 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
Intravenous chemotherapy plus abdominal locoregional hyperthermia is explored as a noninvasive alternative to hyperthermic intraperitoneal chemotherapy (HIPEC) in treatment of peritoneal carcinomatosis (PC). First clinical results demonstrate feasibility, but survival data show mixed results and for pancreatic and gastric origin results are not better than expected for chemotherapy alone. In this study, computer simulations are performed to compare the effectiveness of peritoneal heating for five different locoregional heating systems.
Methods
Simulations of peritoneal heating were performed for a phantom and two pancreatic cancer patients, using the Thermotron RF8, the AMC-4/ALBA-4D system, the BSD Sigma-60 and Sigma-Eye system, and the AMC-8 system. Specific absorption rate (SAR) distributions were optimized and evaluated. Next, to provide an indication of possible enhancement factors, the corresponding temperature distributions and thermal enhancement ratio (TER) of oxaliplatin were estimated.
Results
Both phantom and patient simulations showed a relatively poor SAR coverage for the Thermotron RF8, a fairly good coverage for the AMC-4/ALBA-4D, Sigma-60, and Sigma-Eye systems, and the best and most homogeneous coverage for the AMC-8 system. In at least 50% of the peritoneum, 35–45 W/kg was predicted. Thermal simulations confirmed these favorable peritoneal heating properties of the AMC-8 system and TER values of ∼1.4–1.5 were predicted in at least 50% of the peritoneum.
Conclusion
Locoregional peritoneal heating with the AMC-8 system yields more favorable heating patterns compared to other clinically used locoregional heating devices. Therefore, results of this study may promote the use of the AMC-8 system for locoregional hyperthermia in future multidisciplinary studies for treatment of PC.
Introduction
Peritoneal carcinomatosis (PC) is intraperitoneal dissemination of any form of cancer that does not originate from the peritoneum itself. PC can occur in various gastrointestinal and gynaecological malignancies. Patients with PC have a poor prognosis and in non-gynaecological malignancies such as colorectal, gastric, and pancreatic cancer the overall survival is only a few months [Citation1,Citation2]. PC is considered a locoregional disease and in absence of other systemic metastases, multimodality approaches combining cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) have been proven to significantly increase locoregional control and survival [Citation3–5]. During HIPEC, intraperitoneal chemotherapy is combined with hyperthermia (i.e., heating of the peritoneal cavity to 39–43 °C), thereby increasing the efficacy of selected chemotherapeutics. The thermal enhancement ratio (TER), i.e., the ratio between the treatment time T0 required to reduce tumor cell survival with a factor 1/e for treatment at 37 °C to T0 at a hyperthermic temperature level, increases significantly as a function of thermal dose [Citation6]. Clinical results of HIPEC treatments are very good. For example, a randomized trial demonstrated the efficacy of HIPEC compared to standard treatment in patients with PC of colorectal cancer origin [Citation7]. The median survival almost doubled from 12.6 months in the standard therapy arm to 22.3 months in the HIPEC arm, after a median follow-up period of 21.6 months [Citation7]. A recent phase III study for ovarian cancer comparing cytoreductive surgery with surgery plus HIPEC showed that with HIPEC the median overall survival increased from 33.9 to 45.7 months after a median follow-up of 4.7 years, with no significant difference in side effects [Citation8].
Pancreatic cancer is a biologically aggressive disease and even with a complete visible resection, (peritoneal) micro-metastases are likely to remain behind. HIPEC could therefore be a very effective adjuvant treatment. A prospective study adding HIPEC to microscopically margin-negative (R0) resection included 33 patients and showed a median survival of 13 months, after a median follow-up of 11 months, with a recurrence rate of 60.6% [Citation9]. Recently, Sugarbaker [Citation10] has proposed a regional chemotherapy treatment that consists of HIPEC with gemcitabine combined with long-term normothermic intraperitoneal chemotherapy (gemcitabine) for 6 months postoperatively, which has demonstrated to decrease local-regional failure and seems more adequate to target micro-metastases in the peritoneum, liver, and lymph nodes. A small number of patients have been treated with this regimen so far and results are very promising, with a 3-year survival of 50% and a markedly change in the patterns of surgical treatment failure [Citation10].
HIPEC is an invasive, lengthy and complex procedure, for which patients must be in good clinical condition. As any surgical procedure, HIPEC yields a risk of bleeding and infection. Locoregional hyperthermia of the whole abdomen could be feasible, as first investigated in dogs [Citation11] and subsequently in phase I clinical trials, using intraperitoneal cisplatin and hyperthermia for (relapsed) ovarian carcinoma [Citation12,Citation13]. Therefore, the combination of intravenous chemotherapy with abdominal locoregional hyperthermia is also explored as a noninvasive alternative to HIPEC in treatment of PC [Citation14]. Phase I/II trials with regional hyperthermia applied using the BSD 2000 Sigma-60 and Sigma-Eye systems showed that this treatment combination is well tolerated and survival rates are encouraging in patients with PC originating from colorectal and ovarian cancers [Citation15,Citation16]. The 3-year overall survival was 20–30%, which is comparable to HIPEC [Citation7]. However, the survival data for gastric and pancreatic cancer patients were not better than expected for chemotherapy alone [Citation15]. Locoregional hyperthermia is usually applied for pelvic tumor sites as cervix, rectum, and bladder [Citation17], but the peritoneum extends over a length of 35–40 cm, which is therefore more challenging to heat effectively. The limited axial heating lengths of the locoregional heating systems used in this study probably do not allow adequate heating of the full peritoneum, which could explain the disappointing results for gastric and pancreatic cancer patients.
A loco-regional hyperthermia system with a longer axial heating length could improve results for peritoneal heating of, e.g., pancreatic cancer patients. The 70 MHz AMC-8 system, as developed and clinically used at the Academic Medical Center in Amsterdam, consists of two rings of each four rectangular waveguides [Citation18]. This system was shown to yield a more favorable SAR distribution compared to the single-ring AMC-4 system, which has similar heating properties as the BSD-2000 Sigma-60 system [Citation19].
The AMC-8 system could thus be more effective for locoregional peritoneal heating compared to other clinically used locoregional heating devices. Before evaluating clinical feasibility for this specific purpose, numerical simulations would be very helpful to obtain more insight in the heating properties of this system for peritoneal heating. Hyperthermia treatment planning has been demonstrated a very useful tool to evaluate the heating characteristics of different hyperthermia systems [Citation20–25]. An additional advantage of a simulation study is also the direct comparison between heating systems. All variables can be kept constant in the model and SAR and temperature distributions in realistic anatomies can be evaluated while only the heating system is varied.
