Abstract
Objectives
To prospectively compare the effectiveness and safety of percutaneous microwave ablation (PMWA) and ultrasound-guided radiofrequency ablation (USgRFA) for treating symptomatic uterine adenomyosis.
Methods
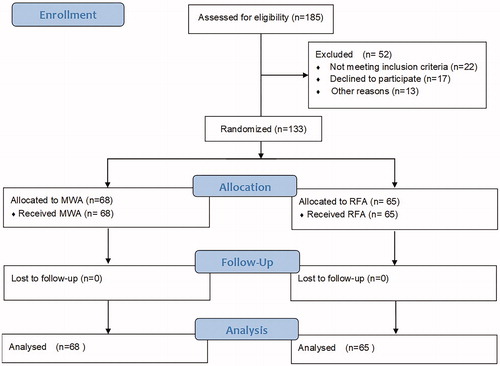
One hundred and thirty-three women with symptomatic uterine adenomyosis who met the inclusion criteria were enrolled in our study from October 2015 to October 2017. Sixty-eight patients underwent PMWA, and sixty-five patients underwent USgRFA. All patients were followed up for 12 months. Assessment endpoints included treatment time, percentage ablation, percentage uterine regression, symptom severity scores (SSSs), dysmenorrhea scores and adverse events.
Results
The mean age of the patients in our study was 39.4 ± 4.2 years (range, 35–50 years), and the median volume of uterine adenomyosis was 124.3 cm3 (range, 28.7–374.5 cm3). The mean ablation time was 16.3 ± 4.9 min (range, 5–23 min) in the MWA group, which was demonstrably superior to that of the RFA group, which was 37.5 ± 6.2 min (range, 5–39 min). The mean percentages of ablation of uterine adenomyosis were 79.7 ± 15.1% and 79.2 ± 14.2% in the MWA group and the RFA group, respectively, and showed no significant difference between the groups. The percentages of regression of uterine volume also showed no marked difference between the two groups. Changes in the dysmenorrhea scores and the SSSs after ablation were similar in the MWA group and in the RFA group, and no significant difference was found between the groups. Finally, the percentage occurrence of adverse events was the same in the two groups.
Conclusions
The safety and effectiveness of PMWA and USgRFA in the treatment of uterine adenomyosis were similar; however, the mean ablation time of PMWA was shorter than that of USgRFA.
Introduction
Uterine adenomyosis is a common gynecological disorder characterized by the presence of heterotopic functional endometrial glands and stroma in the myometrium, accompanied by hypertrophy and proliferation of neighboring myometrial cells and causing diffusive or local thickening of the uterine wall. The typical clinical symptom of adenomyosis is progressive dysmenorrhea, and abnormal uterine bleeding, dysmenorrhea, and uterus enlargement (that produces bulk pressure) are the most common symptoms, which seriously affect the living quality of women of reproductive age [Citation1,Citation2]. Conservative treatment is a challenge. Medical management can be effective; however, its effect is often transient [Citation3]. Separating normal myometrial tissue from myometrial tissue invaded by adenomyosis can be difficult with surgery [Citation4]. The levonorgestrel-releasing intrauterine system appears to be an effective method for alleviating dysmenorrhea and menorrhagia, but the expulsion rate is relatively high due to the enlargement of the patients’ uteri and heavy regular bleeding [Citation5,Citation6]. Uterine artery embolization (UAE) has been demonstrated to be effective and safe, but the impact of this treatment modality on fertility and ovary function remains to be determined [Citation7–9]. On the other hand, rapid regrowth of adenomyosis and relapse of symptoms and signs are the major problems of conservative treatment [Citation10]. Therefore, there is a need for a simple, repeatable, affordable and more precise technique for destroying adenomyosis. Currently, advancements in medical technology have made less invasive or noninvasive treatment options available. One such option is image-guided thermal ablation, the destruction of tissue by a rapid temperature rise. There are various thermal energy source alternatives, such as radiofrequency (RF), microwave (MW), laser and high-intensity focused ultrasound (HIFU). HIFU is effective and noninvasive, but it is time-consuming, and its indication is limited by the location, size