Abstract
Objective
To compare the long-term outcomes of MWA as the first-line treatment for HCC in peribiliary versus non-peribiliary locations using propensity score matching analysis.
Methods
The study participants were recruited between April 2012 and October 2016. In total, 236 patients with HCC <5 cm who underwent ultrasonography-guided percutaneous MWA as the first-line treatment were enrolled. The patients were grouped into two according to tumor location: peribiliary (n = 74) and non-peribiliary (n = 162). The progression-free survival (PFS) and overall survival (OS) rates were compared before and after propensity score matching. Subgroup analyses were conducted for the peribiliary group according to the biliary grading.
Results
Propensity score matching yielded 63 matched pairs of patients. In the two matched groups, cumulative PFS rates were 29.0% and 14.0% in the peribiliary group, and 51.0% and 31.0% in the non-peribiliary group at 3 and 5 years, respectively. Corresponding OS rates were 51.0% and 49.0% in the peribiliary group, and 77.0% and 70.0% in the non-peribiliary group at 3 and 5 years, respectively. In addition, there were significant differences in major complication rates between the two groups (25.7% vs 8.0%; p < .001). In contrast to peribiliary HCCs adjacent to the second-degree branches of intrahepatic bile duct (67.1 ± 5.2 months), subgroup analysis indicated that the mean OS was significantly lower in peribiliary HCCs adjacent to the first-degree branches (51.2 ± 7.5 months) (p = .015).
Conclusion
The application of MWA for peribiliary HCC leads to a higher rate of complications and worse long-term tumor control than for non-peribiliary HCC.
The application of MWA for peribiliary HCC leads to a higher rate of complications than for non-peribiliary HCC.
The application of MWA for peribiliary HCC leads to worse long-term tumor control than for non-peribiliary HCC. Abbreviations: Hepatocellular carcinoma (HCC); microwave ablation (MWA); α-fetoprotein (α-FP); local tumor progression (LTP); intrahepatic distal recurrence (IDR); progression-free survival (PFS); overall survival (OS).
KEY POINTS
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors globally, and it is the third leading cause of cancer-related deaths [Citation1]. Liver transplantation has been successful as the alternative therapy in a field where drug development has been challenging. However, it is not without shortcomings owing to the scarcity of donor organs, high cost, and a longer waiting period for transplantation procedure. Surgical resection is generally regarded as the preferred treatment for patients with very early-stage HCC [Citation2]. Unfortunately, the majority of HCC patients have background chronic liver diseases, especially cirrhosis, and a substantial proportion of these patients are not eligible for surgical resection due to impaired liver functional reserve and/or multi-nodularity of the tumor [Citation3]. Ablation therapy such as radiofrequency ablation and microwave ablation (MWA) is generally considered as one of the most effective treatment modalities [Citation4], with imaging response and tumor recurrence rates similar to those of surgical resection [Citation5]. MWA has recently emerged as a new therapeutic strategy with the capability to perform larger and faster ablations that exceed the limitations of radiofrequency ablation [Citation6].
Thermal ablation has a major limitation; it does not achieve adequate control of HCCs at-risky locations. Concerning tumor location, previous studies have demonstrated the therapeutic efficacy of percutaneous ablation for HCCs adjacent to major hepatic vessels [Citation7], bile duct [Citation8], and subcapsular or subdiaphragmatic localization near the colon or gall bladder [Citation9,Citation10]. However, for peribiliary tumors, thermal ablation of these HCC locations is limited by two major problems. The first problem is whether a peribiliary location affects local tumor control rate because portal veins and intrahepatic bile ducts closely run parallel in the liver Glisson system. The blood flow drives thermal energy away from the targeted tissue and hence not able to obtain enough ablative margin [Citation11,Citation12]. The second problem is the suggested higher risk for major complications due to the thermal and puncture injury of the adjacent biliary structures [Citation13,Citation14].
