Abstract
Background
Non-contact induction heating (NCIH) is a noninvasive treatment modality that can be used to cause thermal damage to bacterial biofilms on a metal implant surface in the context of a prosthetic joint infection. The purpose of this study was (1) to determine the effectiveness of NCIH on killing Staphylococcus aureus from biofilm and (2) to determine the possible synergistic effect of NCIH and cocktails of antibiotics and N-acetylcysteine (NAC).
Methods
Staphylococcus aureus biofilms were grown on titanium alloy (Ti6Al4V) coupons. These coupons were heated to 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C for 3.5 min and subsequently exposed to cocktails of vancomycin, rifampicin and NAC at clinically relevant concentrations over 24 h.
Results
In the control group without induction heating, 2.2*107 colony forming units (CFU)/cm2 were observed. At 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C, a reduction of 0.3-log, 3.9-log, 4.2-log, 4.3-log, and 6.6-log CFU/cm2 were observed, respectively. There was synergy between antibiotics and induction heating that resulted in less than 100 CFU/cm2 remaining after 3.5 min at 60 °C, and exposure to vancomycin and rifampicin. Total eradication was observed at 80 °C. Total eradication was also observed at 60 °C and a cocktail of antibiotics with NAC.
Conclusion
Induction heating of titanium alloy coupons is effective for the reduction of bacterial load in vitro in S. aureus biofilms. Induction heating and cocktails of antibiotics and NAC have a synergistic effect that results in the total eradication of the biofilm at 60 °C and higher for clinically relevant concentrations of vancomycin, rifampicin and NAC.
Introduction
Prosthetic joint infection (PJI) is a major problem in both elective orthopedic and acute trauma surgery. In PJI, the causative micro-organisms are mostly Staphylococci such as Staphylococcus aureus [Citation1]. Due to bacterial biofilms on the implant surface, patients with infected implants will undergo long and extensive treatments that consist of multiple surgical procedures and antibiotic courses for several months [Citation2]. This treatment is maximally invasive; therefore, impossible in patients with high comorbidity factors. Furthermore, the increasing antibiotic resistance of bacteria raises concern and limits the choices of antibiotics [Citation3–6]. Therefore, it is vital that new treatments for the prevention and treatment of biofilm infections in implants are being developed.
Non-contact induction heating (NCIH) of metal implants is a new and emerging treatment for infected metal implants [Citation7–10]. NCIH uses pulsed electromagnetic fields (PEMF) to induce so-called ‘eddy currents’ within metal objects which causes them to heat up. This heat can be used to cause thermal damage to bacterial biofilms on the metal implant, hence, killing the bacteria and weakening the biofilm. NCIH only actively heats the metal implant and has no direct heating effect on the surrounding tissue; thus it can potentially be used as a noninvasive treatment for PJI.
There may be concerns for thermal necrosis when heating metal implants to 60–80 °C. However, such temperatures are not uncommon in orthopedic surgery with cementing, drilling and using diathermia [Citation11–Citation13]. For instance, Samara et al. have shown that bone cement, which achieves durable fixation for e.g., hip and knee implants reaches temperatures of 80 °C for more than 10 min during the curing process [Citation14]. Additionally, special heating techniques such as segmental induction heating can be used to apply localized heating to a segment of an implant, using the remainder of the implant as a heat sink [Citation10].
Presently, several studies have shown the effectiveness of NCIH on metal implant in the reduction of bacterial load in vitro [Citation7–Citation10]. However, it is currently unknown whether NCIH can reduce or even eradicate S. aureus from the biofilm on a titanium alloy, which is commonly used for joint implants. Furthermore, it is important to evaluate the possible synergistic effect of NCIH and antibiotics, because NCIH will very likely be applied to a clinical setting where antibiotics are part of the treatment strategy and heat has been shown to enhance the antibacterial activity of antimicrobial agents against staphylococcal biofilms [Citation15].
Some reports have suggested that N-acetylcysteine (NAC) has a synergistic effect with antibiotics, such as rifampicin, against staphylococcal biofilms and could be used as an adjuvant with antibiotics and NCIH [Citation16,Citation17].