In this study, computer simulations are performed to evaluate the potential of external locoregional heating systems for peritoneal heating and whether the AMC-8 system outperforms the other clinically used locoregional heating devices. To this end, we compared the effectiveness of peritoneal heating for the AMC-8 system with other clinically used phased-array locoregional heating devices, i.e., the AMC-4/ALBA-4D system, the BSD 2000 Sigma-60 system, and the BSD 2000 Sigma-Eye system, as well as with a capacitive system similar to the Thermotron RF8. Basic heating patterns are first evaluated in a homogeneous phantom, which is followed by simulations for two pancreatic cancer patients. Additionally, the theoretical thermal enhancement of oxaliplatin is compared to provide an indication of possible enhancement factors. Oxaliplatin is a component of FOLFIRINOX chemotherapy, which is currently standard for pancreatic cancer patients, and the effectiveness of this platinum-based drug component is known to be very sensitive to hyperthermic temperatures [Citation6].
Methods
Simulations were performed using the treatment planning package Plan2Heat, which uses voxel-based finite difference calculations [Citation26]. The resolution applied for all simulations was 2.5 × 2.5 × 2.5 mm3. The definitions of the phantom, heating systems and water boluses were generated using the Generic Object Format [Citation27]. Five clinically used heating systems were modeled; a capacitive system and four phased-array heating devices.
The Thermotron RF8 [Citation28]. This capacitive system operates at 8 MHz and uses a pair of circular electrodes with variable sizes up to 30 cm diameter, depending on the heating target. Since for peritoneal hyperthermia a large volume should be heated, two 30 cm electrodes (anterior-posterior) were considered. For the electrode bolus filling circulating saline (0.4% NaCl [Citation28,Citation29]) was modeled. An overlay bolus was modeled with a salinity based on the study of Kato et al. [Citation30]. This combination with an overlay bolus was demonstrated to be optimal for deep capacitive heating in a previous study [Citation20]. The overlay bolus was assumed to cover an axial length of 40 cm. A typical thickness of the electrode bolus and overlay bolus of ∼1.5 cm and ∼2.5 cm was assumed, respectively [Citation31]. This system, as well as other capacitive systems, provides no power steering possibilities other than electrode selection.
The 70 MHz AMC-4 system [Citation32], with a single ring of four waveguides with an aperture size of 20 × 34 cm2. The water bolus has a length of 40 cm in axial direction. This AMC-4 system is similar to a single ring of the AMC-8 system and to the ALBA-4D system [Citation18,Citation33]. This system provides 2D phase-amplitude steering.
The BSD 2000 Sigma-60 system [Citation34,Citation35], with eight paired dipole antennas organized in a single ring with a diameter of 58 cm and a length of 50 cm. The dipole antennas have a length of 45 cm. The water bolus is 36 cm long and fills the space between the patient and the antennas. The frequency range is variable between 60 and 120 MHz. In this study, 70 MHz was selected to achieve deep heating with a large focus size. This system also provides 2D phase-amplitude steering.
The BSD 2000 Sigma-Eye system [Citation36], with 24 paired dipoles in three rings, providing 3D phase-amplitude steering. The applicator has an (approximately elliptical) eye shape, with major and minor axes of 53 and 38 cm, respectively, and a length of 50 cm. The dipole antennas are 14 cm long and a water bolus of 50 cm length fills the space between the patient and the antennas. The operating frequency is fixed to 100 MHz.
The 70 MHz AMC-8 system [Citation18], with two rings of each four waveguides with an aperture size of 20 × 34 cm2. The water bolus has a length of 70 cm in axial direction. The position of the waveguides is based on the patient’s body dimensions and according to clinical practice a distance of ∼5 cm is considered, yielding a 5-cm thick water bolus. The distance between the rings can be varied to adjust the axial heating length. A distance between the two rings of 10 cm was considered, i.e., approximately the maximum distance still yielding a single heating focus [Citation18]. This system also provides 3D phase-amplitude steering.
The applicator models of the AMC-4 and AMC-8 system have been validated by comparing SAR measurements and simulations for heating homogeneous and inhomogeneous tissue-equivalent phantoms [Citation37,Citation38]. The models of the Sigma-60 and Sigma-Eye applicators as implemented in Plan2Heat were validated using phantom measurements as published by van Rhoon et al. [Citation35]. Modeling of capacitive electrodes, as used in the Thermotron RF8, was validated using analytical solutions of a phantom problem [Citation29].
SAR calculations and optimization
For treatment planning, the target region (peritoneum) was positioned centrally in axial direction, i.e., at the transversal mid-plane of each applicator system. The power distribution resulting from capacitive heating was calculated by solving the quasi-static formulation of Maxwell’s equations. This is a valid approach because of the low frequency applied for capacitive heating [Citation29]. When writing the electric field vector as with V the potential, the quasi-static formulation yields an elliptic partial differential equation to be solved for V [Citation39]. The electrode plates at the top and bottom were kept at a constant potential of 1 V and −1 V, respectively. The boundaries of the simulation domain were fixed at zero potential.
For the radiative phased array systems, electric fields were calculated for each antenna (pair) separately, with unit amplitude and zero phase, using the finite difference time domain method [Citation40], with perfectly matched layer boundary conditions [Citation41]. To maximize the specific absorption rate (SAR) coverage in the target volume, phases and amplitudes were optimized to yield a maximum SAR50, i.e., the SAR at least achieved in 50% of the peritoneal target volume, using a standard steepest descent method [Citation42]. This optimization method modifies the steering vector containing the amplitudes and phases in small steps from a start vector in the direction of maximal improvement of the objective function, which is defined as:
where Ptot is the total power, Pi the power delivered by antenna (pair) i and fmin and fmax are the lower and upper limit for the relative power, respectively. These constraints to the output power of each antenna (pair) were set to avoid clinically unrealistic optimal settings, e.g., one antenna (pair) delivering almost all power and others almost switched off. An antenna of the AMC-4 or antenna pair of the Sigma-60 system should deliver at least 10% (fmin = 0.1) and at most 40% (fmax = 0.4) of the total amount of applied power delivered by all antennas or antenna pairs together, in order to avoid clinically irrelevant settings. For the AMC-8 system, this was at least 5% and at most 25% and for the Sigma-Eye system at least 3% and at most 15%. Air in the peritoneum was not taken into account during optimization and evaluation.
The (optimized) SAR distribution was calculated for each heating system and for a straight comparison the absorbed power in the phantom or patient was scaled according to the actively heated length, i.e., the axial length covered by the water bolus. For the AMC-8 system, an absorbed power of 1000 W was assumed and by scaling this yields 571 W for the AMC-4/ALBA-4D and the Thermotron RF8, and 514 W and 714 W for the Sigma-60 and the Sigma-Eye system, respectively. The 1 cc average SAR distribution was calculated for evaluation. Dielectric tissue properties were obtained from the literature and are listed in [Citation43–45].
