Abstract
Purpose
To assess the oncologic outcomes of the hepatocellular carcinoma (HCC) patients in challenging locations (CLs) underwent ultrasound-guided percutaneous microwave ablation (US-PMWA) and the efficacy and safety of the advanced assistive technology (AAT).
Materials and methods
Data for 489 treatment-naïve patients with HCC who met Milan criteria and subsequently underwent US-PMWA were reviewed from March 2012 to November 2016. According to the distance (<5 mm) between the tumor and surrounding structures, the patients were divided into two groups: a CL group and a non-CL group. Regarding MWA assisted by AAT, the CL group was further subdivided into two groups: an AAT group and a non-AAT group. Technique effectiveness, complications and survival outcomes (i.e., overall survival [OS] and recurrence-free survival [RFS]) were compared between CL and non-CL groups. Local tumor progression (LTP) was compared between AAT and non-AAT groups.
Results
Technique effectiveness and complications in the CL group were similar to those in the non-CL group (p = .873 and p = .828, respectively). The OS and RFS in six types of CL groups were comparable with those in non-CL group (p = .131–.117) including adjacent vital structures, gallbladder, hepatic hilar regions, major vessels, diaphragm and capsule, respectively. The LTP rates in the AAT group were significantly higher than those in the non-AAT group (p = .001).
Conclusions
US-PMWA assisted by AAT to treat HCC lesions in CLs was safe and effective; also, this technique had comparable success and survival outcomes with those of patients in non-CL.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cancer-related cause of death globally, and its morbidity and mortality are increasing [Citation1,Citation2]. Surgical management is the first-line treatment for HCC. Nevertheless, most patients with HCC are not ideal candidates for surgery due to their poor physical condition or a difficult tumor location [Citation3–5]. Based on the American National Comprehensive Cancer Network (NCCN) guidelines, thermal ablation is recommended for HCC in early stage [Citation6,Citation7]. Microwave ablation (MWA), an alternative option to unresectable HCC with acceptable therapeutic efficiency, has been widely used with several advantages including greater a intratumoral temperature as well as a reduction in operation time and electrical conductivity dependence [Citation8–10]. In spite of the convincing usefulness of MWA, local HCC recurrence is still a key challenge due to a failure to achieve a safe tumor margin of 5–10 mm [Citation11–13]. Additionally, numerous large case series have recommended that topographical factors may impede the therapeutic effectiveness of percutaneous ablation, such as the nodule abutting important organs including the gastrointestinal tract, gallbladder, major vessels, diaphragm, etc. These areas have been regarded as ‘challenging locations’ (CLs) [Citation14]. Due to suboptimal conspicuity, an insufficient electrode path and the possibility of thermal injury to nearby organs, an adequate ablation sphere is difficult to obtain.
Numerous techniques have been established to improve the accuracy and safety of electrode placement and ablation to prevent recurrence including 3-dimensional (3D) visualization ablation planning, intraoperative navigation technology and utilizing artificial ascites or pleural effusion [Citation15–19]. 3D visualization ablation planning incorporates special characteristics such as an interactive manual simulation of the insertion number, a display of the virtual thermal field and a calculation of the distance between the target and surrounding vital structures. Meanwhile, the needle path can be executed accurately by intraoperative navigation technology (e.g., contrast-enhanced ultrasonography and multimodal image fusion guidance), especially in cases where a nodule abutting the diaphragm or rib can result in an image that is occluded via ultrasound, for instance. These two techniques can improve the accuracy of HCC ablations. Moreover, artificial ascites and real-time temperature monitors can be applied to avoid the possibility of ambustion when the lesion is closer to important organs; this approach can improve the safety of HCC ablations.
In this study, we compared different oncologic outcomes among tumors in CLs as well as in easy-to-access locations treated by percutaneous MWA. The objective of this study was to confirm the safety and efficacy of the MWA procedure accompanied by advanced assistive technology (AAT) in HCC lesions.