Therefore, the purpose of this study was (1) to determine the effectiveness of NCIH on killing S. aureus from biofilm and (2) to determine the possible synergistic effect of NCIH and cocktails of antibiotics and NAC.
Materials and methods
Induction heating and temperature control
Staphylococcus aureus biofilms (American Type Culture Collection (ATCC) 29213) were grown on titanium alloy (Ti6Al4V) coupons of 38 mm by 25 mm at 1 mm thick and exposed to a PEMF of 97 kHz at a maximum of 65 W from a custom built induction heater. As the implant material influences bacterial adhesion and total biofilm burden, we used titanium alloy (Ti6Al4V) coupons, which are the main material used in joint implants [Citation18]. The induction heater features a pancake-type coil with 9 turns of copper litz wire and an inductance of 12 millihenries. For non-contact temperature measurement and temperature control, we used a microcontroller board based on the ATmega328 (Arduino Uno, Adafruit Industries, New York, NY) and infrared (IR) temperature sensor (MLX90614, Melexis, Ieper Belgium). The temperature was recorded 4 times per second (4 Hz) in real-time and stored in a data file on a laptop. shows the schematic arrangement of the non-contact induction heater and temperature control system as well as the heating curves.
Figure 1. Schematic of the arrangement of the induction systems and a titanium alloy coupon in a Petri dish (top) and heating curves for target temperatures, Tx (bottom). µC = micro-controller for temperature control and communication (data logging) with the laptop.
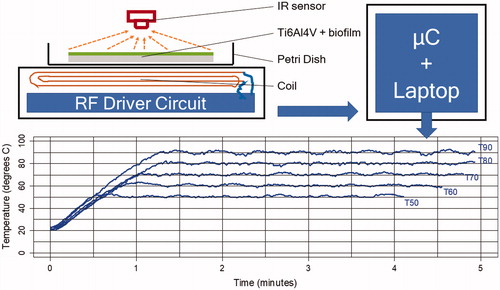
Temperature measurement with the IR sensor is affected by the emissivity of the surface of, in this case, the titanium alloy coupon with biofilm. The emissivity of an object is the ratio of the amount of radiation actually emitted from the surface to that emitted by a blackbody at the same temperature [Citation19]. To validate the IR temperature measurements a K-type thermocouple was used to compare the temperature measured with the IR sensor to the temperature measured with the thermocouple. These measurements were taken after the PEMF had been switched off, because the PEMF can affect the thermocouple measurements [Citation7–10].
Titanium alloy coupons with biofilm were heated to temperatures of 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C for 3.5 min. The duration of 3.5 min was chosen to allow comparison to previous results of induction heating planktonic micro-organisms [Citation9]. Experiments were repeated at least 5 times unless otherwise indicated.
Biofilm growth and quantification
Staphylococcus aureus ATCC 29213, a biofilm forming clinical isolate[Citation20,Citation21], biofilms were grown on titanium alloy (Ti6Al4V) coupons for 24 h in a polypropylene container equipped with bacteria filter (1 micron PTFE hydrophobic membrane, Medical Filtration Solutions Ltd, Preston, UK) to allow for sterile ventilation. A biofilm was produced by immersing the coupons in 100 ml of growth medium (BHI: brain hart infusion), inoculated with S. aureus, for 4 h to allow adherence. Then this was transferred into another 100 ml of medium (BHI: brain hart infusion) and incubated for 24 h at 37 °C. For both the biofilm adherence phase and biofilm growth phase 9–10 coupons were placed in the same container, as a batch, to increase reproducibility and reduce variation in colony forming units (CFU)/cm2.