and vascularity of the lesion [Citation11]. However, in RF and MW ablation, the electrode or antenna is directly inserted into the lesions under ultrasound (US) guidance, and the generated heat acts directly on the target tissues, overcoming the above disadvantages of HIFU [Citation12,Citation13]. RF is a widely used and studied ablative technique worldwide. In radiofrequency ablation (RFA), a high-frequency alternating electrical current is used to create ionic agitation, which produces frictional heat and heat conduction to achieve subsequent tissue necrosis [Citation14]. Microwave ablation (MWA) is one of the most recent and exciting advances in the field of thermoablative technology, which uses electromagnetic energy to rapidly rotate adjacent polar water molecules [Citation15]. With the advantages of minimal invasion, favorable efficacy and low complication rates, both MW and RF ablations are optimal thermal ablation options using needle techniques for uterine fibroids [Citation16–20]. However, there are limited data on MW and RF ablation for adenomyosis, which presents with the same benign uterine lesion as fibroids. In addition, to our knowledge, there have been no studies comparing the effectiveness of RF and MW ablation for uterine adenomyosis; which of the two energy sources has a greater advantage remains unknown. Our study addressed these questions by investigating and comparing the efficacy and safety of RFA and MWA in treating symptomatic uterine adenomyosis.
Materials and methods
Subjects and enrollment
The MWA versus RFA trial was a single-center, prospective, randomized, parallel and controlled clinical trial in China. This study was approved by the institutional review board of the Chinese PLA General Hospital, China. The protocol number is s201613901. This study was registered in Chinese Clinical Trial Registry (ChiCTR-INR-17919471). One hundred and thirty-three women with symptomatic adenomyosis were enrolled in the study between October 2015 and October 2017.
After written informed consent to participate in this study was obtained, patients were randomly stratified to the MWA or RFA group according to the lesion type (focal or diffuse). We stratified the randomization by computer at a 1:1 ratio. Sixty-eight patients underwent PMWA (percutaneous microwave ablation), and 65 patients underwent RFA. All patients met the following criteria: (1) adenomyosis diagnosed by US and contrast-enhanced magnetic resonance imaging (CE-MRI) with at least one clinical syndrome, such as secondary dysmenorrhea and menorrhagia; (2) childbearing completed; and (3) surgery or other conservative treatments declined. The exclusion criteria included the existence of other pelvic or uterine diseases (e.g., pelvic inflammatory diseases, ovarian endometriomas, or uterine fibroids) and previous treatments, such as UAE and HIFU ().
Microwave ablation procedure
The MW tumor treatment device (KV2000; Nanjing Kangyou Microwave Energy Sources Institute, Nanjing City, China) has a MW transmission frequency of 2450 MHz. The MW antenna is 15G, 18 cm long, and uses embedded aperture MW emission. The distance from the aperture of the MW emission to the needle tip is 11 mm. The output energy was set at 50 W. Ablation was performed under intravenous, conscious sedation. The antenna was percutaneously inserted into the lesion under the guidance of real-time US. The entire ablation procedure was performed under real-time US guidance and according to the ‘moving shot’ technique. Once the hyperechogenic signal reached 3–5 mm from the margin of the lesion, the MW energy was discontinued. Then, the effectiveness of the ablation was immediately assessed using contrast-enhanced sonography (CEUS) (SonoVue, Bracco SinePharm). The non-enhanced regions on CEUS indicated necrotic areas. Supplementary MWA was immediately performed in the enhanced regions.