Most intrahepatic small bile duct injury has been considered as minor complication without any effects on clinical outcomes [Citation15]. These bile duct changes after ablation are easily ignored in patients without either clinical or laboratory abnormalities [Citation16]. However, intrahepatic central bile duct injury may cause different degree of biliary complications and indirectly influences prognosis of patients. Severe bile duct dilatation after ablation has been significantly associated with survival and recurrence rate in patients [Citation17,Citation18]. Presently, no study has compared the therapeutic outcomes of peribiliary and non-peribiliary HCC. Only a few studies [Citation17–19] have addressed the efficacy and safety of ablation methods for peribiliary HCC. These studies, however, yielded conflicting results in terms of local and distant tumor progression and therapeutic outcomes. These controversies have not been addressed in a large, comparative study with long-term follow-up. Furthermore, no guidelines on the use of ablation for the treatment of peribiliary HCC have been proposed.
The aim of this study was to compare the long-term outcomes of MWA as a first-line treatment for HCC at a peribiliary or non-peribiliary location using a large retrospective cohort.
Methods
Patients
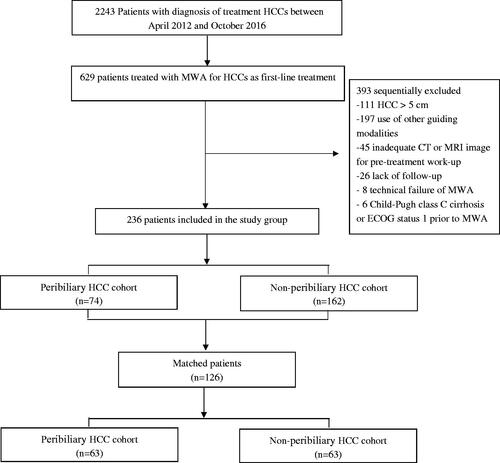
We performed a retrospective analysis of patient database at a single medical institution. This study was approved by the institutional review board, and written informed consents were obtained from all patients prior to MWA treatment. Between April 2012 and October 2016, 629 patients underwent MWA at our medical center. Based on a multidisciplinary panel discussion, the inclusion criteria for the study were as follows: (a) age 18 to 75 years, (b) a solitary HCC < = 5.0 cm in diameter, or multiple (three or fewer) HCC lesions, each < = 3.0 cm in diameter, (c) no radiologic evidence of invasion into major portal/hepatic venous branches and no extrahepatic metastases, (d) dynamic CT/MRI for pretreatment assessment, (e) normal coagulation status, (f) Child-Pugh grade A or B liver function with cirrhosis, and (g) an Eastern Cooperative Oncology Group performance status of 0. The patients were divided into two categories based on the presence of peritumoral biliary: peribiliary and non-peribiliary groups (). The criteria for the diagnosis of HCC in the medical center were based on the most recent guidelines at the time the MWA procedures were performed [Citation2,Citation20]. Histologic examination confirmed the presence of HCC in 90 patients (38.1%) based on US-guided biopsy, among which 31 did not meet the noninvasive diagnostic criteria. In the remaining 146 patients, HCC was diagnosed by imaging and according to the American Association for the Study of Liver Disease recommendations.
First-line treatment was defined as having no prior therapy for HCC at the time of diagnosis. In general, for patients with clinically significant portal hypertension and insufficient postoperative hepatic reserve with the indocyanine green test, MWA was recommended as an alternative therapeutic option [Citation21].
Definition of peribiliary HCC
Based on previous experimental and clinical studies, peribiliary HCC was defined as a tumor with its nearest margin ≤5 mm from the first- or second-degree branches of bile duct on axial or coronal CT and/or magnetic resonance images [Citation17–19]. Because portal veins and intrahepatic bile ducts run parallel in the liver Glisson system, it was assumed that tumors adjacent to portal veins were also adjacent to the corresponding bile ducts. All tumors were retrospectively classified by two radiologists with over 10 to 12 years of experience in abdominal imaging and were blinded to clinical outcomes. Any classification discrepancies between the two radiologists were resolved through inter-observer agreement analysis.
Microwave ablation protocols
MWA was performed using a single water-cooled microwave system (ECO-100C; Nanjing, Jiangsu, China) under real-time US guidance and a 25-cm cooled-shaft electrode probe (15-gauge) with a 1.5-cm expandable tip. The performing physician aimed at generating sufficient ablation zone to encompass the visible mass and at least a 5-mm ablation margin. Multiple overlapping ablations were carried out for the tumors. After administration of analgesia by an anesthesiologist, a 14-Ga antenna was first advanced into the target lesion to reach the deep margin of the tumor. A session ended if the deep region of the lesion was covered by hyperechoic regions on the US. Subsequently, the antenna was gradually withdrawn, and microwave emission restarted. The procedure was terminated when all portions of the index tumor were covered by hyperechoic regions on the US.