Prior to thermal shock by the induction heater, the coupons were washed with phosphate-buffered saline (PBS) solution in a Petri-dish to remove any planktonic bacteria. Subsequently, the coupons were exposed to the thermal shock. After the thermal shock, the coupons were washed again with PBS solution in a Petri-dish and directly afterward placed in a 50 ml centrifuge tube with 20 ml of PBS solution. This tube, including the coupon, was sonicated (Elma, D-78224 Ultrasonic cleaner, Singen, Germany) for 5 min at 35 kHz to dislodge the bacteria from the biofilm into suspension. Afterward a dilution series of the supernatant (centrifuged 5 min at 12,000 rpm) was cultured for 48 h on BHI plates at 37 °C to determine the CFU/cm2.
We also included two control conditions without induction heating: which went through all steps but the induction heater was not switched on. In a second control condition the coupons also went through all the steps except induction heating and were exposed to 0.5% chlorhexidine with 70% alcohol for 3.5 min.
Heat, antibiotics, and NAC experiments
To study the possible synergistic effect of antibiotics, NAC and induction heating, the titanium alloy coupons with biofilm were also exposed (after induction heating) to vancomycin (10 mg/l or 1 mg/l) and rifampicin (1 mg/l) with and without NAC (0.5 mg/l, all from Sigma-Aldrich, Chicago, IL) for 24 h at 37 °C. These concentrations were chosen to represent clinically relevant concentrations [Citation22–24]. To represent clinical practice, we added rifampicin to the antibiotic treatment because the studied S. aureus strain (ATCC 29213) is sensitive to rifampicin [Citation25,Citation26]. After thermal shock by induction heating, instead of proceeding with sonication, the coupons were placed separately into another polypropylene container equipped with bacteria filter with 50 ml of fresh BHI growth medium that contained vancomycin and rifampicin with or without NAC. The coupons were subsequently incubated for 24 h at 37 °C, washed with PBS solution in a Petri-dish, placed into a 50 ml centrifuge tube with 20 ml of PBS and subsequently sonicated and enumerated as described above. Biofilms were exposed to antibiotics and NAC after thermal shock to represent the clinical practice of starting antibiotics after surgical treatment e.g., debridement to allow for (aspiration) cultures. We also included conditions with only antibiotics or NAC and without induction heating.
Statistics
Intraclass correlation (ICC) was used to compare the temperature measured with the IR sensor with the temperature measured with the K-type thermocouple. Statistical analyses, when appropriate, were performed using analysis of variance (ANOVA) (SPSS version 23, IBM, Armonk, NY). In line with recent recommendations means and corresponding confidence intervals are reported, whereas p-values are not reported [Citation27].
Results
The ICC of the temperatures measured with the IR sensor and K-type thermocouple was 0.99 (95%CI 0.99–1.00), which indicated a near perfect agreement.
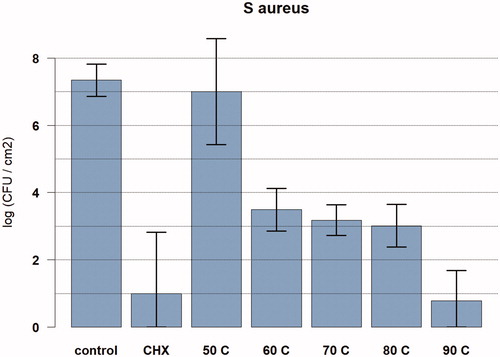
In the control group without induction heating, 2.2*107 CFU/cm2 were observed (n = 17). For 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C, a reduction of 0.3-log, 3.9-log, 4.2-log, 4.3-log, and 6.6-log CFU/cm2 were observed, respectively (ANOVA analysis). In the chlorhexidine-alcohol group, a reduction of 6.3 log CFU/cm2 was observed ().
Figure 2. Graph showing the relationship between temperature exposure and log colony forming units (CFU) per cm2 for Staphylococcus aureus biofilms. The bacteria were exposed to the target temperature for 3.5 min. Data are presented as means and corresponding 95% confidence intervals. CHX: chlorhexidine; C: degrees Celsius.