Radiofrequency ablation procedure
In this study, an RF generator (STARmed, Korea) with a maximum power of 200 W at a frequency of 480 kHz was used. The switching monopolar mode was selected. The electrode (VIVA, STARmed, Korea) we used was 17G, internally cooled, and single tine with a 2.5 cm active tip. The electrode was percutaneously inserted into the lesion under the guidance of transabdominal US. The entire ablation procedure was performed under real-time US guidance and according to the ‘moving shot’ technique: the lesion was ablated unit-by-unit by moving the electrode tip. Once the hyperechogenic signal covered the whole lesion or reached 3–5 mm from the margin of the serosa or uterine endometrium on real-time US, the energy was discontinued. Then, the effectiveness of the ablation was immediately assessed using CEUS (Sono Vue, Bracco SinePharm). Supplementary RFA was also immediately performed in the enhanced regions. Total power time (in minutes) and total energy deposition were automatically recorded by the RF equipment.
Follow-up assessment
Postablation-enhanced MRI was performed within 3 days to accurately evaluate both possible injury to the surrounding organs and the range of the non-perfused area. All patients underwent a thorough clinical evaluation before and 3 months, 6 months, 9 months and 12 months after the treatment, which included dysmenorrhea symptom severity (Verbal Rating Scale, VRS) and symptom severity score (SSS) questionnaires. The SSSs questionnaires included 8 items: amount of menstrual blood loss, menstrual blood clotting, prolonged menstruation, menstrual disorders, pelvic pressure, frequent urination during the day and at night, and fatigue. Response options for the symptom severity subscales ranged from ‘not at all’ to ‘a very great deal’ [Citation21]. The VRS scores ranged from 0 to 5. The SSSs ranged from 0 to 100, with higher scores indicating more severe symptomatology [Citation22].
Statistical analysis
Statistical analysis was performed using SPSS 22.0. Student’s t-test was used to compare data with normal distributions, whereas the Wilcoxon signed-rank test was used to compare data with skewed distributions. The chi-square test was used to compare discrete variables between the two groups.
Results
Baseline information
A total of 133 women with symptomatic uterine adenomyosis underwent either MWA (68 women) or RFA (65 women) therapy in a single session at our institution. The average age of the patients was 39.4 ± 4.2 years (range, 35–50 years), and the median volume of uterine adenomyosis was 124.3 cm3 (range, 28.7–374.5 cm3). The baseline characteristics of the two groups are given in , which shows no significant differences in the baseline data between the MWA group and the RFA group.
Table 1. Baseline data of patients in PMWA and USgRFA.
Post-procedure evaluation of RFA
The USgRFA (US-guided RF ablation) procedure was successfully performed for all 65 patients in this group. The mean ablation time was 37.5 ± 6.2 min (range, 5–39 min). The immediate mean ablation rate after treatment was 79.2 ± 14.2% (range, 70.5–99.1%) (). The mean percentage reduction of adenomyosis was 48.5% and 67.3% at 3 and 12 months postablation, respectively. Consistent with the statistically significant reduction in the adenomyosis volume during the follow-up period, uterus volume also showed a statistically significant reduction. Compared with the mean baseline uterus volume, the percentage volume reduction was 34% at 3 months and 47.6% 12 months postablation. The patient pain scores for dysmenorrhea showed a statistically significant decline from the baseline of 3.91 ± 0.98 to 1.92 ± 0.79 12 months after the procedure. The SSSs declined significantly from 21.8 ± 5.5 to 16.4 ± 4.8 at the 12-month follow-up ().
Figure 2. Representative MRI imaging of uterine adenomyoma before and 3 days after RF (A1: before RFA treatment, A2: after RFA treatment) or MW (B1: before PMWA treatment, B2: after PMWA treatment) ablation. The dark shadow shows the area of lesion ablation (non-perfused volume, NPV). The percentages of NPV were 75% and 81%, respectively.
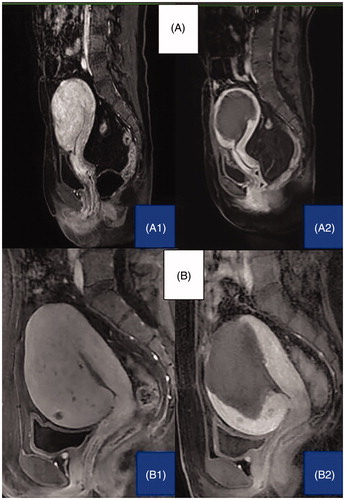
Table 2. Comparison of results between PMWA and USgRFA in treating adenomyosis.