Materials and methods
Study population and design
This study was conducted according to the principles of the Declaration of Helsinki and was sanctioned by the ethics committee of Chinese PLA General Hospital. Due to the retrospective nature of the study, patients were not required to provide consent for participation. Between March 2012 and November 2016, 1238 patients underwent MWA for HCC in the Department of Interventional Ultrasound at Chinese PLA General Hospital. From these patients, 526 who met the Milan criteria and underwent ultrasound-guided percutaneous MWA (US-PMWA) as first-line treatment were included in our study. HCC was diagnosed as per the guidelines of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases [Citation20]. The inclusion criteria were as follows: (a) patients with Child-Pugh class A or B cirrhosis (Eastern Cooperative Oncology Group performance status 0 and Barcelona Clinic Liver Cancer stage 0 or A), (b) a single tumor smaller than 5 cm or 2–3 tumors smaller than 3 cm according to the Milan criteria, (c) no major vascular incursion or extrahepatic metastasis and (d) surgically unresectable HCC lesions or voluntary patient acceptance of local ablation treatment. The exclusion criteria were: (a) patient death due to reasons other than HCC progressions, such as a traffic accident or heart disease or (b) the patient did not follow-up.
MWA was regarded as a technical success when the target lesion was covered entirely by the ablation area on computed tomography (CT) or magnetic resonance imaging (MRI) images taken during an instant follow-up. If the shortest distance between the lesion and adjacent structures was less than 5 mm, the lesion location was defined as a CL. The 489 patients were divided into two groups according to the tumor location: a CL group (n = 334) and a non-CL group (n = 155). Three hundred thirty-four patients in the CL group were subdivided into two groups according to MWA during the follow-up combined with AAT: an AAT group (n = 281) and a non-AAT group (n = 53) (). The US-PMWA procedure was performed as reported in one of our previous studies [Citation21]. For tumors abutting adjacent vital structures, diaphragm or capsule, artificial ascites were used to separate the tumor before as well as throughout MWA.
Definition of challenging locations
As per the previously published literature and based on our clinical knowledge, we defined six types of CLs as a nodule located less than 5 mm from important organs or structures (), including adjacent vital structures (i.e., heart, stomach, colon and kidney), the gallbladder, hepatic hilar regions (i.e., first hepatic hilar and second hepatic hilar), major vessel regions (i.e., a major branch of the portal vein and the inferior vena cava), the diaphragm and the capsule. The distance was measured between the edge of the nodule and around organs using CT or MRI data. The hilum proximity, defined as the shortest distance between edge of the tumor and the surrounding structures such as portal vein and hepatic vein was less than 5 mm.
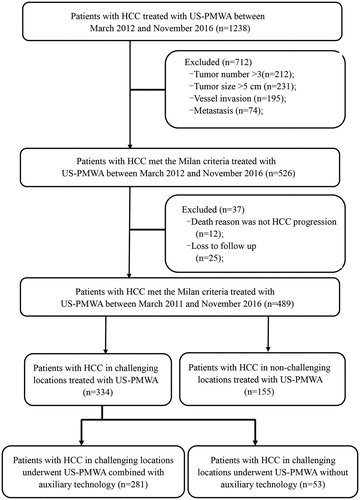
Figure 2. The HCC lesions in six types of CLs underwent US-PMWA. (A) MRI scan showed a nodule (red arrow) abutting to the stomach (yellow arrow) before MWA on the T2-weighted image and an ablation zone (red arrow) after MWA on the delay phase image. (B) MRI scan showed a nodule (red arrow) abutting to the gallbladder (yellow arrow) before MWA on the T2-weighted image and an ablation zone (red arrow) after MWA on the delay phase image. (C) MRI scan showed a nodule (red arrow) abutting to the first hepatic hilar (yellow arrow) before MWA on the T2-weighted image and an ablation zone (red arrow) after MWA on the delay phase image. (D) MRI scan showed a nodule (red arrow) abutting to the hepatic vein (yellow arrow) before MWA on the hepatic arterial phase image and an ablation zone (red arrow) after MWA on the delay phase image. (E) MRI scan showed a nodule (red arrow) abutting to the diaphragm (yellow arrow) before MWA on the T2-weighted image and an ablation zone (red arrow) after MWA on the delay phase image. (F) MRI scan showed a nodule (red arrow) abutting to the capsule (yellow arrow) before MWA on the T2-weighted image and an ablation zone (red arrow) after MWA on the delay phase image.