For antibiotics alone (vancomycin 10 mg/l and rifampicin 1 mg/l), a 3.5-log reduction in CFU/cm2 was observed after 24 h of exposure, whereas an increase in CFU/cm2 of 4.9-log with lower dose of antibiotics (vancomycin 1 mg/l and rifampicin 1 mg/l) was observed. There was a 5.6-log reduction for antibiotics and NAC (n = 3; vancomycin 10 mg/l, rifampicin 1 mg/l and NAC 0.5 mg/l) after 24 h of exposure ().
Figure 3. Graph showing the relationship between temperature exposure and log colony forming units (CFU) per cm2 for Staphylococcus aureus biofilms with and without antibiotics after thermal exposure. The bacteria were exposed to the target temperature for 3.5 min. Data are presented as means and corresponding 95% confidence intervals. AB: vancomycin 10mg/l, and rifampicin 1mg/l for 24 h after thermal shock from induction heater; C: degrees Celsius; *full eradication.
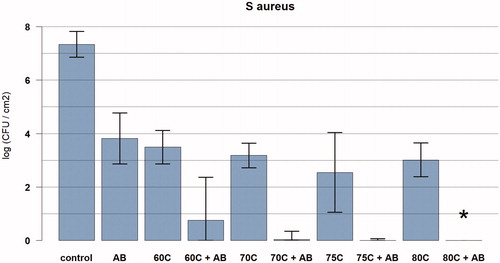
Figure 4. Graph showing the relationship between temperature exposure and log colony forming units (CFU) per cm2 for Staphylococcus aureus biofilms with cocktails of antibiotics with and without NAC. The bacteria were exposed to the target temperature for 3.5 min. Data are presented as means and corresponding 95% confidence intervals. AB: vancomycin 10mg/l, and rifampicin 1mg/l for 24 h after thermal shock from induction heater; C: degrees Celsius; NAC: NAC 0.5 mg/l; *full eradication.
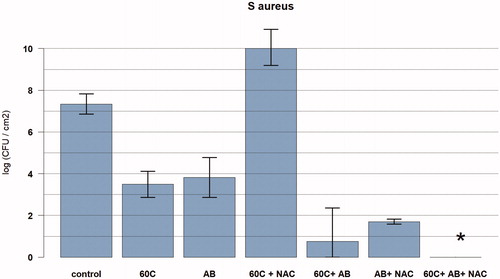
Figure 5. Graph showing the relation between temperature exposure and log colony forming units (CFU) per cm2 for Staphylococcus aureus biofilms for different concentrations of vancomycin (10 mg/l and 1 mg/l), rifampicin (1 mg/l), and NAC (0.5 mg/l). The bacteria were exposed to the target temperature for 3.5 min. Data are presented as means and corresponding 95% confidence intervals. AB: vancomycin 10 mg/l, and rifampicin 1 mg/l OR vancomycin 1 mg/l and rifampicin 1 mg/l for 24 h after thermal shock from induction heater; C: degrees Celsius; NAC: NAC 0.5 mg/l; *full eradication.
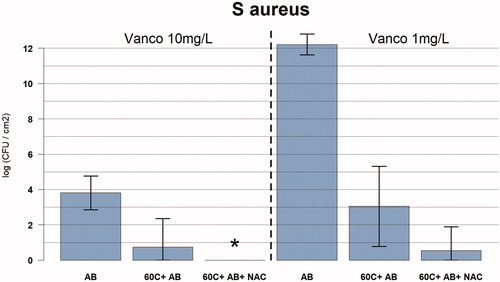
There was synergy between antibiotics and induction heat that resulted in less than 100 CFU/cm2 remaining after 3.5 min at 60 °C (n = 3) and higher with 24 h exposure to antibiotics (vancomycin 10 mg/l and rifampicin 1 mg/l). Total eradication was observed at 80 °C and higher (). For the lower dose of antibiotics (vancomycin 1 mg/l + rifampicin 1 mg/l) there was a 4.3-log reduction in CFU/cm2 with prior induction heating at 60 °C.