Comparison of the results of MWA versus RFA
The PMWA procedure was successfully performed for all 68 patients in this group. The mean ablation time was 16.3 ± 4.9 min (range, 5–23 min), which was demonstrably superior to that of the RFA group. The mean percentage ablation for MWA was 79.7% ± 15.1% (range, 72.5–99.3%), which was similar to that of the RFA group (). The mean percentages of regression of adenomyosis at 3 months and 12 months after the course of treatment were 54.4% and 71.7%, respectively, which were greater than those of the RFA group but not significantly different. The percentages of reduction in uterus volume were 44.8% and 64.9% 3 and 12 months after the procedure, respectively, which were both greater than the corresponding values for the RFA group. In the pretreatment period, both the VRS scores and the SSSs were comparable between the RFA group and the MWA group. The SSSs decreased significantly from 22.0 ± 4.9 to 17.4 ± 5.0 at the 12-month follow-up, which was not significantly different from the change in the RFA group. The VRS declined from the baseline score of 4.02 ± 0.71 to 1.75 ± 1.13 at 12 months postablation. The drop in VRS scores at the 12-month follow-up in the MWA group was greater than that in the RFA group, but no significant difference was found between the groups ().
Efficacy
Since most patients complained of dysmenorrhea in both groups (81.5% in the RFA group and 89.7% in the MWA group), the efficacy was determined by the degree of alleviation of dysmenorrhea 12 months after ablation. In the RFA group, four cases (7.6%) showed no improvement in dysmenorrhea. Twenty-six cases (49.3%) reported complete relief of dysmenorrhea, and 23 (43.1%) cases reported partial relief. In the MWA group, three patients (5.0%) showed no improvement in dysmenorrhea. Forty (65.9%) patients reported complete relief of dysmenorrhea, and 18 (29.1%) patients reported partial relief ().
Table 3. Relief of dysmenorrhea following treatment of adenomyosis.
Complications
The common adverse events after both RFA and MWA treatments were abdominal pain, vaginal discharge and low-grade fever, which were self-limited and lasted for no more than 14 days. They were classified as grade A or B according to the unified standardized Society of Interventional Radiology (SIR) grading system. There was no significant difference in the incidence of severe adverse events between the two groups ().
Discussion
Image-guided thermal ablation is an established treatment for tumors. There is an established history of treating hepatocellular carcinomata and other soft tissue malignancies with RFA or PMWA [Citation23,Citation24]. Hyperthermic ablation results in thermal fixation, which preserves cellular architecture, as well as coagulative necrosis [Citation25]. In the case of uterine fibroids, the presence of coagulative necrosis after treatment with RF or MW energy can result in volume reduction of the lesion and symptomatic relief [Citation16–20]. In the present study, RFA or MWA resulted not only in volume reduction of the adenomyoma but also in volume reduction of the whole uterus. Consistent with the reduction of lesion and uterus volumes, symptoms were dramatically improved in both the RFA and MWA groups, which resulted in a total effective rate of 92.4% and 95.0%, respectively, at the end of the first year. RF and MW ablation both allow flexible approaches, including laparoscopic, transabdominal or transvaginal approaches with US guidance. In the transabdominal approach, percutaneous puncture overcomes the disadvantage of puncture through the cervical canal and endometrium. RFA and MWA employ the same agent for lesion destruction, heat. However, many studies have suggested that MWA possesses several theoretical advantages over RFA: consistently larger ablation volumes, higher intratumoral temperatures, faster ablation times, less dependency on the electrical conductivities of the tissue and energy delivery less limited by the exponentially rising electrical impedances of tumor tissue [Citation14]. In the current study, although RFA and MWA resulted in similar necrosis ratios, MWA was more effective than RFA with respect to uterus volume shrinkage and dysmenorrhea improvement. The possible reasons for this difference between the two modalities are as follows: first, the uterus volume shrank quickly postablation not only from lesion involution but also from the ablation-induced tissue contraction as a result of dehydration [Citation26]. Brace et al. found that MV produced more contraction than RF in the liver [Citation27]. Heat is dissipated from the region by further heat conduction into normothermic