Follow-up
After discharge, patients in both groups underwent multiphase CT/MRI examination with liver protocol, chest radiography, and laboratory tests, including serum α-fetoprotein (α-FP), 1 month after initial treatment, every 3 months during the first 2 years, and every 4–6 months henceforth. For cases where extrahepatic recurrence was suspected on the basis of clinical symptoms or unexplained elevation of tumor marker levels, chest CT, brain MRI, whole-body bone scintigraphy, and positron emission tomography were performed. Where recurrent tumor was identified during follow-up, optimal second-line treatments such as ablative therapies, surgery, TACE, and liver transplantation were performed based on the recommendations of a multidisciplinary team for HCC treatment, the characteristics of the recurrent tumor, hepatic function, and the general condition of the patient.
Outcome assessment
Analysis of therapeutic outcomes was assessed on a patient-by-patient basis. Technical success, primary technique effectiveness, local tumor progression (LTP), intrahepatic distal recurrence (IDR), progression-free survival (PFS), overall survival (OS), and complications were compared between the two groups. Primary technical success was defined as complete ablation of the target tumor. LTP was defined as the appearance of a new tumor at the margin of the ablation zone on follow-up images [Citation22]. PFS was defined as the follow-up time without any event, such as local tumor progression, intrahepatic distal recurrence, extrahepatic recurrence, or death. OS rate was defined as the period from the first MWA procedure to either death or the last visit to the outpatient clinic. MWA-induced complications were assessed in accordance with the Society of Interventional Radiology guidelines [Citation23]. Bile duct injury was confirmed by the presence of upstream distal bile duct dilatation on imaging studies. Intrahepatic bile duct dilatation was confirmed by ultrasound or CT scan and was defined as an event related to the ablation, but not because of other mechanical processes. Mild dilatation was defined as dilatation limited to one hepatic subsegment, whereas severe dilatation was defined as dilatation in two or more subsegments.
Statistical analyses
Continuous variables were analyzed using the two-sample t-test if the assumption of normality was satisfied; otherwise, the Wilcoxon rank-sum test was used. Categorical variables were analyzed using the χ2 test. The effect of selection bias and confounding factors was reduced by calculating the propensity score using logistic regression and performing 1:1 patient matching [Citation24,Citation25]. The standardized mean difference was computed to assess the balance of variables used for matching and confirm whether the values were lower than 0.1. Variables associated with the selection of treatment, such as, age, sex, serum bilirubin and AFP level, platelet count, tumor size, and Child-Pugh class, were included for the generation of propensity scores. Binary logistic regression with selected variables was used to generate continuous propensity scores from 0 to 1. A one-to-one nearest-neighbor match without replacement between two groups was performed to select patients for subsequent analyses [Citation26]. The PFS and OS rate were estimated by the Kaplan–Meier method with the log-rank test, and the cumulative incidence curve for LTP and IDR was estimated using the Fine and Gray regression model for competing risk. Univariate and multivariate analysis of the entire data was performed using the Cox proportional hazards regression model for PFS and OS. The variables with p < .1 on univariate analysis were included in the final multivariate model. The statistical analyses were performed using the SPSS 10.0 statistical software (SPSS, Chicago, IL).
Results
Patients
The baseline characteristics of all patients (overall data, n = 236) are presented in . The median follow-up period was 43 months (range, 5–81 months) in the peribiliary group and 48 months (range, 6–90 months) in the non-peribiliary group (p = .683). The size of the index tumor was larger in the peribiliary group than in the non-peribiliary group (mean ± standard deviation, 3.3 cm ± 0.56 vs 3.1 cm ± 0.60, respectively; p = .08). The peribiliary group had a higher rate of liver cirrhosis (82.4% vs 66.0%, respectively; p = .01), the use of antiviral therapy during follow-up (51.3% vs 37.0%, respectively; p = .038), and the use of biopsy prior to treatment (41.9% vs 60.5%, respectively; p = .008), and they had more overlapping ablations when compared with the non-peribiliary group.