Total eradication was observed at 60 °C followed by a cocktail of antibiotics with NAC (n = 4; vancomycin 10 mg/l, rifampicin 1 mg/l and NAC 0.5 mg/l) (). For the lower dose of antibiotics (vancomycin 1 mg/l + rifampicin 1 mg/l) combined with NAC (0.5 mg/l), there was a 6.8-log reduction in CFU/cm2 with prior induction heating at 60 °C.
For 60 °C followed by NAC alone (NAC 0.5 mg/l, no antibiotics), there was an increase in CFU/cm2 of 2.7-log ().
Discussion
The results of our study showed that induction heating of titanium coupons was effective in the reduction of bacterial load in vitro for S. aureus biofilms. Induction heating with cocktails of antibiotics and NAC had a synergistic effect resulting in total eradication of the biofilm at 60 °C with clinically relevant concentrations. Induction heating uses pulsed electromagnetic fields (PEMF). The PEMF together with eddy currents at the implant (titanium coupon) surface may interfere with the transport of charged molecules within the bacteria possibly making them more susceptible to thermal shock. Furthermore, with induction heating the heat originates at the biofilm-implant interface and travels into the biofilm, whereas the cocktail of antibiotics and NAC diffuses into the biofilm starting at the outer border of the biofilm and ultimately ending at the biofilm–implant interface. This attack from two directions may be a mechanism for the observed synergistic effect that leads to total eradication.
The observed synergistic effect between heat and antibiotics (without NAC) is in accordance with Hajdu et al., who have shown enhancement of antibacterial activity of antimicrobial agents against staphylococcal biofilms by increasing the ambient temperature [Citation15]. Ricker and Nuxoll have also demonstrated this synergistic effect of antibiotics and heat in a Pseudomonas film for erythromycin, tobramycin, and ciprofloxacin [Citation28]. Both Hajdu and Ricker and Nuxoll used heat conduction rather than induction heating [Citation15,Citation28].
The observed synergistic effect between antibiotics and NAC is in accordance with Leite et al., who have shown for Staphylococcus epidermidis (not aureus) a 4 log reduction in the number of biofilm cells with NAC 40 mg/l and rifampicin 10 mg/l [Citation17]. However, with mature biofilms and different concentrations of NAC this effect may be different [Citation16].
Full eradication necessitates a thermal dose of 60–80 °C for 3.5 min. Although this may seem high, such a thermal dose is not uncommon in orthopedic surgery with cementing, drilling and using diathermia [Citation11–13]. There are also animal experiments that confirm the lack of significant necrosis after induction heating up to 60–65 °C. Müller et al. heated a nickel–titanium shape memory rod in the femur of rats at 40–60 °C using induction heating and observed no necrosis in the surrounding bone and tissue [Citation29]. They also heated an osteosynthesis plate in a rabbit model with induction heating and noted that all osteotomies underneath the plate healed [Citation30]. Chopra et al. have shown, in a mouse model, that thermal damage is confined to a localized region (< 2 mm) around the implant [Citation8]. Fang et al. heated metal implants in a rat model to 75 °C without any significant thermal damage to the surrounding tissue [Citation31]. These studies are in agreement with Samara et al., who have shown that bone cement achieves durable fixation despite the temperature reaching 80 °C for more than 10 min, caused by the curing process of the cement [Citation14]. Furthermore special heating techniques such as segmental induction heating can be used to apply localized heating to a segment of an implant using the remainder of the implant as a heat sink and to avoid damage to vital areas of the implant necessary to maintain fixation [Citation10].
We should note some limitations. Regarding non-contact temperature control, an IR sensor such as the one used in our experiments cannot be readily used in clinical situations because of the absence of a direct line of sight: there is tissue and bone between the implant and the IR sensor. However, there are already noninvasive temperature safety systems that rely on different mechanisms. Cheng et al. developed remote acoustic sensing, which uses remote acoustic sensors to detect sounds associated with boiling on the implant surface [Citation7].