tissue during the ablation. RF ablation is fundamentally restricted by the need to conduct electric energy into the body. As temperatures rise, increasing impedance limits further deposition of electricity into the tissue. This becomes even more pronounced if charring occurs [Citation14]. Since MW ablation does not rely on the conduction of electricity into tissue, it is not limited by charring. Moreover, MW ablation is less affected by the heat-sink effect than RF ablation [Citation28]. Therefore, the radial depth of heating is greater for MW than RF [Citation28,Citation29]. A previous study proved that MW ablation has a much broader power field than RF ablation, up to 2 cm in diameter [Citation30]. In addition, in an in vivo study, measurement of the mean temperature at a site 5 mm from the electrode showed that tissue temperatures were driven considerably higher (up to 80–90 °C) with MW ablation than with RF ablation (approximately 60–65 °C) [Citation31]. Higher temperatures allow a larger thermal gradient to be created, which suggests that the thermal effect of MWA influences more tissue beyond the necrosis zone to a greater extent than RFA. Although MWA resulted in more uterus volume shrinkage than RFA, the change in the bulk pressure-related SSS in the present study was similar in the MWA group and the RFA group. The reason for this might be that the sample of the patients that presented with bulk pressure was small in both groups (3 in the MW group and 2 in the RF group). Postprocedural imaging findings are only a rough guide to the success of ablation therapy because microscopic foci of residual disease cannot be expected to be identified with standard imaging [Citation32], especially for adenomyosis, where the depths of nidi vary throughout the uterine wall and their borders are poorly defined. Although RFA and MWA resulted in similar necrosis ratios as assessed by enhanced MRI in the current study, the microscopic foci of residual lesions in the two groups are difficult to compare. The thermal effect not only results in coagulative necrosis in the target tissue but also affects the living environment and leads to changes at the cytomolecular level. Luo et al. observed the features of lesions caused by RFA in leiomyoma tissue and found that the thermal effect of RFA can depress the expression of estrogen receptors (ERs) and progesterone receptors (PRs) [Citation33]. Ying et al. found that the thermal effect can depress the expression of the Survivin, ER and PR genes in the endometrium and that the effect was permanent [Citation34]. The mechanisms of adenomyosis-associated pain are complex and diverse. Numerous mechanisms, such as oxytocin receptor overexpression in myometrial smooth muscle cells and increased innervation in the endometrium or the myometrium, have been shown to be responsible for adenomyosis-associated dysmenorrhea [Citation35–38]. We hypothesize that thermal effects can influence adjacent tissues, such as residual lesions and myometrium, and inhibit certain mechanisms, thus relieving dysmenorrhea. In that respect, MWA, with its larger thermal gradient, is more effective than RFA. A previous study of endometrial ablation for adenomyosis showed that the improvement in dysmenorrhea has a strong correlation with the depth of the endometrial penetration [Citation39]. Superficial adenomyosis can be treated definitively with ablation, while deep adenomyosis responds poorly to ablation. Conversely, MWA, with its greater radial depth of heating, is more effective than RFA.
Our study has a number of limitations. First, similar to most studies, the RF device in this study used a monopolar electrode. There are various types of electrodes, and the type of electrode used clearly influences the extent of ablation. However, our study implies that an energy source with greater thermal efficiency would be more effective for the treatment of adenomyosis. Further comparison is necessary for other types of electrodes. Second, our study is a single-center and small-sample controlled clinical trial. Multi-center and large-sample controlled trials are required before these techniques can be widely applied in the gynecology field.
Conclusion
Based on our results, both MWA and RFA are safe and effective modalities in the treatment of uterine adenomyosis; however, the mean ablation time of PMWA was shorter than that of USgRFA. Hence, these clinically effective and safe treatments are alternatives for women with uterine adenomyosis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Graziano A, Lo Monte G, Piva I, et al. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci. 2015;19:1146–1154.