Table 1. Baseline characteristics of the study population.
Major complications
After 236 MWA treatments, no procedure-related deaths occurred in either group (). For delayed major complications, there was a significantly higher incidence of intrahepatic bile duct dilatation and biloma in the peribiliary group compared to the non-peribiliary group (24.3% vs 2.5%, respectively, p < .001). In the peribiliary group, 16 patients (16/74 21.6%) developed intrahepatic bile duct dilatation and two patients (2/74, 2.7%) developed both biloma and intrahepatic bile duct dilatation. In the non-peribiliary group, four patients (4/162 2.5%) developed intrahepatic bile duct dilatation and one patient (1/162, 0.1%) had bilio-pleural fistula. The number of patients who underwent biliary drainage was 10 and 2 patients between the peribiliary and non-peribiliary group.
Table 2. Major complications after MWA.
Comparison of therapeutic outcomes before propensity score matching
LTP and IDR. During follow-up, LTP occurred in 10 out of 74 patients (13.5%) in the peribiliary group and in 28 out of 162 patients (17.3%) in the non-peribiliary group. The cumulative LTP rates at 1, 3, and 5 years were 3.0%, 11.0% and 17.0%, respectively, for the peribiliary group, and 4.0%, 16.0%, and 21%, respectively, for the non-peribiliary group (p = .439). IDR was identified in 42 patients (56.8%) in the peribiliary group and 52 patients (32.1%) in the non-peribiliary group. The 1-, 3-, and 5-year cumulative IDR rates were 30%, 60%, and 73% in the peribiliary group and 10.0%, 38.0%, and 42% in the non-peribiliary group, respectively (p < .001).
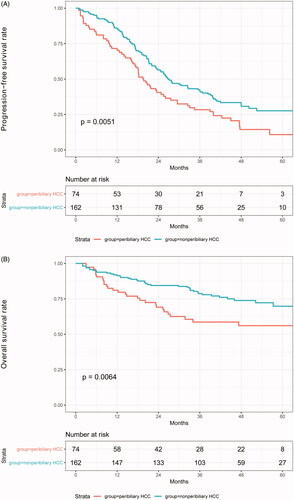
PFS and OS. During follow-up, 29 (39%) out of 74 patients in the peribiliary group and 40 (24.5%) out of 163 patients in the non-peribiliary group died. The 1-, 3-, and 5-year PFS rates were 69%, 28%, and 11% in the peribiliary group and 83%, 40%, and 28% in the non-peribiliary group, respectively (p = .005) (). The 1-, 3- and 5-year OS rates were 80%, 59%, and 56% in the peribiliary group and 91%, 78%, and 70% in the non-peribiliary group, respectively (p = .005) ().
Comparison of therapeutic outcomes after propensity score matching
Sixty-three patients were matched in each group, and the baseline characteristics were well-balanced between the two groups (). Based on the matched data, the cumulative LTP rates at 1, 3, and 5 years were 3%, 11%, and 17%, respectively, in the peribiliary group and 3%, 16%, and 20% in the non-peribiliary group, respectively (p = .524). The cumulative IDR rates at 1, 3, and 5 years were 29%, 62%, and 71% for the peribiliary group and 10%, 36%, and 44% for the non-peribiliary group, respectively (p = .002). The PFS rates at 1, 3, and 5-years were 73%, 29%, and 14% in the peribiliary group and 90%, 51%, and 31% in the non-peribiliary group, respectively (p = .006) (). The estimated OS rates at 1, 3, and 5-years were 79%, 51%, and 49% in the peribiliary group and 90%, 77%, and 70% in the non-peribiliary group, respectively (p = .01) ().
Figure 3. Types of recurrence and survival curves in matched patients from the two groups. (A) Progression-free survival rates and (B) overall survival rates.
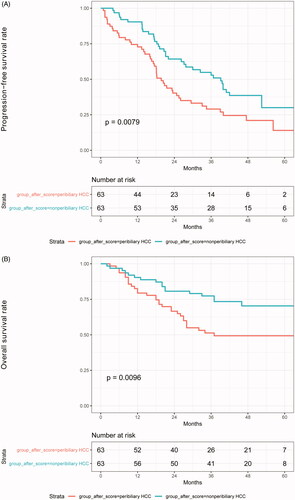
Table 3. Baseline characteristics of study patients after propensity score analysis.