Definition of advanced assistive technology
AAT methods including hydrodissection, thermal monitoring, 3D planning and multimodal image fusion guidance were reviewed in this study (). High temperatures and a large sphere volume may inadvertently burn non-target structures, especially in hepatic tumors abutting the gastrointestinal tract. If lesions abutting the gastrointestinal tract or diaphragm were found before MWA, hydrodissection was applied. Hydrodissection was defined as injecting fluid including 0.9% saline solution or 5% dextrose in water implemented by a 21-gauge needle (Chiba needle; Cook Incorporated, Bloomington, IN) attached to a 50-ml syringe by a connecting tube (BD Angiocath; Sandy, UT) to separate the tumor from the surrounding organs under ultrasonography guidance. Chilled normal saline was delivered until separation of more than 0.5 cm was achieved and maintained during the procedure. Thermal monitoring was defined as the use of 21-gauge thermocouple needles, equipped on the MWA system, that is easily seen by ultrasonography used during the ablation of HCC lesions abutting vital structures (i.e., heart, stomach, colon and kidney). Based on our experimental evidence, the ablation temperature cutoff was set at 60 °C. If the measured temperature reached 60 °C, ablation was immediately stopped and activated again after the temperature decreased to 50 °C. Also, puncture path or 3D planning involved an interactive manual simulation according to the tumor size, location and the relationship of the tumor and the surrounding organs in a 3D model by interventional radiologists [Citation22]. Multimodal image fusion guidance was defined as puncture guidance using a fused image from the registration of pre-operatively acquired images (CT or MRI) with intra-operative images (ultrasonography) based on a specified anatomical landmark.
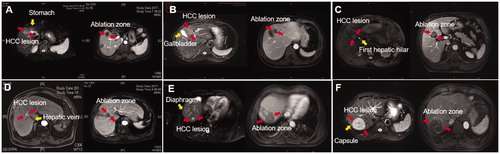
Figure 3. The four types of AATs were applied to assistance of US-PMWA for HCC lesions in CL. (A) A 65-years old, female patient had a HCC nodule (4.4 cm × 4.1 cm × 2.9 cm in diameter) located in S6 who underwent 3 D visualization preoperative planning; (B) A patient with HCC nodule (1.4 cm × 1.1 cm × 0.9 cm in diameter) located in S7 who under multimodal image fusion guided MWA; (C) A thermal monitoring needle was inserted the location abutting to the gallbladder (red arrow) for real-time temperature monitoring for the duration of ablation under US guidance; (D) The artificial ascites (yellow arrow) generated to separate the tumor from the hepatic capsule under US guidance during ablation procedure (red arrow).
Follow-up and oncological outcomes
Three days after the latest course of a well-defined ablation procedure, CT or MRI was used to assess treatment effectiveness. Asymmetrical peripheral enhancement including a dispersed, nodular or unusual pattern signified an inadequate ablation; thus, another ablation was required in these cases. Ablation margins were evaluated by tumor map based on registration between 3D target tumor before MWA and 3D ablation zone after MWA in our previous report [Citation23]. If a thorough ablation was accomplished, then routine CT or MRI data and serum tumor markers were evaluated at 1 and 3 months after US-PMWA, then at 6-month intervals. To confirm suspected metastasis, a chest CT, bone scan or positron emission tomography-CT was performed. Technique effectiveness was defined as the absence of contrast-enhancement on ablation zone images of the mass after 1 month. A recurrence, including local tumor recurrence (LTR), intrahepatic distant recurrence (IDR) and extrahepatic distant recurrence (EDR), was diagnosed as a new tumor using contrast-enhanced images. The endpoints of this study were death or recurrence. Overall survival (OS) was computed from the day of the first session of MWA treatment to the day of death or the day of final follow-up. Recurrence-free survival (RFS) was computed from the first session of MWA treatment to the day of tumor reappearance or the last day of follow-up. Local tumor progression (LTP) was defined the appearance of tumor foci at the edge of the ablation zone after at least one contrast-enhanced follow-up study had documented adequate ablation and there was an absence of viable tissue in the target tumor and the surrounding AM assessed using imaging criteria. IDR was defined as the appearance of tumor foci distant from the edge of the ablation zone after at least one contrast-enhanced follow-up study had documented adequate ablation and an absence of viable tissue in the target tumor and surrounding AM using imaging criteria [Citation24]. EDR was defined as an asymmetrical, dispersed or unusual pattern of enhancement in the extrahepatic lesion at least one contrast-enhanced follow-up study. Complications were classified according to the Society of Interventional Radiology Classification system for Complications by Outcome [Citation25].