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus revisited. Am J Obstet Gynecol. 1972;112:583–593.
- Levgur M. Therapeutic options for adenomyosis: a review. Arch Gynecol Obstet. 2007;276:1–15.
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril. 2014;101:472–487.
- Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189–193.
- Bragheto AM, Caserta N, Bahamondes L, at al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195–199.
- Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297–302.
- Holub Z, Mara M, Kuzel D, et al. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90:1886–1891.
- Homer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril. 2010;94:324–330.
- Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4:323–336.
- Arleo K, Khilnani M, Ng A, et al. Features influencing patient selection for fibroid treatment with magnetic resonance-guided focused ultrasound. J Vasc Interv Radiol. 2007;18:681–685.
- Meng X, He G, Zhang J, et al. A comparative study of fibroid ablation rates using radiofrequency or high-intensity focused ultrasound. Cardiovasc Intervent Radiol. 2010;33:794–799.
- Zhao W-P, Han Z-Y, Zhang J, et al. A retrospective comparison of microwave ablation and high intensityfocused ultrasound for treating symptomatic uterine fibroids. Eur J Radiol. 2015;84:413–417.
- Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol. 1976;39:69–76.
- Tabuse K, Katsumi M, Kobayashi Y, et al. Microwave surgery: hepatectomy using a microwave tissue coagulator. World J Surg. 1985;9:136–143.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Berman M, Guido RS, Leal JGG, et al. Three-year outcome of the halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21:767–774.
- Bongers M, Brölmann H, Gupta J, et al. Transcervical, intrauterine ultrasound-guided adiofrequency ablation of uterine fibroids with the VizAblate System: three- and six-month endpoint results from the FAST-EU study. Gynecol Surg. 2015;12:61–70.
- Zhang J, Feng L, Zhang BS, et al. Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment – a clinical study. Int J Hyperthermia. 2011;27:510–516.
- Liu H, Zhang J, Han ZY, et al. Effectiveness of ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroids: a multicentre study in China. Int J Hyperthermia. 2016;32:876–880.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300.
- Holdgate A, Asha S, Craig J, et al. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg Med. 2003;15:441–446.
- Carrafiello G, Lagana D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6:S65–S69.
- Zerlauth JB, Meuli R, Dunet V. Renal cell carcinoma metastasis involving vertebral hemangioma: dual percutaneous treatment by navigational bipolar radiofrequency ablation and high viscosity cement vertebroplasty. BMJ Case Rep. 2017.
- Rabinovici J, Stewart EA. New interventional techniques for adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:617–636.
- Wall MS, Deng X-H, Torzilli PA, et al. Thermal modification of collagen. J Shoulder Elbow Surg. 1999;8:339–344.
- Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol. 2010;21:1280–1286. Aug
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139.
- Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–644.
- Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol. 2013;36:505–511.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124–130.
- Goldberg SN, Gazelle GS, Compton CC, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88 :2452–2463.
- Luo X, Shen Y, Song W-X, et al. Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int J Gynaecol Obstet. 2007;99:9–13.
- Ge-Ping YIN, Juan LI, Tong-Yu ZHU, et al. Expression of survivin, estrogen receptor and progesterone receptor of endometrium of patients with an ovulatory dysfunctional uterine bleeding before and after radiofrequency heat-coagulation treatment. Chin J Obstet Gynecol Pediatr. 2010;6:175–181.
- Zhang X, Lu B, Huang X, et al. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril. 2009;92:1799–1801.
- Zhang X, Lu B, Huang X, et al. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril. 2010;94:730–737.
- Woodbury RA, Torpin R, Child GP, et al. Myometrial physiology and its relation to pelvic pain. J Am Med Assoc. 1947;134:1081–1085.
- Guo S-W, Mao X, Ma Q, et al. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99:231–240.
- McCausland AM, McCausland VM. Depth of endometrial penetration in adenomyosis helps determine outcome of rollerball ablation. Am J Obstet Gynecol. 1996;174:1786–1793.