Analysis of risk factors for therapeutic outcomes
Multivariate analysis of all patients (n = 236) revealed that peribiliary HCC (hazard ratio [HR], 2.563; 95% CI, 1.880–3.496; p < .001) and Child-Pugh class B (HR, 1.536; 95% CI, 1.065–2.215; p = .022) were significantly associated with poor PFS (). For OS, peribiliary HCC (HR, 2.347; 95% CI, 1.256–2.570; p < .001), Child-Pugh class B (HR, 2.142; 95% CI, 1.021–1.987; p < .001), and tumor size (HR, 1.382; 95% CI, 0.886–1.367; p = .011) were found to be independent poor prognostic factors for OS in small HCC patients ().
Table 4. Risk factor analysis for recurrence-free survival after MWA.
Table 5. Risk factor analysis for overall survival after MWA.
Subgroup analysis of the peribiliary group
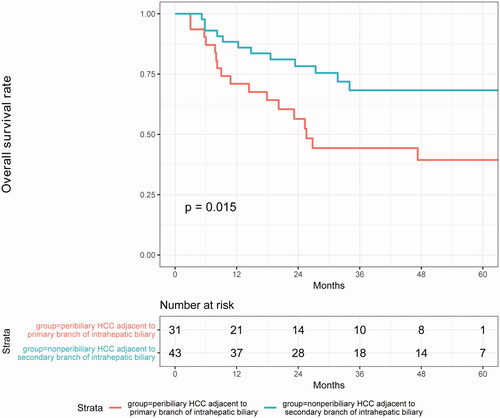
To examine the relationship between tumor location and the incidence of severe bile duct dilatation after MWA, 74 patients in the peribiliary group were analyzed. We found that 12 patients had mild dilatation (16.2%) and 6 patients had severe dilatation (8.1%). Among the six patients with severe dilatation, five had the tumor adjacent to the first branch of intrahepatic biliary and one patient had a tumor adjacent to the second subsegmental branches.
In the peribiliary group, patients were divided into two groups according to the relationship between peribiliary tumor location and intrahepatic bile duct system grading. The mean survival time of patients with tumor adjacent to the first branch of intrahepatic biliary was 51.2 ± 7.5 months. Compared with patients with tumor adjacent to the second branch (67.1 ± 5.2 months), the mean OS of patients with tumor adjacent to the first branch of intrahepatic biliary was significantly decreased (p = .015) ().
Discussion
With an adequate follow-up duration, this study indicates that MWA for peribiliary HCC leads to a higher rate of complications and worse long-term tumor control than for non-peribiliary HCC. In addition, multivariate analysis showed that tumor location was an independent prognostic factor for PFS and OS in HCC patients. First, MWA uses thermal energy generated by an electric current to kill cancer cells. However, this thermal energy may affect intra- and extrahepatic tissues, resulting in complications [Citation27]. The cooling effect of circulating fluid in vascular is thought to be relatively immune to the thermal injury; however, the velocity of bile juice in the bile duct is much slower. When the index tumor is near the intrahepatic bile duct, thermal injury may occur during ablation [Citation28]. Secondly, a portion of the surrounding liver is ablated intentionally to achieve a sufficient safety margin. Postoperative hyperemia and edema often occur around the ablation site after thermal ablation, and this may lead to compression of the adjacent bile duct in the short term [Citation8]. Thirdly, during the MWA, mechanical puncture may also cause intrahepatic bile duct injury [Citation14]. The incidence rate of intrahepatic bile duct injury has been reported to range from 0.1% to 12% per puncture session [Citation29,Citation30]. The above factors increase the risk of biliary complications such as intrahepatic bile duct dilatation, biliary tract stricture, bilomas, and hepatic infarction. To guarantee safety and efficacy, precise electrode placement near the biliary requires accurate adjustment to avoid puncturing the biliary or vessels. To avoid thermal injury, the ablative strategy should be modified to a low power or a radiofrequency electrode. It is beneficial to perform transarterial chemoembolization or percutaneous ethanol injection because application of these procedures prior to the ablation decreases tumor size and makes the ablation safer with a higher margin.
