Abstract
Purpose
The aim of this study was to determine whether moderate hyperthermic doses, routinely encountered in the periablational zone during thermal ablation, activate tumor cells sufficiently to secrete pro-tumorigenic factors that can induce increased proliferation.
Material and methods
R3230 rat mammary tumor cells and human cancer cell lines, MCF7 breast adenocarcinoma, HepG2 and Huh7 HCC, and HT-29 and SW480 colon adenocarcinoma, were heated in to 45 ± 1 °C or 43 ± 1 °C in vitro for 5-10 min and incubated thereafter at 37 °C for 1.5, 3 or 8 hr (n = 3 trials each; total N = 135). mRNA expression profiles of cytokines implicated in RF-induced tumorigenesis including IL-6, TNFα, STAT3, HGF, and VEGF, were evaluated by relative quantitative real-time PCR. HSP70 was used as control. c-Met and STAT3 levels were assessed by Western blot. Finally, naïve cancer cells were incubated with medium from R3230 and human cancer cells that were subjected to 43–45 °C for 5 or 10 min and incubated for 3 or 8 h at 37 °C in an xCELLigence or incuCyte detection system.
Results
Cell-line-specific dose and time-dependent elevations of at least a doubling in HSP70, IL-6, TNFα, STAT3, and HGF gene expression were observed in R3230 and human cancer cells subjected to moderate hyperthermia. R3230 and several human cell lines showed increased phosphorylation of STAT3 3 h post-heating and increased c-Met following heating. Medium of cancer cells subject to moderate hyperthermia induced statistically significant accelerated cell growth of all cell lines compared to non-heated media (p < 0.01, all comparisons).
Conclusion
Heat-damaged human tumor cells by themselves can induce proliferation of tumor by releasing pro-tumorigenic factors.
Introduction
Image-guided percutaneous minimally invasive thermal ablation using various energy sources (predominantly radiofrequency (RF) and microwave (MW) energies) is commonly used to successfully treat a wide range of focal primary and metastatic tumors in the liver, lung, kidney, and other sites due to their high efficiency, and low risks of complication [Citation1–5]. Yet, further gains in clinical efficacy may be possible as there is compelling evidence that systemic pro-oncogenic effects from tumor ablation [Citation6,Citation7], while incompletely characterized, may play a significant, deleterious role in clinical outcome in a substantial, but poorly quantified, number of clinical cases.
Although it has been previously shown that partial injury of normal liver or kidney from non-lethal heating, as routinely seen following complete thermal ablation, can cause accelerated growth in distant tumor, more recently it has been shown that even partial ablation of R3230 mammary tumors in rats and subcutaneous CT26 and MC38 mouse colorectal adenocarcinoma tumors can cause similar accelerated tumor growth and increased intratumoral angiogenesis of local or distant tumors [Citation8]. Moreover, multiple cytokine, growth factor, and transcription factor mediators, including IL-6, STAT3, HGF/c-Met, and VEGF/VEGFR, that contribute to downstream ablation-induced tumor growth stimulation are upregulated in the periablational rim surrounding the ablation zone and in the serum after complete and partial RFA of normal and tumor tissues [Citation6,Citation7,Citation9–12]. Likewise, colorectal cancer patients with high levels of HGF and IL-6 in the serum after RFA are associated with tumor progression, metastases and poor survival [Citation13]. Yet, there are significant gaps in our understanding of this pro-tumorigenic cascade including determination as to which cells are key mediators of this process. Specifically, within the periablational zone of normal tissue, multiple cell populations, including native hepatocytes and infiltrating myofibroblasts and macrophages, contribute to increased expression of factors that contribute to off-target distant tumor stimulation post-ablation [Citation14]. However, in cases of partial tumor ablation it is unknown whether residual tumor cancer subjected to partial treatment is a primary determinant of this process or whether the process is due to increased cytokinetic activity in the tumor of the infiltrative inflammatory cells or baseline stroma cells that are part of the tumor microenvironment (i.e., intratumoral fibroblasts and vasculature). Indeed, residual viable tumor cells have been identified in up to 20% of clinical cases following ablation [Citation15,Citation16], confirming the need to further study the potential influence of heated tumor cells on production of pro-oncogenic factors following ablation.
Characterizing the cellular origins of this phenomenon within tumor is a necessary next step in developing strategies to suppress the pro-oncogenic effects of thermal ablation. Given that these periablational cells are closely linked with key pro-oncogenic mediators, elucidating their precise role in ‘off-target’ effects of RFA in in vitro studies may offer better overall therapeutic strategies. Moreover, it is necessary to determine whether this phenomenon occurs also in any of the multiple types of human cancer cell populations commonly treated by ablation. Accordingly, direct in vitro assessment of cultured cancer cell lines could provide further evidence of this phenomenon in humans.
Here, we studied whether moderate hyperthermic doses, routinely encountered in the periablational zone during thermal ablation, induce pro-tumorigenic genetic and proteomic activation of tumor cells. To accomplish this, we used in vitro methods to assess the gene expression profile of heated cells, followed by determination of protein secretion and activation of these tumor cells, and performed an in vitro proliferation assay in an animal cancer cell line and five representative human cancer cell lines to assess whether human cancer cells display similar pro-tumorigenic properties.
