Abstract
Purpose
To evaluate the safety and efficacy of ultrasound-guided RFA for the treatment of papillary thyroid microcarcinoma (PTMC).
Materials and methods
The data of 204 nodules from 198 PTMC patients who were treated using RFA were retrospectively reviewed in this study. Demographic variables, complication details and CEUS results in different time points were collected. The volumes and volume reduction rate (VRR) of the ablated area under CEUS at different follow-up time points were calculated and compared.
Results
All the patients were successfully treated without major complication. Mild complications included cervical discomfort in three cases, postoperative cervical pain in one case, and transient hoarse voice in five cases. The volume of the ablated area in the 1st, 3rd, 6th, 12th, 18th and 24th month postoperatively were 241.7 ± 298.3mm3, 89.8 ± 147.2 mm3, 37.6 ± 87.2 mm3, 13.6 ± 59.8 mm3, 2.4 ± 14.4 mm3, and 0.2 ± 2.0 mm3 respectively, with a statistically significant decrease (F = 138.1, p = .000), and the VRR in those time points were 73.9 ± 13.7%, 90.5 ± 8.2%, 96.1 ± 5.9%, 98.8 ± 3.2%, 99.6 ± 1.9% and 99.8 ± 1.0% respectively, with a statistically significant decrease (F = 695.3, p = .000).
Conclusions
US-guided RFA is safe and effective for PTMC, with a good oncological outcome and VRR. Further randomized controlled prospective trials are still needed to compare the value of RFA and surgery.
Introduction
Papillary thyroid carcinoma (PTC), which is the most common pathological type of primary thyroid cancer and accounted for about 85% of the tumors, showed an increasing incidence in recent years [Citation1]. PTC is generally asymptomatic, well differentiated, less invasive, and unlikely to have regional lymphatic metastasis, thus the majority of patients tended to have good prognosis [Citation2].
Surgery remained to be the dominant approach for PTC, which radically resect both thyroid glands and regional lymph node [Citation3,Citation4]. However, considering the good prognosis, the necessity of extensive radical resection is questioned for a certain of patients because of the risk of surgical complications and impair of postoperative life quality [Citation5]. This promoted the raise of the idea of individualized treatment for PTC [Citation6], which mainly emphasized on reducing surgical extension, protecting normal gland tissues, decreasing lymphatic dissection, and lessening frequency of I-131 radiotherapy.
As an alternative option to surgery, image guided ablation has the advantage of minimal invasiveness, and had been successfully performed using different techniques in the past few years, including ethanol ablation [Citation7], radiofrequency ablation (RFA) [Citation8], microwave ablation [Citation9], laser ablation [Citation10] and so on. Among those techniques, RFA was most commonly applied and had been proved to be safe and effective for the treatment of benign thyroid nodules [Citation8], and a recently published guideline based on Delphi method suggested that thermal ablation may be proposed as a first line treatment for solid nonfunctioning thyroid nodules that are benign at cytology when they become symptomatic [Citation11]. Very recently, it was also shown that RFA could be an effective alternative to repeat surgery in the treatment of locally recurrent thyroid cancers, with a significantly decreased complication rate [Citation12].
Currently, indications of RFA recommended by several guidelines are generally benign thyroid nodules for symptomatic or cosmetic reason, and the recommendations for malignant diseases were restricted to palliative treatment for recurrent thyroid cancers or metastatic lymph nodes when surgery is contraindicated or declined [Citation13–15]. However, several previous studies also showed that image-guided RFA was also safe and effective in the treatment of primary thyroid microcarcinoma (PTMC) [Citation16,Citation17], and in 2017 RFA guideline of Korean society of thyroid radiology, it was accepted that RFA can be used for patients with primary thyroid cancer who refuse surgery or who cannot undergo an operation [Citation18].
Previously, we reported a study showing that RFA was safe and effective for the treatment of low-risk PTMC [Citation19]. However, in that preliminary study, patients with aggressive histological PTMC were ruled out, who might also benefit from RFA. Besides, the mean follow-up time were only 7.8 ± 2.9 months, which was still relatively short. Thus, in the present study, we retrospectively reviewed the results of RFA for patients with PTMC, to verify whether RFA is effective for treatment of PTMC (not only for low-risk patients).