Thermal injury to the biliary tract after ablation has been widely reported (0.1%–12%) [Citation13,Citation31]. In the current study, thermal ablation was well tolerated by many HCC patients, with a morbidity rate of 9.3% for major biliary tract complications. The incidence of biliary tract complications adjacent to intrahepatic bile duct after MWA reached 24% in the group of tumors adjacent to the bile duct. These results are comparable with those reported in the medical literature. However, tumor diameter was 5 cm in this study. Biliary tract complications are among the common outcomes of percutaneous ablation. For asymptomatic biliary branch expansion, no special treatment is required and regular follow-up is sufficient. In cases of obstructive jaundice caused by severe biliary expansion, especially if the tumor is located in the hilum of the liver, the bile duct should be avoided as much as possible during ablation. Percutaneous hepatic biliary drainage occurs in cases of postoperative obstructive jaundice. If the jaundice index decreases, further biliary stent implantation may be performed. Cholelioma is another common biliary tract complication. For clinically symptomatic cholelioma, suction and drainage should be conducted under the guidance of imaging equipment.
In this study, the PFS incidence rates were significantly different between the two groups before and after the propensity score analysis. Notably, PFS and IDR were significantly different between the two groups, whereas LTP was not different between the two groups. Each type of recurrence has a distinct mechanism of pathogenesis. The recurrence of peribiliary tumors can be associated with the fact that portal veins and intrahepatic bile ducts run parallel closely in the liver Glisson system. The heat-sink effect mediating convective heat loss into hepatic vessels adjacent to the tumor would theoretically make the ablation of perivascular tumor tissue insufficient leading to a decrease in local tumor control [Citation32]. However, previous studies found that long-term therapeutic outcomes of ablation for small HCC were equivalent between the perivascular and non-perivascular groups in terms of local tumor progression, disease-free survival, and overall survival. These findings are consistent with those of the present research. Concerning IDR, tumors associated with bile duct injury on ablation are likely to be invasive, namely, microscopic invasion into Glisson’s capsule, portal vein, or bile duct, which may be accompanied by undetectable intrahepatic or extrahepatic metastases at the time of MWA [Citation33]. Patients with bile duct dilatation showed slow progressive impairment in hepatic function due to bile duct injury and the deterioration of liver function, and this is likely to worsen in the long term. These findings are consistent with those obtained previous showing that the bile duct dilatation is a high-risk factor for tumor recurrence after MWA.
In this study, the OS rates were different between the two groups regardless of the propensity score analysis. The OS rate is affected by bile duct dilatation and liver function. Kondo et al. [Citation18] conducted a single-center study to evaluate the effects of thermal injuries on intrahepatic bile ducts between patients with and without visible bile duct dilatation after ablation. They assessed the survival rate and concluded that bile duct dilatation affecting two or more subsegments should be regarded as a complication modifying the prognosis. A study in Japan evaluated the impact of ablation-induced Glisson’s capsule-associated complications on liver function and prognosis of HCC patients [Citation34]. The study found that early-stage HCC patients with ablation-induced Glisson’s capsule-associated complications, including major intrahepatic bile duct dilatation, may have a poor prognosis. A possible explanation for these results is that patients with severe dilatation are characterized by impaired liver function because of bile duct injury itself and a higher HCC recurrence rate.
In the multivariate analysis of all patients, Child-Pugh class B was associated with poor RFS and OS. The serum total bilirubin and lower albumin were higher in peribiliary lesions group compared with non-peribiliary lesions. Previous studies showed that higher serum total bilirubin and lower albumin, which reflect severe liver dysfunction, are linked to higher recurrence rates and worse long-term outcomes in patients with early HCC [Citation18,Citation19,Citation34]. Herein, we found that Child-Pugh class B is a significant predictor for poor overall survival and a high rate of recurrence which is in line with findings of previous studies [Citation35,Citation36]. The severity of the underlying liver disease may also be a risk factor for the development and recurrence of HCC, and thus reinforces the role of liver function in hepatocarcinogenesis [Citation3]. Likewise, it was previously reported that tumor location is a predictive covariate related to recurrence, which correlated with worse prognosis of patients. However, these results were not comparable to non-peribiliary tumor lesions. In agreement with previous reports, we found that tumor location adjacent to peribiliary is an independent predictor of the prognosis of HCC patients. These findings provide more comprehensive and powerful comparative results. However, further prospective randomized controlled trials are required to clarify the relative efficacy of MWA for peribiliary and non-peribiliary groups.