Table 1. Values of the electrical tissue properties at 8, 70, and 100 MHz used in the simulations [Citation43–45]; electrical conductivity (σ [S m−1]); and relative permittivity (εr [−]).
Simulation of basic heating characteristics
Basic characteristics for heating a large peritoneal region were first evaluated for a homogeneous muscle-equivalent structure. For this purpose, heating of a standard muscle-equivalent elliptical quality assurance phantom was modeled. The phantom has minor and major axes of 24 and 36 cm, respectively, and a 2-mm thick PVC shell. To mimic a peritoneal target region, a 40-cm long elliptical region was defined with minor and major axes of 10 and 25 cm, respectively, shifted 4 cm off-center toward the top antenna(s). SAR calculations and optimization were performed as described above. The difference in (1 cc) SAR coverage between the five systems was evaluated.
Patient simulations
To evaluate clinically realistic heating patterns in the peritoneum clinical CT scans of two representative (i.e., normal BMI and relatively low fat percentage [Citation46,Citation47]), pancreatic cancer patients, one male and one female, were used for hyperthermia treatment planning. The anonymized CT datasets were analyzed from patients treated within the HEAT trial (NCT01077427) in accordance with regulations of the local ethical committee. The scans were sufficiently long to contain the full peritoneum. The CT data set was segmented automatically into muscle, fat, bone, lung tissue, and air, based on the CT Hounsfield Units [Citation48]. The available delineations made by a clinician included the liver, kidneys, tumor target region and the peritoneum. Liver and kidney delineations were rasterized in the segmented model. The tumor target region and the peritoneum were modeled as inhomogeneous regions according to the automatic Hounsfield Unit based segmentation and the delineations were used to identify these regions during optimization and evaluation. This yields a realistic patient model with eight different tissues/organs in the abdominal region with the highest contrast in dielectric and thermal properties, allowing a fair qualitative comparison between the different heating devices. SAR calculations and optimizations were performed. The difference in SAR coverage between the different devices was evaluated, both for the tumor target region and the peritoneum separately.
Next, the temperature distributions corresponding to the (optimized) SAR distributions were predicted by solving Pennes’ bio heat equation [Citation49]:
(1)
(1)
with c (J kg−1 °C−1) the specific heat capacity, ktis (W m−1 °C−1) the thermal conductivity, cb (J kg−1 °C−1) the specific heat capacity of blood, Wb (kg m−3 s−1) the volumetric perfusion rate, and Tart (°C) the local arterial or body core temperature. The first term on the right hand side represents the heat conduction in tissue, the second term models the heat removal by blood perfusion. Thermal properties were taken from the literature and are listed in [Citation14,Citation43,Citation45]. Perfusion values were enhanced with a factor 6–8 for fat and muscle tissue to mimic hyperthermic conditions [Citation14,Citation50], which is based on in vivo data from a historical patient group [Citation51]. Constant perfusion levels representative for the high end of the hyperthermia temperature range were modeled to represent a worst-case prediction. The temperatures of the overlay and electrode boluses of the Thermotron RF8 system were fixed at 0 °C (antifreeze is added in clinical practice to lower the freezing point [Citation52]). The water bolus temperature of the AMC-4/ALBA-4D and AMC-8 systems was 12 °C; and for the Sigma-60 and Sigma-Eye systems this was 20 °C, according to clinical practice for locoregional heating with these devices [Citation28,Citation52–54]. Predicted temperature distributions for all systems were evaluated by means of T10, T50, and T90; the temperature at least achieved in 10, 50, and 90% of the target volume, respectively, for both the tumor region and the peritoneum.
Table 2. Values of the thermal tissue properties used in the simulations [Citation14,Citation43,Citation45]; density (ρ [kg m−3]), specific heat capacity (c [J kg−1 °C−1]), thermal conductivity (k [W m−1 °C−1]), and perfusion (Wb [ml 100 g−1 min−1]).
Chemosensitization by hyperthermia is known to be temperature-dependent [Citation6,Citation55]. Standard chemotherapy treatment for pancreatic cancer patients is presently FOLFIRINOX, of which oxaliplatin is a drug component that is very sensitive to heat. Therefore, as an indication of the difference in TER for heating with the various devices, the theoretical TER values of oxaliplatin were evaluated. To estimate the temperature-dependent TER, the extensive experimental data published by Urano and Ling were used, reporting TER values for oxaliplatin at six temperature levels (37, 39, 41, 42, 43, and 44.5 °C) [Citation6]. The TER was described as a function of temperature by fitting two second-order polynomials to the data points; one for the ranges 37–42 °C and one for the range >42 °C. These fits provided an accurate description of the published measurement data (R2 = 1), and allow to estimate the TER for any temperature in the hyperthermia range. The theoretical TER values for the different heating devices in the peritoneum were evaluated.
Treatment planning yields an uncertainty in temperature predictions due to the variation in dielectric and thermal tissue properties [Citation56]. To obtain an indication of how these uncertainties in temperature predictions affect predicted TER values, 1 °C was added and subtracted from the predicted temperature distribution, based on results by De Greef et al. [Citation56], and the theoretical TER values for these temperature distributions were estimated as an upper and lower range of the variation in TER. Although in regions with relatively low temperatures (38–39 °C) a deviation of −1 °C might be quite large, this uniform subtraction and addition was chosen to provide a worst case prediction how uncertainties in temperature predictions affect predicted TER values, and especially the minimum TER that can be expected.
Results
Basic heating characteristics
Results of the phantom simulations demonstrate the basic characteristics for heating a large peritoneal region with different locoregional heating devices and the 1 cc average SAR distributions are shown in . For capacitive heating using the Thermotron RF8, the SAR distribution in the target region is fairly homogeneous, though the absolute SAR values are rather low in comparison to the other heating devices. This can be explained by the fact that the PVC shell of the phantom has more or less similar dielectric properties as a fat layer, which means that a large amount of power is absorbed in this superficial layer, thereby limiting target heating (see also Discussion section). This is in line with previously published clinical and numerical results [Citation20,Citation57,Citation58]. Simulated (axial) SAR coverage for the AMC-4/ALBA-4D, the BSD Sigma-60, and Sigma-Eye systems are fairly reasonable though with these systems the SAR distribution does not cover the full 40 cm axial length of the target region. The AMC-8 system clearly shows the best axial SAR coverage for this large target region.
Figure 1. Predicted (1 cc average) SAR distributions for the five different hyperthermia systems heating a homogeneous muscle-equivalent quality assurance phantom with a PVC shell. A cylindrical target region (10 × 25 × 40 cm) was defined to mimic peritoneal heating, as indicated by the pink contour. Slices were taken through the center of the target.