Statistical analysis
The data from patients meeting the eligibility criteria at baseline were analyzed. SPSS version 19.0 (SPSS, Chicago, IL) was used for the analyses. The quantitative data were presented as the mean ± standard deviation and qualitative data were presented as frequencies. OS and RFS rates were evaluated by the Kaplan–Meier method with a log-rank test. A Cox proportional hazards model was used to elucidate the significant impacts of risk factors on survival as well as recurrence. Univariate and multivariate analyses of independent prognostic factors were evaluated by means of the forward stepwise Cox regression model. p < .05 signified statistical significance.
Results
Baseline characteristics and treatments parameters
Of the 526 patients who underwent US-PMWA, 489 consecutive patients (73 females, 416 males; average age 57.8 ± 9.2 years) were enrolled in our study. The clinical and pathological characteristics of 489 patients with 681 HCC nodules who met the Milan criteria are summarized in . Among these patients, data were reviewed for the 334 patients placed in the CL group and the 155 patients assigned to the non-CL group. The mean age and sex were equivalent among both groups (p = .266 and p = .970, respectively). The mean maximum tumor diameter and tumor number were equivalent among both groups (p = .432 and p = .179, respectively). Comorbidities, cirrhosis, etiology, Child-Pugh grade and α-fetoprotein (AFP) levels were comparable between the two groups (p = .087, p = .379, p = .724, p = .982 and p = .619, respectively). LTP and technique effectiveness were analogs among both groups (p = .828 and p = .873, respectively). The treatment parameters are summarized in . The antenna number, insertion number, ablation duration, ablation power and ablation sessions were analogs among both groups (p = 1.000, p = .872, p = .667, p = .992 and p = .429, respectively). The clinical and pathological characteristics of the 334 patients with HCC in CLs are summarized in Supplementary Table 1. AAT use in the CL group was significantly greater than that in the non-CL group (p< .001). The patients in CL group was stratified in six subgroups, patients with HCC lesions adjacent to vital structures (n = 59, 17.7%) or gallbladder (n = 40, 12%), in the hilar region (n = 18, 5.4%) or adjacent to major vessels (n = 59, 17.7%), diaphragm (n = 64, 19.2%) or capsule (n = 94, 28.1%). In the CL group, 78 (16.1%) patients had hydrodissection, 55 (11.4%) patients underwent multimodal image fusion guidance, 209 (43.3%) patients underwent thermal monitoring and 67 (13.9%) underwent 3D visualization planning.
Table 1. Baseline patient characteristics.
Table 2. Comparation of ablation parameters between CL and Non-CL group.
Comparison of survival outcomes between patients with HCC in CL or non-CL
The median follow-up period was 24.5 months (range, 4.2–57.2 months). Based on follow-up imaging, no significant statistical differences were detected in the technique effectiveness rate between the CL and non-CL groups (95.2% versus 94.9%; p = .873). Comparison of survival outcomes between patients with HCC in CL or non-CL is showed in . The plot Kaplan–Meier curves show the comparation of the 1-, 2- and 3-years OS between CL and non-CL group (). The plot Kaplan–Meier curves show the comparation of the 1-, 2- and 3-years RFS between CL and non-CL group ().
Figure 4. Comparation of the 1-, 2-, and 3-year OS between CL and non-CL group used to plot Kaplan-Meier curves. (A) lesion abutting adjacent vital; (B) lesion abutting gallbladder; (C) lesion abutting hepatic hilar regions; (D) lesions abutting major vessel; (E) lesions abutting diaphragm; (F) lesions abutting capsule.