This study had several limitations. First, it was performed using a retrospective approach with a limited sample size in a single center. Therefore, it is likely to have selection and indication bias. To overcome this, a large cohort multicenter clinical trial should be performed. Secondly, both CT and MRI were used to classify the tumor location relative to other vessels. The difference between CT and MRI images may slightly influence the image outcomes. Thirdly, the portal veins and intrahepatic bile ducts run parallel in the liver Glisson system, and thus, the influence of blood vessel circulation after tumor thermal ablation cannot be completely ignored. Recently, Kang et al. compared the long-term therapeutic outcomes of thermal ablation for perivascular and non-perivascular HCC [Citation28]. The results indicated the long-term outcomes of ablation were similar between perivascular and non-perivascular HCC. Finally, although the current study attempted to accurately define peribiliary HCC logically, the definition was consistent with that used in previous reports [Citation14,Citation34] and thus ignored the true positional relationship between the tumor and the bile ducts.
Conclusion
In conclusion, HCC patients with peribiliary type undergoing MWA showed worse long-term tumor control and OS rate compared to those with non-peribiliary HCC, and in particular, those with tumors adjacent to the primary branch of intrahepatic biliary. Therefore, clinicians should consider the tumor location when balancing the risk benefit of MWA as a first-line treatment for HCCs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108.
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases: management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054–1060.
- Poggi G, Montagna B, DI Cesare P, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res. 2013;33:1221–1227.
- Metcalfe MS, Mullin EJ, Texler M, et al. The safety and efficacy of radiofrequency and electrolytic ablation created adjacent to large hepatic veins in a porcine model. Eur J Surg Oncol. 2007;33:662–667.
- Kim SH, Lim HK, Choi D, et al. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: frequency and clinical significance. AJR Am J Roentgenol. 2004;183:1611–1617.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108.
- Kondo Y, Yoshida H, Shiina S, et al. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006;93:1277–1282.
- Hori T, Nagata K, Hasuike S, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981.
- Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262.
- Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458.
- Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222.
- Dromain C, de Baere T, Elias D, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology. 2002;223:255–262.
- Rhim H, Choi D, Kim YS, et al. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010;75:253–258.
- Lin MX, Ye JY, Tian WS, et al. Risk factors for bile duct injury after percutaneous thermal ablation of malignant liver tumors: a retrospective case–control study. Dig Dis Sci. 2017;62:1086–1094.
- Kondo Y, Shiina S, Tateishi R, et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: impact on patient’s prognosis. Liver Int. 2011;31:197–205.
- Van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus MWA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39:1438–1446.
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430.
- Cucchetti A, Cescon M, Trevisani F, et al. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol. 2012;18:6398–6408.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739.
- Rhim H, Dodd GD, 3rd, Chintapalli KN, et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. RadioGraphics. 2004;24:41–52.
- McDonald RJ, McDonald JS, Kallmes DF, et al. Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology. 2013;269:640–645.
- Pearl J. Causality: models, reasoning, and inference. 2nd ed. Cambridge (UK): Cambridge University Press; 2009.
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceut Statist. 2011;10:150–161.
- Thanos L, Mylona S, Galani P, et al. Overcoming the heat-sink phenomenon: successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn Interv Radiol. 2008;14:51–56.
- Kang TW, Lim HK, Lee MW, et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270:888–899.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451.
- Shibata T, Yamamoto Y, Yamamoto N, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol. 2003;14:1535–1542.
- Ohnishi T, Yasuda I, Nishigaki Y, et al. Intraductal chilled saline perfusion to prevent bile duct injury during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e410–e415.
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95:996–1004.
- Dominique E, El Otmany A, Goharin A, et al. Intraductal cooling of the main bile ducts during intraoperative radiofrequency ablation. J Surg Oncol. 2001;76:297–300.
- Wakamatsu T, Ogasawara S, Chiba T, et al. Impact of radiofrequency ablation-induced Glisson’s capsule-associated complications in patients with hepatocellular carcinoma. PLoS One. 2017;12:e0170153.
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967.
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692.