Patients and methods
Patients and study design
The clinical results of all the patients with pathologically proved PTMC who were treated with RFA from February 2014 to August 2016 were retrospectively reviewed in this study. Medical records were reviewed for demographic variables, complication details and CEUS results in different time points. Approval for the study was obtained from Institutional Review Board of Chinese PLA General Hospital, with the approval ID of S2019-211-01. All the patients signed written consent before RFA stating that they were well informed of the technical details of RFA, the priority of treatments, and the possibility of their clinical data being used in further retrospective researches. To avoid misunderstanding or confusion, as well as to facilitate future secondary data analysis, the terminologies in our study strictly followed the standardization of terminology and reporting criteria published in 2019 [Citation20].
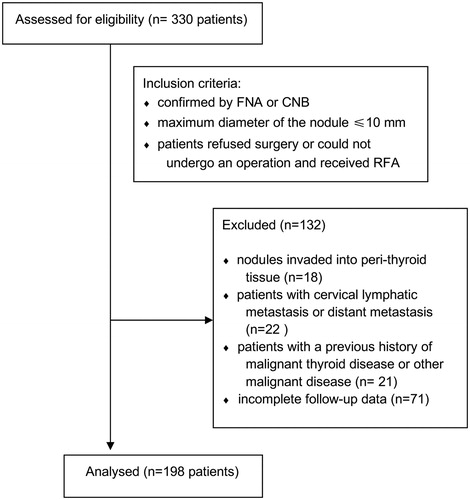
Inclusion criteria were: (1) PTC was confirmed by FNA or CNB; (2) The maximum diameter of the nodule ≤10 mm; (3) Patients who refused surgery or could not undergo an operation, and received RFA. Exclusion criteria were as followed: (1) nodules invaded into peri-thyroid tissue; (2) patients with cervical lymphatic metastasis or distant metastasis; (3) patients with a previous history of malignant thyroid disease or other malignant disease; (4) incomplete follow-up data. The CONSORT flow diagram was shown in .
Instruments and methods
The instruments and methods were generally consistent with those in our previous work. Briefly, RFA was performed using a 18 G bipolar radiofrequency electrode, with a 9 mm or 15 mm active component (CelonProSurge and Celon LabPOWER, Olympus Surgical Technologies Europe, Hamburg, Germany). Ultrasound-guided biopsy and RFA were performed using Siemens 512 Ultrasound System with 6L3 linear array probe. CEUS and conventional ultrasound were performed using Siemens 512 Ultrasound System with 15L8W linear array probe, or Philips iu22 Ultrasound System with L12-5 linear array probe, or Mindray M9 Ultrasound System with L12-4 linear array probe.
Ultrasound contrast agent was Sulfur hexafluoride with a phospholipid shell (SonoVue®, Bracco, Milan, Italy), which was diluted using normal saline (0.9%, 5 ml), and then administered as a bolus injection (2.4 ml) followed by a flush of normal saline (0.9%, 5 ml). The mechanical index of ultrasound system for contrast-enhanced ultrasound was 0.19–0.24.
All the RFA procedures were performed by sonographers with more than 20 years’ experience of thyroid ultrasonography and intervention ultrasound. A supine position was taken with neck fully exposed, and local anesthesia was performed by injection of 1% lidocaine hydrochloride in subcutaneous and peri-thyroid space. If the distance between the lesion and surrounding critical anatomical structures (such as trachea, major vessel, esophagus, recurrent laryngeal nerve and so on) was shorter than 5 mm, hydrodissection was performed with injection of normal saline to prevent heat injury.
Electrode was positioned into the deepest conceptual ablation unit of a thyroid nodule under the guidance of ultrasound, and the output RF power was 3 W-5W. Moving shot technique was used, with the location of the RF electrode tip continuously monitored via real-time US, and the procedure was terminated when all the conceptual units were covered by transient hyperechoic areas.
Evaluation before and after ablation
Cervical CEUS examination was performed preoperatively, immediately after RFA, 1st month postoperatively, 3rd month postoperatively, 6th month postoperatively, and one time every 6 months thereafter. Ultrasound-guided biopsy was performed at central and margin of ablation area, as well as peripheral thyroid parenchyma in the 3rd month postoperatively. Location, number, size, inner echo (calcifications) and vascularity of nodules or ablation area, state of cervical lymph node, and extension of non-enhanced area under CEUS were carefully recorded.