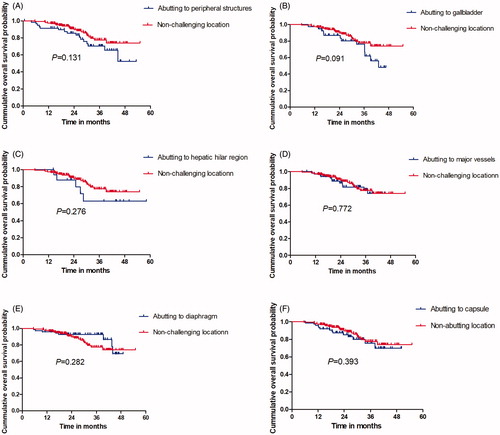
Figure 5. Comparation of the 1-, 2- and 3-year RFS between CL and non-CL group used to plot Kaplan–Meier curves. (A) lesion abutting adjacent vital; (B) lesion abutting gallbladder; (C) lesion abutting hepatic hilar regions; (D) lesions abutting major vessel; (E) lesions abutting diaphragm; (F) lesions abutting capsule.
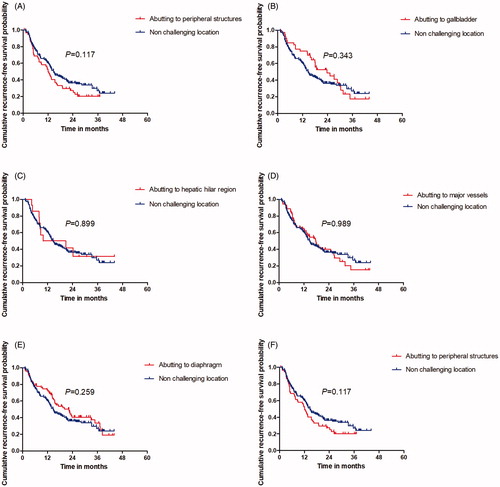
Table 3. Comparation of survival outcomes between CL and non-CL group.
Oncological outcomes after US-PMWA with AAT for HCC in CLs
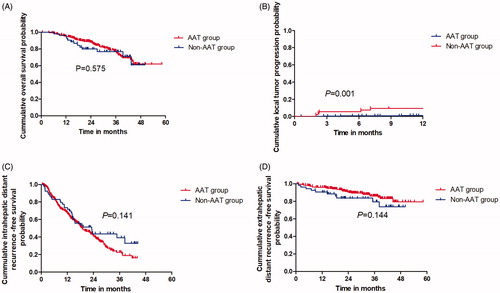
The median follow-up period was 21.2 months (range, 5.8–51.2 months). Notably, 96.1% (270/281) of the patients obtained a 5 mm AM in the AAT group while only 43.4% (23/53) obtained a 5 mm AM in the non-AAT group, with a statistically significant difference (p< .001). The 1-, 2- and 3-year OS rates of patients in the AAT and non-AAT groups were 95.3%, 88.2% and 77.8% vs. 96.2%, 80.0% and 76.9%, respectively (), with no substantial differences (p=.575). The 3-, 6-, 9- and 12-month LTP rates of patients in the AAT and non-AAT groups were 0.4%, 0.7%, 0.7% and 1.1% vs. 5.7%, 7.5%, 9.4% and 9.4%, respectively (), with significant differences (p = .001). The 1-, 2- and 3-year IDR rates of patients in the AAT and non-AAT groups were 66.7%, 41.5% and 22.7% vs. 73.6%, 43.6% and 33.8%, respectively (), with no substantial differences (p = .141). The 1-, 2- and 3-year EDR rates of patients in the AAT and non-AAT groups were 96.0%, 90.7% and 86.0% vs. 90.5%, 83.6% and 79.5%, respectively (), with no substantial differences (p = .144).