Materials and methods
Experimental overview
The study was performed in three phases. In the first phase, to determine whether growth factors associated with tumorigenesis can be induced by tumor cells themselves, an in vitro assay was established to assess the effect of moderate hyperthermia doses found in the periablational zone on tumorigenesis factor induction, protein secretion, and activation post- thermal ablation. R3230 cells were heated to 43–45 °C for 5–10 min as these thermal doses occur in the periablation region where there is induction of substantial cell stress and heat shock protein (HSP) production in this model [Citation17–21]. Cells were incubated thereafter at 37 °C for 1.5, 3, and 8 h (N = 135). mRNA expression profile of factors and cytokines implicated in RFA-induced tumorigenesis including IL-6, STAT3, HGF, and VEGF were evaluated by relative quantitative real-time PCR. Heat shock protein 70 (HSP70) was used to confirm cellular response to thermal activation with tumor necrosis factor (TNFα) used as a control for inflammation [Citation22]. Secretion of cytokine IL-6 was assessed by ELISA, with intracellular transcription factor STAT3 and its active phosphorylated form and c-Met assessed by Western blot [Citation23–27].
In the second phase, to determine whether this phenomenon extends to human cancers, representative human cancer cell lines of some of the most common tumor types treated with thermal ablation in the liver were subject to moderate hyperthermic doses as above to assess its effect on pro-tumorigenesis factor induction post-ablation. Tumor lines included HepG2 and Huh7 hepatocellular carcinoma (HCC), HT-29 and SW480 colon adenocarcinoma, and MCF7 breast adenocarcinoma. mRNA expression profile of factors and cytokines implicated in ablation-induced tumorigenesis were evaluated.
In the third phase, studies were performed to determine whether moderate hyperthermia induces sufficient secretion of proliferative factors to induce increased tumor growth. R3230 and human cancer cells were subjected to 43 °C for 5 min and 45 °C for 5 min or 10 min, respectively, followed by incubation for 8 h and 3 h at 37 °C, respectively. This medium was harvested and used to incubate 10,000 untreated cancer cells for each line, which were then monitored for 30 – 144 h in either an xCELLigence detection system for rodents [Citation28] or the incuCyte detection assay for human cells [Citation29] to assess growth kinetics beyond confluency of the cellular wells. These experiments were performed at least in duplicate for every cell line.
Cell culture
R3230 cells were grown at 37 °C in 5% CO2 in RPMI medium supplemented with 10% fetal calf serum (FCS) (Biological Industries, Israel), 100 IU/ml penicillin, 100 mg/ml streptomycin, and 2% L-glutamine. Human cancer cell lines were grown in DMEM medium supplemented with 10% FCS, 100 IU/ml penicillin, 100 mg/ml streptomycin, and 2% L-glutamine. All cell lines tested negative for Mycoplasma.
In vitro moderate hyperthermia assays
Five milliliters of the medium was pre-heated in a water bath for ∼20min to achieve a regulated temperature of 45 ± 1 °C or 43 ± 1 °C. A 50 ml conical tube containing 8x105 cells in 50 µl was incubated in the water bath for 5 or 10 min. 200 µl of pre-heated medium was added in order to rapidly bring the cells to the desired temperature. At the conclusion of heating, 50 µl room temperature RPMI was added. Cells were subsequently incubated at 37 °C from 1.5 to 24 h. Control cell suspensions were incubated continuously at 37 °C.
mRNA expression analysis
For quantitative real-time PCR, RNA was purified from heated cells using standardized Trizol RNA extraction protocols. Expression of IL-6, TNFα, STAT3, HGF, and VEGF was tested using the CFX384 TouchTM Real-Time PCR Detection System [Citation30]. Additionally, HPRT, GAPDH (ubiquitous endogenous housekeeping genes), and HSP70 expressions were assessed to document normal cellular functioning and response to heating, respectively. Results of mRNA expression are expressed as ratio to the mRNA present in unheated cells and HPRT or GAPDH controls.
Quantification of c-Met, phosphorylated STAT3, and IL-6
c-Met and STAT3 quantification were performed using Western blot analysis for all lines. Heated cells were homogenized using RIPA buffer. Protein was quantified by Bradford protein assay with 40 µg total protein loaded on 10% SDS-polyacrylamide gels and blotted onto PVDF transfer membranes. Nonspecific binding was blocked with 1% skim milk for 1 h followed by incubation with c-Met 1:100 (SC-162; Santa Cruz Biotechnology), P-STAT3 1:100 (#9131- Cell signaling tech) and STAT3 1:1000 (SC-8019; Santa Cruz) antibodies. Standardization of protein quantities was performed using β-actin with results for the assessed protein expressed as the ratio to control specimens. Protein levels of IL-6 and HGF in the heated cells medium were determined using ELISA (DuoSet R&D Systems). Results are expressed as percentage increase in optical density over control cells incubated at 37 °C.