We used only one frequency, 97 kHz, with a maximum of 65 W. These values were chosen to allow for controlled heating of the titanium alloy coupons. For induction heating of orthopedic implants different frequencies may be required depending on the size and geometry of the implants [Citation8,Citation10]. Final parameters to be used in the clinic should also take into consideration possible the side effects of peripheral nerve stimulation and tissue heating, which are frequency dependent [Citation32].
Additionally, our experiments were in vitro and may not translate entirely to in vivo situations with more mature biofilms. For example, physiological and molecular effects of hyperthermia are not accounted for. Localized hyperthermia has been shown to increase blood flow and vessel permeability, which could result in better availability of antibiotics and the immune system [Citation33]. Localized hyperthermia may also activate the immune system and increase the fluidity and permeability of membranes, which are helpful in relieving prosthetic joint infection [Citation33].
As neither induction heat nor antibiotics alone were able to achieve full eradication on their own, a multi-modality treatment of PJI would likely be most successful. Thermo-microbiology and thermo-infectiology are emerging fields and induction heating may have an important future role in the multi-modality treatment of PJI together with antibiotics, surgery, peptides, phage therapy, and local radiation [Citation34].
The future clinical application of NCIH may be divided into noninvasive use and surgical use. During noninvasive use, the metal implant is heated noninvasively and great care is taken to avoid excessive heating to areas where the implant is fixed to the bone or areas that are in close contact with important anatomical structures such as nerves and major blood vessels. This may be performed in a segmental way with tailored heating protocols for each segment: segmental induction heating [Citation10]. The noninvasive application of induction heating may be particularly beneficial in patients for whom surgical treatment is not possible and who for instance receive suppression antibiotic therapy. The ultimate goal is to replace surgical treatment with NCIH. As such, NCIH will most likely be part of a multi-modality treatment that also involves antibiotics and adjuvants (non-antibiotic chemical agents).
Alternatively, NCIH can be used during surgery to increase the effectiveness of the surgical procedure such as in Debridement, Antibiotics and Implant Retention (DAIR): NCIH kills micro-organisms by heat and allows heating of parts of the implant that cannot be reached and mechanically cleaned. During surgery, soft tissue can be protected by keeping it away from the heated part of the implant. Additionally, surgery allows for direct temperature control by direct temperature measurement (e.g., thermocouple) or IR thermal imaging.
In conclusion induction heating of titanium alloy coupons is effective for the reduction of bacterial load in vitro for S. aureus biofilms. Induction heating and cocktails of antibiotics and NAC have a synergistic effect that results in total eradication of the biofilm at 60 °C and higher for clinically relevant concentrations of vancomycin, rifampicin, and NAC.
Acknowledgments
The authors thank ZonMw for providing financial support (“off road” Grant 451001003 and Veni Grant 09150161810084).
Disclosure statement
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Data availability statement
The data set for this work will be made available on EASY by Data Archiving and Networked Services (DANS): https://easy.dans.knaw.nl/ui/home.
Additional information
Funding
References
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794.
- Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–345.
- Anguita-Alonso P, Hanssen AD, Osmon DR, et al. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin Orthop Relat Res. 2005;439:43–47.
- Gomes DM, Ward KE, LaPlante KL. Clinical implications of vancomycin heteroresistant and intermediately susceptible Staphylococcus aureus. Pharmacotherapy. 2015;35:424–432.
- Lee JYH, Monk IR, Gonçalves da Silva A, et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat Microbiol. 2018;3:1175–1185.
- Ravi S, Zhu M, Luey C, et al. Antibiotic resistance in early periprosthetic joint infection. ANZ J Surg. 2016;86:1014–1018.
- Cheng B, Chatzinoff Y, Szczepanski D, et al. Remote acoustic sensing as a safety mechanism during exposure of metal implants to alternating magnetic fields. PLoS One. 2018;13:e0197380.
- Chopra R, Shaikh S, Chatzinoff Y, et al. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci Rep. 2017;7:7520.
- Pijls BG, Sanders IMJG, Kuijper EJ, et al. Non-contact electromagnetic induction heating for eradicating bacteria and yeasts on biomaterials and possible relevance to orthopaedic implant infections: in vitro findings. Bone Joint Res. 2017;6:323–330.