Nodule volume was calculated using the formula of ‘V = length × width × depth × 0.524’, and VRR of the nodule was calculated using the formula of ‘(V1–V2)/V1 × 100%’ (V1 represents initial volume and V2 represents final volume [Citation21]).
Treatment success was defined as 1, the nodule and at least 3 mm surrounding tissue was ablated intraoperatively, which presented as nonenhancement area under CEUS immediately after RFA (additional procedure were performed if insufficient extension of nonenhancement area were revealed) [Citation22]; 2, no recurrence of tumor or cervical lymph node metastasis were observed during follow-up.
Statistical analysis
SPSS 17.0 statistic software was used for data analysis. Quantitative data were expressed using mean ± standard deviation (m ± SD). Analysis of variance for repeated measurement of general linear model was used to analyze the differences among volume and VRR at different follow-up time points. Student’s t-test for compared data were used to compare volume and VRR at an exact time point and its next time point. Difference was considered to be significant when p < .05.
Results
General characteristics
A total of 198 patients were enrolled in the present study, including 141 females and 57 males, with an average age of 42.5 ± 9.5 (range from 22 to 65). A total of 204 nodules were treated for those patients, with an average maximum diameter of 6.34 ± 1.8 mm (range from 3 to 9.4 mm) and an average volume of 99.42 ± 84 mm3 (range from 6.28 to 398.5 mm3). Twenty-two nodules were located in the isthmus, 101 nodules were in the right lobe, and 81 nodules were in the left lobe. Characteristics of the nodules under US and CEUS were shown in .
Table 1. Characteristics of nodules proved to be PTMC under US or CEUS.
Technical details and complications
All the 204 nodules were successfully treated. RFA was performed with 3-W power for 195 nodules and with 5 W power for nine patients. The average ablation time was (257.5 ± 131.4) s (range from 45 to 605 s) and the average deposited energy was (0.91 ± 0.59) kJ (range from 0.13 to 3.53 kJ). Three patients complained of cervical burning feel during the ablation process, and the symptom was relieved a few seconds after ablation. One patient complained of postoperative cervical pain, which was tolerable with oral painkillers and was relieved 2 days later. Five patients suffered from a transient hoarse voice and all of them recovered within 1 month. None of the patients suffered from major complications.
Follow-up and outcomes
The average follow-up duration was (25.9 ± 4.5) m (range from 24 m to 54 m). The data of one patient was also included in our previous study [Citation19], but we only reported the follow-up result in 6 months postoperatively in that study, while in the present study, the follow-up time of this case was 54 months. One patient received a second ablation due to recurrence during follow-up, and the lesion was completely absorbed after two years. No other recurrence or lymph node metastasis was found during follow-up.
CEUS at different follow-up time points showed a nonenhanced area of the ablated zone, which gradually shrank. There was a significant difference among volume and VRR of ablated area under CEUS in different follow-up time points (F = 138.1, p = .000, and F = 695.3, p = .000 respectively), which significantly decreased during follow-up (p < .05). Detailed volume of ablated area and VRR in different follow-up time points were showed in . Complete images of the same case, including preoperative imaging, intraoperative imaging, and post-operative imaging were showed in , Citation3, and Citation4 ( showed the preoperative and postoperative pathology result of the case, showed the preoperative imaging and CEUS imaging immediately after RFA, showed the CEUS images during follow-up).
Figure 2. The preoperative and postoperative pathological images of the patient. (A) PTC was diagnosed by preoperative biopsy pathology; (B) degenerative changes were revealed in postoperative biopsy pathology.
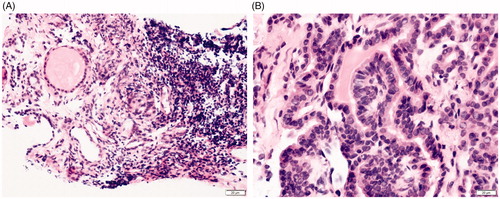
Figure 3. Preoperative imaging and CEUS imaging immediately after RFA. (A) Preoperative CEUS imaging showing the hypoechoic nodule was low-enhanced; (B) CEUS imaging immediately after RFA revealed a non-enhanced area of 1.6 cm × 1.4 cm ×1.2 cm in the ablated area.