Figure 6. The oncological outcomes of patients with HCC in CL who underwent US-PMWA assisted by AATs. (A) The 1-, 2- and 3-year OS rates were compared between ATT and non-ATT group; (B) The 3-, 6-, 9- and 12-months LTP rates were compared between ATT and non-ATT group; (C) The 1-, 2- and 3-year IDR rates were compared between ATT and non-ATT group; (D) The 1-, 2- and 3-year EDR rates were compared between ATT and non-ATT group.
Univariate and multivariate analyses for oncological outcomes
Univariate and multivariate logistic regression analyses were performed to identify predictors of oncological outcomes in patients with HCC in CLs who underwent US-PMWA. The univariate analysis indicated significant changes in terms of OS rates based on the tumor size (hazard ratio [HR] = 2.285; p = .043), the number of tumors (HR = 1.372; p = .017) and AFP level (HR =2.109; p = .007). The multivariate analysis revealed significant changes in terms of OS rates based on the number of tumors (HR = 1.758; p = .008) and AFP level (HR = 1.971; p = .002) (). The univariate analysis indicated significant changes in terms of LTP rates based on the cirrhosis (HR = 1.916; p = .017), tumor number (HR = 3.782; p = .001) and AM (HR = 5.385; p< .001). The multivariate analysis revealed significant changes in terms of LTP rates based on AM (HR = 3.342; p = .001) ().
Table 4. Factors associated with poor OS after MWA for HCC in CLs according to univariate and multivariate analysis.
Table 5. Factors associated with poor LTP after MWA for HCC in CLs according to univariate and multivariate analysis.
Complications
In this study, 8 (1.6%, 8/489) patients had major complications (). Of these patients, 6 (1.8%, 6/334) were in the CL group and also all occurred in AAT group. Hemoperitoneum was detected in 1 patient, liver abscess was noted in 2 patients who developed cholangiectasis and had jaundice and 3 patients had lesions abutting hepatic hilar regions; these patients experienced percutaneous transhepatic cholangial drainage and jaundice was resolved after the treatment. Also, 2 (1.2%, 2/155) patients in the non-CL group had a large pleural effusion that was resolved after drainage. There were comparable major complications between CL and non-CL group (p=.828).
Table 6. Comparison of major complications between the CL group and non-CL group.
Discussion
Most of the patients analyzed in this study had lesions located in CLs (334/489, 68.3%) and received MWA. High-power MWA was less affected by a ‘heat-sink’ effect and it produced a larger ablation volume compared with radiofrequency ablation (RFA) [Citation26–29]. Therefore, it is more suitable to treat HCC in CLs. Some studies have reported using an elliptical ablation zone with a major axis over 3 cm and a short axis over 2 cm when specific ablation parameters are used, such as 10 min of ablation time and 60 W of ablation power [Citation30]. Filippiadis et al. [Citation31] reported that 36 HCC lesions in CLs that underwent high-power MWA showed very satisfactory efficacy, with 91.6% of complete ablation rate. The technical effectiveness rate, 95.2% in the CL group, is higher in this study.
Four types of AATs were commonly used and most patients (83.5%) with HCC in CLs underwent MWA assisted by AAT. The OS and RFS were not significantly different between the six subgroups among the CL and non-CL groups. Chen et al. [Citation32] also reported that the OS and RFS rates after RFA were not significantly different between peri-hepatic-vein (pHV) and non-pHV groups. Though there was large number of procedures with AAT in this study, there were no differences in the oncologic outcomes including OS, IDR and EDR between the AAT and non-AAT groups. However, the higher LTP rate in the non-AAT group may result from a failure to achieve a safe 5–10 mm margin [Citation27]. The occurrence rate of ablation-related complications in the CL group was higher than that in the non-CL group. Among them, lesions in hepatic hilar regions led to cholangiectasis with jaundice even if transhepatic cholangial drainage with intraductal chilled saline perfusion was used to protect bile ducts; this suggests that MWA with rapid warming may burn the bile duct. In addition, the cause of abscesses and pleural effusions after MWA is mainly related to poor liver function when the parenchyma is subjected to high-temperature stimulation.