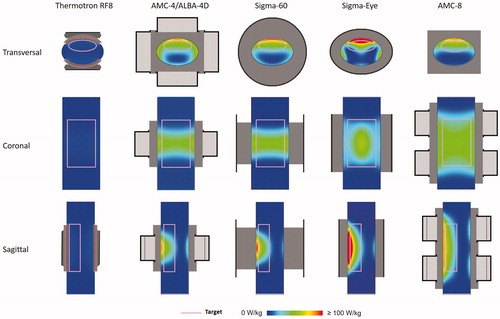
shows the (average 1 cc) SAR-volume histogram for the cylindrical target region in this phantom. An overview of the target SAR50 values, the maximum SAR values in the target, as well as the maximum SAR values elsewhere, are listed in . The SAR50 value for the Thermotron RF8 is 5.7 W/kg. For the AMC-4/ALBA-4D system and the BSD Sigma-60 and Sigma-Eye systems, the target SAR values are much better, with a SAR50 between 21 and 27 W/kg. Although SAR coverage with these systems is more or less comparable, the BSD Sigma-Eye system shows an increased inhomogeneity with maximum overall SAR values reaching up to 195 W/kg. The AMC-8 system provides most adequate heating of this large region, with a predicted SAR50 of 38.2 W/kg and also a relatively homogeneous distribution compared to the other phased array heating systems, with maximum overall SAR values up to ∼90 W/kg. This would be favorable for peritoneal heating: effectively covering a large region, without a high risk of treatment limiting hot spots elsewhere.
Figure 2. Predicted (1 cc average) SAR volume histogram of the cylindrical peritoneum-mimicking target region (10 × 25 × 40 cm), simulated in a standard homogeneous muscle-equivalent quality assurance phantom with a PVC shell, for different locoregional heating devices.
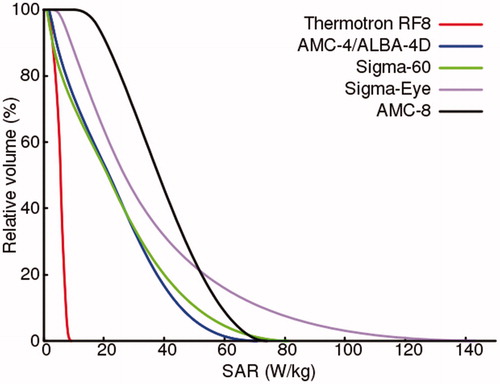
Table 3. Values of the simulated target (1 cc average) SAR50 values, the maximum SAR values in the target, as well as the overall maximum SAR values for heating a homogeneous phantom with a PVC shell using different locoregional heating devices.
Patient simulations
To determine whether these favorable peritoneal heating properties of the AMC-8 system compared to other locoregional heating devices also apply for realistic patient anatomies, simulations were performed for peritoneal heating of two pancreatic cancer patients, one female (patient 1) and one male (patient 2). and show the simulated 1 cc average SAR distributions for both patients and all five heating systems. Results are in line with the phantom simulations. For capacitive heating (Thermotron RF8), the large power absorption in the superficial fat layers limits adequate target heating. A reasonably good SAR coverage is observed for the AMC-4/ALBA-4D and the BSD Sigma-60 and Sigma-Eye systems, for both the tumor target region and the peritoneum, but the AMC-8 system yields the most favorable SAR patterns.
Figure 3. Predicted (1 cc average) SAR distributions for the five different hyperthermia systems heating the peritoneum for patient 1 (female).
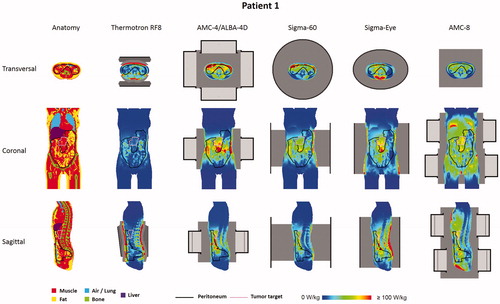
Figure 4. Predicted (1 cc average) SAR distributions for the five different hyperthermia systems heating the peritoneum for patient 2 (male).
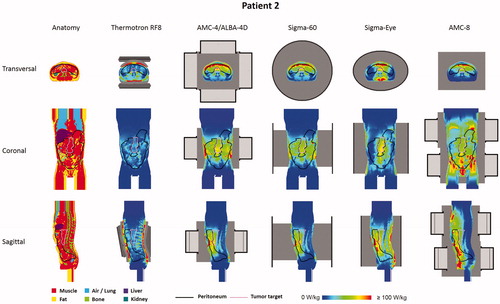
shows (1 cc average) SAR-volume histograms for both the peritoneum and the tumor target. summarizes the SAR50 and maximum SAR values in the tumor target and the peritoneum, as well as the overall maximum SAR values, for both patients. These values quantify the observations from the distributions in and . With capacitive heating SAR50 values in both the tumor target and the peritoneum are relatively low (∼15 W/kg), while the maximum SAR values in the superficial fat layer are very high (>650 W/kg). Although the most superficially located SAR maxima will be (partly) suppressed by bolus cooling during locoregional heating, this absorption is relatively high and still yields a risk of inducing treatment limiting hot spots. With the AMC-4/ALBA-4D and the BSD Sigma-60 and Sigma-Eye systems, more adequate SAR coverage of the peritoneum and the tumor target is achieved with SAR50 values for the peritoneum and the tumor target typically in the range of 25–35 W/kg and 45–55 W/kg, respectively. Superficial SAR values with these systems are a factor 2.5–4.5 lower compared with the Thermotron RF8 and the most superficial SAR hot spots will not result in temperature hot spots due to bolus cooling, similar to locoregional pelvic heating. As indicated by the SAR-volume histograms, the AMC-8 system yields the best and most homogeneous SAR coverage of the peritoneum, also with a good coverage of the tumor target. The SAR50 values in the peritoneum were 36.3 and 44.7 W/kg for patients 1 and 2, respectively, with maximum overall SAR values of 234.4 and 255 W/kg, respectively, which is more or less comparable to the maximum SAR values predicted for the other locoregional heating devices.
Figure 5. Predicted (1 cc average) SAR–volume histogram of the peritoneum and tumor target (insert) for both patients, for locoregional heating with different hyperthermia devices.
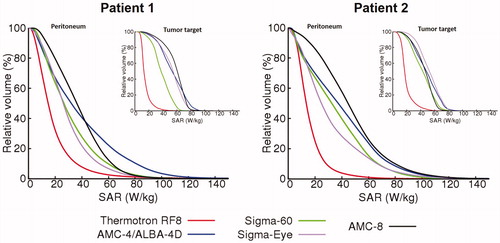
Table 4. Values of the simulated (1 cc average) SAR50 and maximum SAR values in the peritoneum and the tumor target, as well as the overall maximum SAR for heating two pancreatic cancer patients with five different locoregional devices.