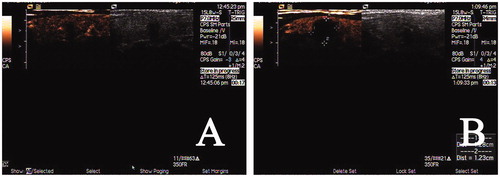
Figure 4. Post-operative CEUS images during follow-up. (A) the non-enhanced area was 1.4cmx1.3cmx1.1cm 1 month after RFA; (B) the non-enhanced area was 0.9cmx0.9cmx0.8cm 3 months after RFA; (C) the non-enhanced area was 0.2cmx0.3cmx0.3cm 18 months after RFA (VRR was 99%).
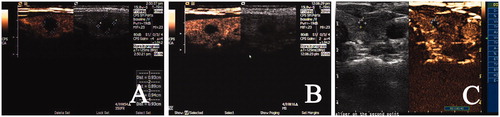
Table 2. Changes of volume and VRR after RFA in the follow-ups.
Discussion
The main results of the present study are the good outcome of 198 patients with PTMC treated by RFA, with satisfying complication rate. Of all the cases, recurrence was found in only one patient during the (25.9 ± 4.5) m follow-up, and almost all the nodules were absorbed eventually. The VRR was 90.5 ± 8.2% after 3 months, 96.1 ± 5.9% after 6 months, and was 99.6 ± 1.9% after 18 months. Although VRR continued to decrease after 18 months (99.8 ± 1.0% in 24 months after RFA, p = .005), the difference is not clinically significant.
Image-guided thermal ablation had been applied for the treatment of tumors for several decades, and its efficiency for the treatment of benign thyroid nodules was widely accepted. Numerous studies had shown that RFA was safe for the treatment of benign thyroid nodules and recurrent thyroid cancers, which can effectively reduce the volume of nodules and relieve the symptoms [Citation8,Citation14]. However, its application for PTC remained controversial. Early guidelines did not recommend RFA for PTC because of lacking of evidence [Citation13–15]. In recent years, several studies showed that thermal was also effective for PTC [Citation16,Citation19,Citation23,Citation24], and in 2017, thyroid RFA guideline of Korean society of thyroid radiology, RFA was recommended as an alternative of surgery for patients who refuse surgery or who cannot undergo an operation [Citation18]. In the present study, it was also showed that RFA can effectively eliminate PTMC, with only one case of recurrence in a (25.9 ± 4.5) m follow-up. The VRR was also satisfying, with the majority of the ablated area disappeared in two years.
Satisfying results of image-guided thermal ablation for primary thyroid cancer were also reported using other means, like laser and microwave. In 2000, Pacella et al. [Citation25] used percutaneous laser photocoagulation to treat recurrent thyroid cancer and functional adenoma. It was suggested by Valcavi et al. [Citation26] that laser ablation has the best effect on small and medium-sized nodules (maximum length of 40 ∼ 50 mm, maximum width of 25 ∼ 35 mm and a maximum height of 15 ∼ 20 mm). Microwave thermal ablation treatment for PTMC was studied in 21 patients [Citation17]. After 11 months of follow-up, no recurrence and metastasis were observed in all cases and the nodule reduction rate was 90%. However, whether there is a difference among the efficiency of different techniques remained unclear, which could be investigated in further study.
In the present study, CEUS was used for the evaluation of the ablated area. After RFA, the tissue of the ablation area would undergo heat coagulation necrosis, resulting in embolism of microcirculation and loss of blood supply. Thus, CEUS could accurately evaluate the extension of ablated area and the efficacy of the treatment, which was also stated in previous studies [Citation27,Citation28]. Besides, CEUS could also be used in locating the actual recurrent area in an enlarged nodule during follow-up and was valuable for an accurate second ablation.