In the six subgroups, it was important to keep adjacent vital structures and the gallbladder secure to prevent damage to the gastrointestinal tract or gallbladder as well as avoid heart perforation by thermal ablation. Hydrodissection is a safe and effective method for HCC lesions on the above-mentioned locations undergoing MWA as it can protect adjacent vital organs from thermal injury during the ablation procedure. In cases with a nodule abutting the diaphragm and capsule, the tumor is generally non-visualized or conspicuous via ultrasonography since sonic wave transmission is hindered by air in the lung or rib. Multimodal image fusion or contrast-enhanced ultrasound guidance can overcome the disadvantages of conventional ultrasound guidance and can clearly show the tumors in these CLs. Zhang et al. reported a comparison of HCC treated by MWA guided by a 3D fusion image navigation system and ultrasonography. The complete ablation rate of the first session was greater in the 3D group (94.7%) compared to the ultrasound group (62.5%). Moreover, artificial ascites offer an effective technique to enlarge the extrahepatic space and block the heat propagating to the periphery when the HCC lesions underwent MWA, which can increase the range of the thermal field; this not only provides a clear sonic view but also prevents any thermal injuries to the vital organs [Citation33]. Wang et al. reported, in a systemic review of artificial ascites, a great accomplishment rate (>90%) without the clear presence of severe adverse events when RFA is used for HCC that is difficult to ablate. Also, Wu et al. [Citation34] reported that artificial ascites could reduce abdominal wall injuries during RFA for HCCs abutting the liver surface and abdominal wall.
For a nodule abutting hepatic hilar regions and major vessels, a more precise calculation of the distance between the edge of the thermal field and the surrounding vessel structure is needed. To address the difficulty of low spatial resolution in 2D image planning, 3D visualization of the target tumor and vessel structures give essential support in 3D visualization software with sharp-cut characteristics including interactive manual simulation of the insertion number, a virtual thermal field display and the calculation of the distance between the target and surrounding vital structures [Citation35,Citation36]. Notably, Li et al. [Citation15] reported that the clinical efficiency of combining a 3D visualization operative treatment planning system and US-PMWA to manage large hepatic hilar HCCs achieved promising prognoses. Also, Zhang et al. [Citation37] reported that 3D visualization preoperative planning assisted surgical resection for progressive hilar cholangiocarcinoma and improved the success rate of operations. Moreover, 43.3% of HCC nodules respond to MWA combined with real-time temperature monitoring technology; this approach guards the surrounding organs from thermal injury and decreases the burn risk.
In univariate and multivariate analyses for oncological outcomes of HCC in CLs underwent MWA, a large tumor diameter (3–5 cm), multiple tumors and high AFP levels predicated poor OS. This result suggested that the tumor characteristics were the main independent prognostic factors of OS, which was consistent with previous reports. Furthermore, AM and multiple tumor characteristics were independent prognostic factors of LTP. This result suggests that insufficient thermal field range results in residual tumor cells, which may propagate though vascular invasion or untreated micrometastases to adjacent organs during the MWA procedure. Major complications were comparable between the CL and non-CL groups, indicating that AAT plays an imperative part in reducing the incidence of major complications of HCC in CLs in patients who underwent MWA.
This study had several limitations. First, as a retrospective study, treatments were selected according to the specific physician’s experience, triggering possible bias that may not have been evaluated. Second, this is a single-center study and there were few distinct CL types. Hence, a multi-center clinical trial with a larger cohort is essential. Third, CT and MRI data were used to categorize the tumor site relative to vital organs. The difference between CT and MRI data might impact the image results. At last, AM is an independent risk factors associated with LTP after ablation for HCC. However, as a new evaluation method, the assessment ability of tumor map in our study needs further verification.
In conclusion, HCC lesions in CLs have similar oncological outcomes compared with those in non-CLs after US-PMWA. Also, AAT has a relatively high clinical application value and reduces LTP rate of patient with MWA treatment used for HCC in CLs.
Author contributions
C.A. participated in the data analysis and drafted the manuscript. P.L. and J.Y. conceived of the study and carried out the editorial support for this manuscript. P.L., J.Y., X.L.Y., Z.G.C., Z.Y.H. and F.Y.L. participated in the ablation procedure. L.Z. and F.Y.L. provided assistance in data analysis. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (207.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Oliveri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma. Lancet. 2012; 380:470.