Temperature distributions and thermal enhancement
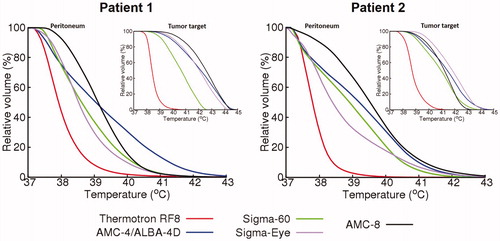
The temperature distributions corresponding to the (optimized) SAR distributions were simulated and the temperature-volume histograms predicted for the peritoneum and the tumor target are shown in . These graphs confirm the conclusions from the SAR distributions and show that the AMC-8 system provides the most homogeneous and effective heating of the peritoneum, with a T50 value of 39–39.5 °C. Although heating was optimized for the peritoneum, the tumor target is also heated effectively for all phased-array systems.
Figure 6. Predicted temperature–volume histogram of the peritoneum and tumor target (insert) for both patients, for locoregional heating with different hyperthermia devices.
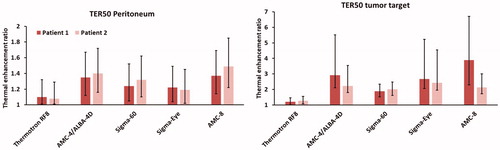
As an indication of the difference in TER for heating with the various devices, the theoretical TER values of oxaliplatin were evaluated. The TER values at least achieved in 50% of the peritoneum or tumor target are listed in . These values show that reasonable TER values are predicted for all phased-array systems, but for the AMC-8 system the highest TER values are predicted in the peritoneum, with a TER50 of 1.4 and 1.5 for patient 1 and 2, respectively. This would imply an effective treatment, since clinical TER values for hyperthermia are typically around 1.2–1.3. A TER50 of ∼2 or higher is predicted for the tumor target for all phased-array systems.
Table 5. Predicted TER for oxaliplatin achieved in at least 50% of the peritoneum or tumor target (TER50) for both patients, for locoregional heating with different hyperthermia devices.
shows how uncertainties in temperature predictions of ± 1 °C affect the predicted TER values. The colored bars represent the values listed in and the error bars represent the TER50 values for temperature distributions 1 °C lower or higher than the predicted temperature distributions. Although the uncertainty becomes larger for higher TER values, a trend remains that the TER50 is higher with the AMC-8 system compared to the other systems. Adding this uncertainty, the minimum expected TER50 value in the peritoneum is 1.14 and 1.22 for patients 1 and 2, respectively, which is still higher compared to the other devices. The minimum expected TER50 for the tumor target is still ∼1.5 or higher for all phased array systems.
Figure 7. Predicted TER and the variation in TER for oxaliplatin achieved in at least 50% of the peritoneum or tumor target (TER50) for both patients, for locoregional heating with different hyperthermia devices, when assuming an uncertainty in the temperature distribution of ± 1 °C. The Colored bars represent the values listed in and the error bars represent the TER50 values for temperature distributions 1 °C lower or higher than the predicted temperature distributions.
Discussion
This study indicated that peritoneal heating with the AMC-8 system provides a better target coverage compared with commonly used locoregional heating devices: the capacitive Thermotron RF8 system and the radiative AMC-4, BSD Sigma-60 and BSD Sigma-Eye systems. The AMC-4 and Sigma-60 systems both provide 2D steering, while the Sigma-Eye and the AMC-8 system both provide 3D steering and thus more degrees of freedom to steer the power distribution according to treatment requirements. The main differences between the AMC-8 and the Sigma-Eye system are the operating frequency and the heating length. A fair comparison was made, with an equivalent amount of absorbed power in the patient for each heating system, relative to the heating length. Cross-coupling between antennas was not accounted for in the simulations. This phenomenon can reduce efficiency and affect control during clinical applications, especially for the dipole arrays [Citation59,Citation60], but on-line adjustments can overcome this [Citation61]. The improved peritoneal coverage demonstrated for the AMC-8 system is mainly because of the larger axial heating length of 70 cm covered by the waveguides and the water boluses, which is 40–95% larger compared with the 36–50 cm for the other heating devices. Because of this long heating length both the primary tumor location and the peritoneal (micro)metastases could be heated effectively in the same hyperthermia session using the AMC-8 system. So far, the AMC-8 system has been applied clinically for pelvic heating and treatments were well tolerated, without signs of unacceptable systemic heating [Citation62]. The AMC-4 system has recently been commercialized as the ALBA-4D system [Citation33]. When clinical results are supportive, the AMC-8 system may be expected to be commercialized also in the near future.
For heating with the Thermotron RF8 significantly large power absorption in fatty tissue is a limiting factor to achieve a high SAR50 in the peritoneum. This can be explained by the fact that the electrodes produce an electric field (E-field) that is oriented mainly perpendicular to the (superficial) fat–muscle interfaces, while for the radiative antennas the main E-field direction is parallel to the superficial fat–muscle interfaces. The interface conditions derived from Maxwell’s equations prescribe that the tangential E-field is continuous, while the normal E-field component undergoes a discontinuity proportional to the dielectric properties of the two different tissues. This yields a relatively high E-field value in fatty tissue for the capacitive heating, and thus, despite the low electrical conductivity, a relatively high energy absorption. Therefore, this is not unique for the Thermotron RF8, but applies to other capacitive devices as well. The choice of bolus is important for capacitive heating to reduce heating of the superficial fat layers [Citation30]. The set-up modeled in this study was optimized by Kato et al. [Citation30] and a previous simulation study showed that this indeed yields more favorable heating patterns than saline electrode boluses alone or electrode boluses with distilled water [Citation20].
Since effective heating of the peritoneum substantially improves for the AMC-8 system compared with the other systems, also the homogeneity of the temperature distribution and the TER improves. Nevertheless, also with the AMC-8 system the temperature distribution will still be somewhat heterogeneous, mainly due to the heterogeneity of the power deposition in combination with heterogeneities in tissue properties and blood perfusion. This is inherent to locoregional heating, though similar temperature (and thus TER) heterogeneities are also present during HIPEC treatments. Temperature measurements during HIPEC are usually sparse, though Rettenmaier et al. prospectively evaluated the temperature at five upper abdominal, mid-abdominal, and supra-pubic sites in 11 advanced stage ovarian cancer patients treated with HIPEC [Citation63]. The median temperature at the inflow locations was 42.6 °C. The median temperatures at the abdominal locations ranged between 41.1 °C and 41.9 °C, with outliers as low as 40.3 °C. Thus, the median abdominal temperature was 1–1.5 °C lower than the inflow temperature, with outliers of more than 2 °C, demonstrating that temperature heterogeneity also occurs during HIPEC.