- Pijls BG, Sanders IMJG, Kuijper EJ, et al. Segmental induction heating of orthopaedic metal implants. Bone Joint Res. 2018;7:609–619.
- Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999;25:17S–21S.
- Eriksson AR, Albrektsson T, Albrektsson B. Heat caused by drilling cortical bone. Temperature measured in vivo in patients and animals. Acta Orthop Scand. 1984;55:629–631.
- Sutton PA, Awad S, Perkins AC, et al. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel and the Ligasure. Br J Surg. 2010;97:428–433.
- Samara E, Moriarty TF, Decosterd LA, et al. Antibiotic stability over six weeks in aqueous solution at body temperature with and without heat treatment that mimics the curing of bone cement. Bone Joint Res. 2017;6:296–306.
- Hajdu S, Holinka J, Reichmann S, et al. Increased temperature enhances the antimicrobial effects of daptomycin, vancomycin, tigecycline, fosfomycin, and cefamandole on staphylococcal biofilms. Antimicrob Agents Chemother. 2010;54:4078–4084.
- Eroshenko D, Polyudova T, Korobov V. N-acetylcysteine inhibits growth, adhesion and biofilm formation of Gram-positive skin pathogens. Microb Pathog. 2017;105:145–152.
- Leite B, Gomes F, Teixeira P, et al. Staphylococcus epidermidis biofilms control by N-acetylcysteine and rifampicin. Am J Ther. 2013;20:322–328.
- Koseki H, Yonekura A, Shida T, et al. Early staphylococcal biofilm formation on solid orthopaedic implant materials: in vitro study. PLoS One. 2014;9:e107588.
- Vollmer M, Mollmann KP. Infrared thermal imaging. Fundamentals, research and applications. 2nd ed. Weinheim: Wiley; 2010.
- Pettit RK, Weber CA, Pettit GR. Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob. 2009;8:28.
- Soni I, Chakrapani H, Chopra S. Draft genome sequence of methicillin-sensitive Staphylococcus aureus ATCC 29213. Genome Announc. 2015;3:e01095–15.
- Aarnoutse RE, Kibiki GS, Reither K, et al. Pharmacokinetics, tolerability, and bacteriological response of Rifampin administered at 600, 900, and 1,200 milligrams daily in patients with pulmonary Tuberculosis. Antimicrob Agents Chemother. 2017;61:e01054–17.
- Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–134.
- Thabit AK, Fatani DF, Bamakhrama MS, et al. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136.
- Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25.
- Scheper H, van Hooven D, van de Sande M, et al. Outcome of acute staphylococcal prosthetic joint infection treated with debridement, implant retention and antimicrobial treatment with short duration of rifampicin. J Infect. 2018;76:498–500.
- Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–307.
- Ricker EB, Nuxoll E. Synergistic effects of heat and antibiotics on Pseudomonas aeruginosa biofilms. Biofouling. 2017;33:855–866.
- Muller CW, ElKashef T, Pfeifer R, et al. Transcutaneous electromagnetic induction heating of an intramedullary nickel-titanium shape memory implant. Int Orthop. 2014;38:2551–2557.
- Muller CW, Pfeifer R, Meier K, et al. A novel shape memory plate osteosynthesis for noninvasive modulation of fixation stiffness in a rabbit Tibia Osteotomy model. Biomed Res Int. 2015;2015:652940.
- Fang C-H, Tsai P-I, Huang S-W, et al. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect Dis. 2017;17:516.
- ICNIRP. ICNIRP Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (100kHz to 300GHz). 2018 [cited 2019 Oct 7]. Available from: https://www.icnirp.org/cms/upload/consultation_upload/ICNIRP_RF_Guidelines_PCD_2018_07_11.pd
- van den Tempel N, Horsman MR, Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperthermia. 2016;32:446–454.
- Gazel D, Yilmaz M. Are infectious diseases and microbiology new fields for thermal therapy research? Int J Hyperthermia. 2018;34:918–924.