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853.
- Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: from diagnosis to treatment. World J Hepatol. 2015;7:1020–1029.
- Benson AB, D’Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;117:302–310.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72:124–131.
- Liu X, Wang Z, Chen Z, et al. Efficacy and safety of transcatheter arterial chemoembolization and transcatheter arterial chemotherapy infusion in hepatocellular carcinoma: a systematic review and meta-analysis. Oncol Res. 2017;26:231–239.
- Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61:1100–1101.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19:5430–5438.
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Solbiati M, Muglia R, Goldberg SN, et al. A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia. 2019;36:337–343.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29:2698–2705.
- Kim JS, Kim W, So YH, et al. Topographical impact of hepatitis B-related hepatocellular carcinoma on local recurrence after radiofrequency ablation. J Clin Gastroenterol. 2014;48:66–72.
- Li X, Yu J, Liang P, et al. Ultrasound-guided percutaneous microwave ablation assisted by three-dimensional visualization operative treatment planning system and percutaneous transhepatic cholangial drainage with intraductal chilled saline perfusion for larger hepatic hilum hepatocellular (D ≥ 3 cm): preliminary results. Oncotarget. 2017;8:79742–79749.
- Zhang D, Liang W, Zhang M, et al. Multiple antenna placement in microwave ablation assisted by a three-dimensional fusion image navigation system for hepatocellular carcinoma. Int J Hyperthermia. 2018;35:122–132.
- Cheng Z, Yu X, Han Z, et al. Ultrasound-guided hydrodissection for assisting percutaneous microwave ablation of renal cell carcinomas adjacent to intestinal tracts: a preliminary clinical study. Int J Hyperthermia. 2018;34:315–320.
- Zhi-Yu H, Ping L, Xiao-Ling Y, et al. A clinical study of thermal monitoring techniques of ultrasound-guided microwave ablation for hepatocellular carcinoma in high-risk locations. Sci Rep. 2017;7:41246.
- Liu F, Liang P, Yu X, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: a preliminary clinical application. Int J Hyperthermia. 2013;29:671–677.
- Sherman M, Bruix J, Porayko M, et al. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology. 2012;56:793–796.
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307.
- Liu F, Liang P, Yu X, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: a preliminary clinical application. Int J Hyperthermia. 2013;29:671–677.
- An C, Li X, Liang P, et al. A tumor map generated from three-dimensional visualization of image fusion for the assessment of microwave ablation of hepatocellular carcinoma: a preliminary study. Cancer Manag Res. 2019;11:1569–1578.
- Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25:1706–1708.
- Fang C, Cortis K, Yusuf GT, et al. Complications from percutaneous microwave ablation of liver tumours: - a pictorial review. Br J Radiol. 2019;92:20180864.
- Heerink WJ, Solouki AM, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol. 2018;28:3228–3236.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172–1173.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011; 79:124–130.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–275.e1.
- Jiao D, Qian L, Zhang Y, et al. Microwave ablation treatment of liver cancer with 2,450-MHz cooled-shaft antenna: an experimental and clinical study. J Cancer Res Clin Oncol. 2010;136:1507–1516.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34:863–869.
- Chen J, Peng K, Hu D, et al. Tumor location influences oncologic outcomes of hepatocellular Carcinoma patients undergoing radiofrequency ablation. Cancers (Basel). 2018;10:378.
- Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int. 2015;9:514–519.
- Wu CC, Chen WS, Ho MC, et al. Minimizing abdominal wall damage during high-intensity focused ultrasound ablation by inducing artificial ascites. J Acoust Soc Am. 2008;124:674–679.
- Seitel A, Engel M, Sommer CM, et al. Computer-assisted trajectory planning for percutaneous needle insertions. Med Phys. 2011;38:3246–3259.
- Fang CH, Tao HS, Yang J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. 2015;220:28–37.
- Zhang J, Qiao QL, Guo XC, et al. Application of three-dimensional visualization technique in preoperative planning of progressive hilar cholangiocarcinoma. Am J Transl Res. 2018;10:1730–1735.