In theory, the temperature homogeneity with locoregional heating could be improved by switching between different antenna settings during treatment or by changing the frequency (if possible). The potential benefit of such a strategy has been explored for locoregional pelvic heating to suppress hot spots while maintaining tumor coverage [Citation64]. However, this strategy has not yet been applied in clinical practice, which might also be challenging because of the impact of uncertainties in pretreatment planning. Furthermore, the strategy should be adapted for peritoneal heating to aim at homogeneous heating of a large region, rather than hot spot suppression. This would require further research on novel optimization strategies, from which typically 3D heating systems as the Sigma-Eye and the AMC-8 system will benefit because of the larger number of degrees of freedom.
Whole body hyperthermia (WBHT) increases the body core temperature to 39–40 °C (fever range) or 41–42 °C (extreme WBHT) with systems using non-ionizing, electromagnetic energy, such as infrared or microwave. This could be an alternative to realize homogeneous temperatures throughout the peritoneum and phase I/II studies showed promising results for, e.g., colorectal and ovarian cancer [Citation65,Citation66]. However, whole body heating is quite a burdening treatment for the patient, is associated with relatively large toxicity and the target temperature is limited to 41.8 °C. Furthermore, it requires general anesthesia or deep analgesic sedation, which makes whole body heating also logistically demanding. Under clinical conditions the number of WBHT sessions is limited to 3–6. Therefore, whole body hyperthermia would not be very suitable as standard treatment option for patients with peritoneal metastases.
A less demanding strategy to improve the homogeneity of the temperature and drug distributions in the peritoneum would be to combine a closed HIPEC procedure with external locoregional hyperthermia. With this combination, the chemotherapy perfusate will be preheated before entering the body and the internal heat distribution will be enhanced by a mild external heating; a strategy rather similar to hyperthermia treatments performed for non-muscle invasive bladder cancer at some departments [Citation67,Citation68]. When inflow and outflow positions are chosen adequately, a more homogeneous distribution could be realized. Advanced numerical modeling including fluid dynamics to accurately model convective heat transport in fluids would be very helpful to provide more insight in the potential of such a treatment combination. Recently, we developed a thermodynamic fluid model as an extension to Plan2Heat to improve temperature predictions for bladder hyperthermia. This fluid dynamics modeling module has been validated experimentally, showing an accuracy of ∼0.1 °C, and has been applied successfully for (retrospective) hyperthermia treatment evaluation of bladder cancer patients [Citation69]. These fluid dynamics simulations are computationally intensive, which currently limits routine clinical application. Nevertheless, this module provides an excellent basis for HIPEC modeling, which is subject of ongoing research and techniques to optimize mesh generation and reduce computation times are investigated.
With the AMC-8 system, a larger volume is heated in comparison with the other systems, which means that the inflowing arterial blood is likely to be subject to some level of preheating and some (mild) systemic temperature increase might occur, which has an important impact on the heat transfer via the vasculature. This explains why clinical use of the AMC-8 system for standard pelvic tumor heating shows similar tumor temperatures compared with the AMC-4 system when using the same amount of total power for both systems [Citation62], which is not predicted by the widely adopted Pennes model used in this study [Citation49]. According to the Pennes model, almost twice the amount of total power should be required for the AMC-8 system to reach the same tumor temperature [Citation24], as was also modeled in this study. Advanced discrete vasculature modeling would improve temperature predictions, as it accounts for the interaction between large blood vessels and tissue [Citation70,Citation71]. This larger role of perfusion related heat transport within the heated region is also likely to result in more homogenous tissue temperature distributions for the AMC-8 system compared to temperature uniformity in the relatively smaller heated regions of the other hyperthermia devices discussed. Thus, because of these favorable perfusion related heat transport effects when heating a longer volume in one single session, an alternative approach to overcome the suboptimal heating length of the other radiative devices by dividing treatment of PC into two separate hyperthermia sessions for the more distal and the more proximal part of the peritoneum would result in a combined temperature distribution that would be less homogeneous than the temperature distribution achieved in a single session of the AMC-8 system.
Recording the temperature in the target volume or surrounding tissue is essential to monitor treatment quality, as prescribed in the Quality Assurance (QA) guidelines for locoregional hyperthermia [Citation72]. Ideally, noninvasive MR-thermometry would provide detailed 3D temperature information. However, the AMC-8 system contains large metal waveguides and is not MR compatible. Furthermore, MR thermometry in the abdomen is still very challenging and does not provide sufficient accuracy because of artifacts due to bowel motion and heterogeneities [Citation73]. The mean MR temperature in the liver could be used as a QA reference parameter, since the liver is the drainage area of the portal vein. Therefore, liver temperature is correlated to the mean temperature increase in the peritoneum. In case MR thermometry would be applied, e.g., when using the Sigma-Eye applicator in a hybrid system, QA guidelines prescribe that additional thermometry using temperature probes is still required [Citation72]. Invasive temperature measurements in the peritoneum would require laparoscopic placement or radiological intervention, which is associated with discomfort and a serious risk of complications. This makes invasive thermometry less suitable for routine clinical use in these patients. During locoregional heating of pelvic tumors, usually standard minimally invasive thermometry in existing body cavities (vagina, rectum, bladder) is applied for treatment monitoring. For peritoneal heating of pancreatic cancer patients, an additional minimally invasive probe could be inserted under endoscopic guidance via the nose, through the esophagus, stomach and duodenum to the approximate location of the pancreas [Citation74] to provide maximum minimally invasive thermometry feedback for treatment guidance.
Minimally invasive thermometry in the pelvic region was also applied in the first clinical applications of locoregional hyperthermia for patients with PC [Citation14]. To obtain a more detailed estimation about the 3D temperature distribution, the noninvasive measurements can be combined with sophisticated treatment planning tools, where simulations are matched to the reference temperature measurements as described by Beck et al. [Citation14]. During treatment, similar on-line adaptive treatment planning strategies as have been reported for pelvic heating can be used to optimize the target temperature distribution and avoid treatment limiting hot spots [Citation75,Citation76], thereby optimizing treatment quality.
This simulation study evaluated peritoneal heating for pancreatic cancer patients, but there is a much wider spectrum of oncological indications for peritoneal heating, including colorectal cancer, ovarian cancer and gastric cancer. Since feasibility of locoregional hyperthermia with the BSD Sigma-60 and Sigma-Eye systems was already demonstrated in phase I/II trials in patients with PC originating from colorectal and ovarian cancer, with encouraging patient survival [Citation15,Citation16], further clinical application using the AMC-8 system would be a logical next step to further improve clinical results for locoregional hyperthermia in patients with PC.