The advantage of minimal invasiveness of image-guided thermal ablation had been reported previously and widely accepted [Citation16,Citation19,Citation23,Citation24]. Some authors even thought that images-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis [Citation29]. In recent years, improved imaging could detect PTC in their very initial stage [Citation1], which was thought to be overdiagnosis because the medical treatment might not be beneficial [Citation2]. As a minimally invasive treatment, image-guided thermal ablation might be a good solution for the above situation [Citation29]. In our study, none of the patients had severe complications. Mild complications included cervical discomfort in three cases, postoperative cervical pain in one case, and transient hoarse voice in five cases. The satisfying results also suggested the important value of RFA in the treatment of PTMC, which reduces the aggressiveness of treatment and its unavoidable complications.
In the present study, we also showed the efficiency of RFA in the treatment of multiple malignant nodules. There were six patients who had two nodules proved to be PTMC at the same time, and all the 12 nodules were successfully treated. According to our experience, sufficient preoperative examination was extremely important for the treatment of those patients, which helped to identify and radically treat all the tumors. Besides, the detailed preoperative examination was helpful for precise planning of the ablation routes, which is important because the high-echoic area during ablation shielded structures beneath, and made it impossible to evaluate the sufficiency of RFA in the deeper area.
Another interesting result of the present study is the dynamic change of calcifications in the nodules. In our study, 96 (47.1%) PTMC nodules containing calcifications, and after RFA, 93 (45.6%) was absorbed within 12 months. After 24 months, no calcification was identified in any of the ablated nodules. In a previous study of low power microwave ablation for PTMC, the calcification was not fully absorbed in one patient, which might be either a result of selection bias or different techniques [Citation30].
We have to admit that there are still some limitations to our study. First, as a retrospective study, sample bias is inevitable. Besides, considering the good prognosis of PTC, a 2-year follow-up might still be not long enough. Thus, randomized controlled prospective trials are still needed to compare the value of RFA and surgery in the future. However, considering the good outcomes of the cases, it can still be concluded that US-guided RFA is safe and effective for PTMC.
Acknowledgments
All the staff in Chinese PLA General Hospital who participated in the treatment are acknowledged for their work, and Dr. Tang Wenbo is acknowledged for his help in language editing. We would also like to express our gratefulness for all the patients who agreed to participate in the present study.
Disclosure statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted. All the authors have no conflicts of interest or financial ties to disclose.
Additional information
Funding
References
- Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347(aug27 4):f4706–f4706.
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30.
- Gharib H, Papini E, Garber JR, Nodules AAATFoT, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules–2016 Update. Endocr Pract. 2016;22(Supplement 1):1–639.
- Haugen BR. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123(3):372–381.
- Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–276.
- Fallahi P, Ferrari SM, Mazzi V, et al. Personalization of targeted therapy in advanced thyroid cancer. Cg. 2014;15(3):190–202.
- Jeong SY, Baek JH, Choi YJ, et al. Ethanol and thermal ablation for malignant thyroid tumours. Int J Hyperthermia. 2017; 33(8):938–945.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017; 33(8):920–930.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018; 34(5):653–659.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography (Seoul, Korea). 2019; 38(1):25–36.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019; 36(1):375– 382.
- Choi Y, Jung SL, Bae JS, Lee SH, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019; 36(1):358–367.
- Na DG, Korean Society of Thyroid Radiology (KSThR), Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13(2):117–125.
- Dietrich CF, Muller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018; 44(1):14–36.
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015; 18(4):423–430.
- Valcavi R, Piana S, Bortolan GS, et al. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013; 23(12):1578–1582.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014; 30(2):150–157.
- Kim JH, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017. Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018; 19(4):632–655.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR. 2010;194(4):1137–1142.
- Guang Y, Luo Y, Zhang Y, et al. Efficacy and safety of percutaneous ultrasound guided radiofrequency ablation for treating cervical metastatic lymph nodes from papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(8):1555–1562.
- Kim JH, Baek JH, Sung JY, Min HS, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33(2):212–219.
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21(8):917–920.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217(3):673–677.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Solbiati L, Ierace T, Tonolini M, et al. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51(Suppl):S19–S23.
- Kim JH, Baek JH, Lim HK, et al. Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography (Seoul, Korea). 2019;38(2):125–134.
- Mauri G, Sconfienza LM. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int J Hyperthermia. 2017;33(4):489–490.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.