outcomes of patients with ovarian cancer, including the residual tumor after CRS, the immune status in receiving HIPEC. In the future, immune cell therapy using T cells or dendritic cells might also play an important role along with HIPEC and warrant further investigation.
Conclusions
In our mouse study, the use of HIPEC to treat ovarian cancer increased progenitor cell production, decreased bone marrow suppression, and improved neutrophil recovery and immune response through chemotaxis. The mechanism potential underlying this phenomenon is that hyperthermia induces HSP release from necrotic tissue and proinflammatory cytokine release from the bone marrow or lymphoid tissues. HSPs and related cytokines can induce bone marrow-derived MSC proliferation [Citation24] and leukocyte release in serum, similar to leukocytosis during inflammation. The increased number of peripheral neutrophils caused the release of chemotactic cytokines to improve neutrophil recruitment. Current evidence indicates that the potential survival benefit of HIPEC may be the largest when combined with CRS for advanced ovarian cancer and salvage CRS for recurrent ovarian cancer [Citation42]. Thus, administering HIPEC in patients with advanced ovarian, tubal, and peritoneal cancer after optimal debulking surgery may better compensate neutropenia than conventional IP chemotherapy.
Supplemental Material
Download JPEG Image (5.2 MB)Disclosure statement
The authors report no conflict of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 2014;15(1):23–34.
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet (London, England). 2015;385(9972):977–1010.
- Armstrong DK, Bundy B, Wenzel L, for the Gynecologic Oncology Group, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34.
- Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100(1):27–32.
- Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016. 11:CD005340.
- De Bree E, Rosing H, Filis D, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15(4):1183–1192.
- Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7(1):18–28.
- Markman M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev Anticancer Therapy. 2001;1(1):142–148.
- El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia. 2004;6(2):117–127.
- Steuperaert M, Debbaut C, Segers P, et al. Modelling drug transport during intraperitoneal chemotherapy. Pleura Peritoneum. 2017;2(2):73–83.
- Cascales-Campos P, Gil J, Feliciangeli E, et al. HIPEC in ovarian cancer: treatment of a new era or is it the end of the pipeline? Gynecol Oncol. 2015;139(2):363–368.
- Halkia E, Spiliotis J. The role of cytoreductive surgery and HIPEC in epithelial ovarian cancer. J Buon. 2015;20 (Suppl 1):S12–S28.
- Roviello F, Pinto E, Corso G, et al. Safety and potential benefit of hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal carcinomatosis from primary or recurrent ovarian cancer. J Surg Oncol. 2010;102(6):663–670.
- Cascales-Campos PA, Gil J, Gil E, et al. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann Surg Oncol. 2014;21(7):2383–2389.
- Bhatt A, Glehen O. The role of cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in ovarian cancer: a review. Indian J Surg Oncol. 2016;7(2):188–197.
- Hotouras A, Desai D, Bhan C, et al. Heated IntraPEritoneal Chemotherapy (HIPEC) for patients with recurrent ovarian cancer: a systematic literature review. Int J Gynecol Cancer. 2016;26(4):661–670.
- Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. New Engl J Med. 2018;378:230–240.
- Huo YR, Richards A, Liauw W, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(12):1578–1589.
- Votanopoulos K, Ihemelandu C, Shen P, et al. A comparison of hematologic toxicity profiles after heated intraperitoneal chemotherapy with oxaliplatin and mitomycin C. J Surg Res. 2013;179(1):e133–e139.
- Wu CC, Chuang YT, Hsu YT, et al. Intra-peritoneal hyperthermia combining alpha-galactosylceramide in the treatment of ovarian cancer. PLoS One. 2013;8(7):e69336.
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20(1):395–425.
- Singh IS, Hasday JD. Fever, hyperthermia and the heat shock response. Int J Hyperthermia. 2013;29(5):423–435.
- Moloney TC, Hoban DB, Barry FP, et al. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012;21(6):904–909.
- Ellis GS, Carlson DE, Hester L, et al. G-CSF, but not corticosterone, mediates circulating neutrophilia induced by febrile-range hyperthermia. J Appl Physiol. 2005;98(5):1799–1804.
- Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: a new family of heat-shock proteins? Immunol Investig. 2005;34(3):381–398.
- Rice P, Martin E, He JR, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174(6):3676–3685.
- Tulapurkar ME, Hasday JD, Singh IS. Prolonged exposure to hyperthermic stress augments neutrophil recruitment to lung during the post-exposure recovery period. Int J Hyperthermia. 2011;27(7):717–725.
- Clark OA, Lyman G, Castro AA, et al. Colony stimulating factors for chemotherapy induced febrile neutropenia. Cochrane Database Syst Rev. 2003;10:Cd003039.
- Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460.
- De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–4937.
- Shen L, Smith JM, Shen Z, et al. Inhibition of human neutrophil degranulation by transforming growth factor-β1. Clin Exp Immunol. 2007;149:155–161.
- Allen SS, Mackie JT, Russell K, et al. Altered inflammatory responses following transforming growth factor-β neutralization in experimental guinea pig tuberculous pleurisy. Tuberculosis. 2008;88(5):430–436.
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194.
- Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521.
- Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2019. DOI:10.1038/s41577-019-0210-z
- Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol. 2012;3:63.
- Thomas E, Dudar R. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984;44:605–612.
- Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242–246.
- Coccolini F, Corbella D, Finazzi P, et al. Time course of cytokines, hemodynamic and metabolic parameters during hyperthermic intraperitoneal chemotherapy. Minerva Anestesiol. 2016;82(3):310–319.
- Boettcher S, Manz MG. Regulation of inflammation- and infection-driven hematopoiesis. Trends Immunol. 2017;38(5):345–357.
- Mulier S, Claes JP, Dierieck V, et al. Survival benefit of adding Hyperthermic IntraPEritoneal Chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: review of evidence. Curr Pharm Des. 2012;18(25):3793–3803.