Conclusion
The results of this study suggest that locoregional peritoneal heating with the AMC-8 system yields the most homogeneous SAR coverage of the peritoneum compared to other clinically used locoregional heating devices. Thermal simulations confirmed these favorable peritoneal heating properties of the AMC-8 system and TER values of ∼1.4–1.5 were predicted in at least 50% of the peritoneum. Previous phase I/II studies showed that locoregional heating of the peritoneum is feasible, but suboptimal in terms of temperature and clinical results for gastric and pancreatic cancer patients. Therefore, results of this study may promote the use of the AMC-8 system for locoregional hyperthermia in future multidisciplinary studies for the treatment of PC in gastric and pancreatic cancer patients, as well as for PC originating from other tumor sites.
Disclosure statement
P. Ghadjar received consulting fees from BYTEC Medizintechnik GmbH and Dr. Sennewald Medizintechnik GmbH.
Additional information
Funding
References
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363.
- Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. WJG. 2013;19(41):6979–6994.
- Helderman R, Loke DR, Kok HP, et al. Variation in clinical application of hyperthermic intraperitoneal chemotherapy: a review. Cancers. 2019;11(1):78.
- Wu Q, Wu Q, Xu J, et al. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: a meta-analysis. Int J Hyperthermia. 2019;36(1):562–572.
- Wisselink DD, Braakhuis LLF, Gallo G, et al. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol/Hematol. 2019;142:119–129.
- Urano M, Ling CC. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int J Hyperthermia. 2002;18(4):307–315.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Tentes AA, Stamou K, Pallas N, et al. The effect of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int J Hyperthermia. 2016;32(8):895–899.
- Sugarbaker PH. Strategies to improve local control of resected pancreas adenocarcinoma. Surg Oncol. 2017;26(1):63–70.
- Zakris EL, Dewhirst MW, Riviere JE, et al. Pharmacokinetics and toxicity of intraperitoneal cisplatin combined with regional hyperthermia. JCO. 1987;5(10):1613–1620.
- Jones E, Alvarez Secord A, Prosnitz LR, et al. Intra-peritoneal cisplatin and whole abdomen hyperthermia for relapsed ovarian carcinoma. Int J Hyperthermia. 2006;22(2):161–172.
- Leopold KA, Oleson JR, Clarke-Pearson D, et al. Intraperitoneal cisplatin and regional hyperthermia for ovarian carcinoma. Int J Radiat Oncol Biol Phys. 1993;27(5):1245–1251.
- Beck M, Ghadjar P, Weihrauch M, et al. Regional hyperthermia of the abdomen, a pilot study towards the treatment of peritoneal carcinomatosis. Radiat Oncol. 2015;10(1):157.
- Cho C, Wust P, Hildebrandt B, et al. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: evaluation of two annular-phased-array applicators. Int J Hyperthermia. 2008;24(5):399–408.
- Fotopoulou C, Cho CH, Kraetschell R, et al. Regional abdominal hyperthermia combined with systemic chemotherapy for the treatment of patients with ovarian cancer relapse: results of a pilot study. Int J Hyperthermia. 2010;26(2):118–126.
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497.
- Crezee J, Van Haaren PMA, Westendorp H, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: a preclinical study. Int J Hyperthermia. 2009;25(7):581–592.
- Schneider CJ, van Dijk JD, De Leeuw AA, et al. Quality assurance in various radiative hyperthermia systems applying a phantom with LED matrix. Int J Hyperthermia. 1994;10(5):733–747.
- Kok HP, Navarro F, Strigari L, et al. Locoregional hyperthermia of deep-seated tumours applied with capacitive and radiative systems: a simulation study. Int J Hyperthermia. 2018;34(6):714–730.
- Paulsen KD, Geimer S, Tang J, et al. Optimization of pelvic heating rate distributions with electromagnetic phased arrays. Int J Hyperthermia. 1999;15(3):157–186.
- Kroeze H, van de Kamer JB, De Leeuw AAC, et al. Regional hyperthermia applicator design using FDTD modelling. Phys Med Biol. 2001;46(7):1919–1935.
- Wust P, Seebass M, Nadobny J, et al. Simulation studies promote technological development of radiofrequency phased array hyperthermia. Int J Hyperthermia. 1996;12(4):477–494.
- De Greef M, Kok HP, Bel A, et al. 3-D versus 2-D steering in patient anatomies: a comparison using hyperthermia treatment planning. Int J Hyperthermia. 2011;27(1):74–85.
- Canters RA, Paulides MM, Franckena M, et al. Benefit of replacing the Sigma-60 by the Sigma-Eye applicator. A Monte Carlo-based uncertainty analysis. Strahlenther Onkol. 2013;189(1):74–80.
- Kok HP, Kotte A, Crezee J. Planning, optimisation and evaluation of hyperthermia treatments. Int J Hyperthermia. 2017;33(6):593–607.
- De Bree J. A 3-D anatomy based treatment planning system for interstitial hyperthermia [PhD thesis]. Utrecht: Utrecht University; 1998.
- Abe M, Hiraoka M, Takahashi M, et al. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer. 1986;58(8):1589–1595.
- Kroeze H, van de Kamer JB, de Leeuw AA, et al. Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia. 2003;19(1):58–73.
- Kato H, Hyodo K, Akasaka N, et al. Optimization of bolus for capacitive type heating. Jpn J Hyperthermic Oncol. 1997;13(1):10–17.
- Kroeze H, Kokubo M, van de Kamer JB, et al. Comparison of a capacitive and a cavity slot radiative applicator for regional hyperthermia. Jpn J Hyperthermic Oncol. 2002;18(2):75–91.
- van Dijk JD, Schneider C, van Os R, et al. Results of deep body hyperthermia with large waveguide radiators. Adv Exp Med Biol. 1990;267:315–319.
- Zweije R, Kok HP, Bakker A, et al. Technical and clinical evaluation of the ALBA-4D 70MHz loco-regional hyperthermia system. Proceedings of the 48th European Microwave Conference; 2018. p. 328–331.
- Turner PF, Tumeh A, Schaefermeyer T. BSD-2000 approach for deep local and regional hyperthermia: physics and technology. Strahlenther Onkol. 1989;165(10):738–741.
- Van Rhoon GC, Van Der Heuvel DJ, Ameziane A, et al. Characterization of the SAR-distribution of the Sigma-60 applicator for regional hyperthermia using a Schottky diode sheet. Int J Hyperthermia. 2003;19(6):642–654.
- Fatehi D, van Rhoon GC. SAR characteristics of the Sigma-60-Ellipse applicator. Int J Hyperthermia. 2008;24(4):347–356.
- Van Haaren PMA, Kok HP, Wiersma J, et al. Faster EM-field calculations for locoregional hyperthermia treatment planning using the FDTD method. 21st Annual Meeting of the European Society for Hyperthermic Oncology, Abstract book, Munich, Germany; 2003. p. 137.
- Van Haaren P, Kok P, Van Stam G, et al. Measurements and FDTD calculations in inhomogeneous phantom models. 9th International Congress on Hyperthermic Oncology, St.Louis, USA. Abstracts; 2004. p. 167.
- de Bree J, van der Koijk JF, et al. A 3-D SAR model for current source interstitial hyperthermia. IEEE Trans Biomed Eng. 1996;43(10):1038–1045.
- Taflove A, Hagness SC. Computational electrodynamics. 2nd ed. Boston, London: Artech House; 2000.
- Berenger JP. A perfectly matched layer for the absorption of electromagnetic waves. Comput Phys. 1994;114(2):185–200.
- Press WH, Flannery BP, Teukolsky SA, et al. Numerical recipes in C. Cambridge: Cambridge University Press; 1988.
- ESHO Taskgroup Committee. Treatment planning and modelling in hyperthermia, a task group report of the European Society for Hyperthermic Oncology. Rome (Italy): Tor Vergata; 1992.
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41(11):2251–2269.
- Hasgall PA, Di Gennaro F, Baumgarter C, et al. IT’IS database for thermal and electromagnetic parameters of biological tissues. Version 3.0. Available from: www.itis.ethz.ch/database. 2015.
- Hendifar AE, Petzel MQB, Zimmers TA, et al. on behalf of the Precision Promise Consortium. Pancreas cancer-associated weight loss. Oncologist. 2019;24(5):691–701.
- Okura T, Fujii M, Shiode J, et al. Impact of body mass index on survival of pancreatic cancer patients in Japan. Acta Med Okayama. 2018;72(2):129–135.
- Hornsleth SN, Mella O, Dahl O. A new segmentation algorithm for finite difference based treatment planning systems. In: Franconi C, Arcangeli G, Cavaliere R, editors. Hyperthermic oncology. Vol. 2. Rome (Italy): Tor Vergata; 1996. p. 521–523.
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122.
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 Suppl):4721s–4730s.
- Sreenivasa G, Gellermann J, Rau B, et al. Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys. 2003;55(2):407–419.
- Ohguri T, Imada H, Yahara K, et al. Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. Int J Hyperthermia. 2004;20(5):465–475.
- Fatehi D, van der Zee J, Van Rhoon GC. Intra-patient comparison between two annular phased array applicators, Sigma-60 and Sigma-Eye: applied RF powers and intraluminally measured temperatures. Int J Hyperthermia. 2011;27(3):214–223.
- Van Haaren PMA, Kok HP, Van den Berg CAT, et al. On verification of hyperthermia treatment planning for cervical carcinoma patients. Int J Hyperthermia. 2007;23(3):303–314.
- Bergs JW, Haveman J, Ten Cate R, et al. Effect of 41 degrees C and 43 degrees C on cisplatin radiosensitization in two human carcinoma cell lines with different sensitivities for cisplatin. Oncol Rep. 2007;18(1):219–226.
- De Greef M, Kok HP, Correia D, et al. Optimization in hyperthermia treatment planning: the impact of tissue perfusion uncertainty. Med Phys. 2010;37(9):4540–4550.
- Kok HP, Crezee J. A comparison of the heating characteristics of capacitive and radiative superficial hyperthermia. Int J Hyperthermia. 2017;33(4):378–386.
- Hiraoka M, Jo S, Akuta K, et al. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer. 1987;60(1):121–127.
- Wust P, Beck R, Berger J, et al. Electric field distributions in a phased-array applicator with 12 channels: measurements and numerical simulations. Med Phys. 2000;27(11):2565–2579.
- Wust P, Fahling H, Wlodarczyk W, et al. Antenna arrays in the SIGMA-eye applicator: interactions and transforming networks. Med Phys. 2001;28(8):1793–1805.
- Weihrauch M, Wust P, Weiser M, et al. Adaptation of antenna profiles for control of MR guided hyperthermia (HT) in a hybrid MR-HT system. Med Phys. 2007;34(12):4717–4725.
- Crezee J, Van Stam G, Sijbrands J, et al. Hyperthermia of deep seated pelvic tumors with a phased array of eight versus four 70 MHz waveguides. Proceedings of the 47th European Microwave Conference; 2017. p. 876–879.
- Rettenmaier MA, Mendivil AA, Gray CM, et al. Intra-abdominal temperature distribution during consolidation hyperthermic intraperitoneal chemotherapy with carboplatin in the treatment of advanced stage ovarian carcinoma. Int J Hyperthermia. 2015;31(4):396–402.
- De Greef M, Correia D, Kok HP, et al. Hyperthermia treatment optimization: a search for alternative optimal settings as a backup during treatment. 25th Annual meeting of the European Society for Hyperthermic Oncology.Abstract book, Verona; June 2009.
- Westermann AM, Grosen EA, Katschinski DM, et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer. 2001;37(9):1111–1117.
- Hildebrandt B, Drager J, Kerner T, et al. Whole-body hyperthermia in the scope of von Ardenne's systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: a phase I/II study. Int J Hyperthermia. 2004;20(3):317–333.
- Geijsen ED, De Reijke TM, Koning CCE, et al. Combining mitomycin C and regional 70 MHz hyperthermia in patients with nonmuscle invasive bladder cancer: a pilot study. J Urol. 2015;194(5):1202–1208.
- Inman BA, Stauffer PR, Craciunescu OA, et al. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2014;30(3):171–175.
- Schooneveldt G, Kok HP, Bakker A, et al. Clinical validation of a novel thermophysical bladder model designed to improve the accuracy of hyperthermia treatment planning in the pelvic region. Int J Hyperthermia. 2018;35(1):383–397.
- Kok HP, Van den Berg CAT, Bel A, et al. Fast thermal simulations and temperature optimization for hyperthermia treatment planning, including realistic 3D vessel networks. Med Phys. 2013;40(10):103303.
- Kotte AN, van Leeuwen GM, Lagendijk JJ. Modelling the thermal impact of a discrete vessel tree. Phys Med Biol. 1999;44(1):57–74.
- Bruggmoser G, Bauchowitz S, Canters R, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol. 2011;187(10):605–610.
- Winter L, Oberacker E, Paul K, et al. Magnetic resonance thermometry: methodology, pitfalls and practical solutions. Int J Hyperthermia. 2016;32(1):63–75.
- Kok HP, de Kroon-Oldenhof R, van Straten LK, et al. RF heating of pancreatic tumours guided by hyperthermia treatment planning and limited thermometry. Proceedings of the 48th European Microwave Conference; 2018. p. 332–335.
- Kok HP, Korshuize-van Straten L, Bakker A, et al. On-line adaptive hyperthermia treatment planning during locoregional heating to suppress treatment limiting hot spots. Int J Radiat Oncol Biol Phys. 2017;99(4):1039–1047.
- Kok HP, Korshuize-van Straten L, Bakker A, et al. Feasibility of on-line temperature-based hyperthermia treatment planning to improve tumour temperatures during locoregional hyperthermia. Int J Hyperthermia. 2018;34(7):1082–1091.