In vitro tumor growth assessment
To prepare conditional medium (i.e., media containing factors excreted by tumor cells subject to moderate hyperthermia), R3230 was heated at 43 ± 1 °C and human cancer lines at 45 ± 1 °C for 5 min and 10 min and subsequently incubated at 37 °C for 3 h. Heating was initially performed in culture medium without FCS, with FCS added to a final 5% concentration immediately post heating. Controls were prepared in a similar manner, with incubation to 37 °C. The effect of conditional medium on cell growth was assessed by an xCELLigence detection system (ACEA Bioscience [Citation28]) or incuCyte detection system (Essen BioScience [Citation29]) for 1.5–6 days. Untreated cells were incubated in growth medium without FCS and conditional media was added v/v (final 2.5%FCS). All experiments were performed in triplicate.
Statistical analysis
For in vitro moderate hyperthermia experiments, each individual group result was averaged and standard error of the mean (SEM) calculated. All P-values were calculated by a two-tailed t-test. For xCELLigence experiments, the results are presented as graph of average measured values ± SEM, with statistical significance assessed by Student's t-test. For incuCyte experiments, the results are presented as graph as measured and calculated by incuCyte software (Essen BioScience).
Results
Moderate hyperthermia induces multiple pro-tumorigenic factors in R3230
Cell survival studies showed 80–90% viability following 43–45 °C × 5–20min heating (). HSP70 mRNA was markedly elevated up to 152 ± 53 fold over the thermal doses studied, denoting marked cell stress (). Dose and time-dependent elevation of HSP70, IL-6, TNFα, and HGF gene expression were observed with maximum expression of each cytokine seen at different thermal doses and incubation times (; ). For R3230, HSP70 showed greatest expression overall peaking at 3 h following 43°Cx5min heating at 152 ± 53 fold increase. TNFα expression was also greatest at 43 °C × 10 min, peaking at 1.5 h. IL-6 mRNA level increased the most at 3 h following the highest heating dose, 45 °C × 10min (10.3 ± 2.9 fold increase, p < 0.05). STAT3 doubled expression at 1.5 h at 43 °C × 10 min. HGF expression peaked at 5.5 ± 3.5 fold 3 h following 43 °C × 10min. IL-6 receptor while VEGF expression showed minimal to no upregulation.
Figure 1. R3230 cells survival curves post moderate hyperthermia. In vitro heating of R3230 to 43 °C (A) or 45 °C (B) for 5, 10 or 20 min leads to 80–90% cell survival.
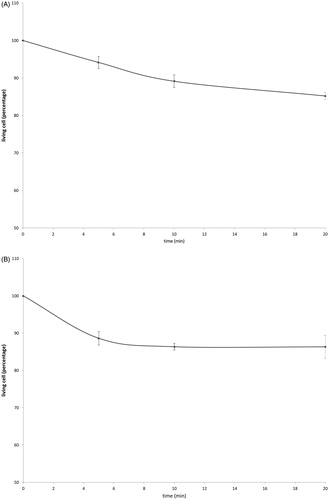
Figure 2. Moderate hyperthermia induces pro-tumorigenic factors mRNA expression in R3230. In vitro heating of R3230 leads to increased expression of cytokines and growth factors. R3230 cells were heated to 43 °C for 5 or 10 min and incubated thereafter at 37 °C for 1.5, 3, and 8 h. The chart presents mRNA fold induction of each labeled cytokine.
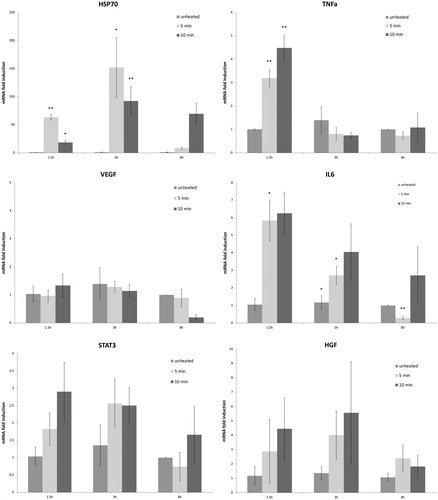
Table 1. mRNA expression levels of studied cytokines following moderate hyperthermia for R3230 and human cancer cell lines.
Following 43°Cx5min heating, R3230 also showed protein activation based upon an increase of phosphorylation of STAT3 at 3 h (26 ± 10%, p < 0.05) (). An increase in total STAT3 protein (20 ± 10%, p < 0.05) and c-Met (25 ± 10%, p < 0.05) were also observed at 8 h following 43 °C × 5min heating compared to unheated controls (). A significant time-dependent increase of IL-6 secretion of 14 ± 3% over controls at 8 h (0.09 ± 0.00od vs. 0.10 ± 0.00od; p < 0.05) was also observed (). However, HGF secretion was not detected through 8 h.
Figure 3. Moderate hyperthermia induces pro-tumorigenic factors in R3230. (A) In vitro heating of R3230 leads to increase in IL-6 secretion, showed by ELISA. Bar charts indicate a significant increase in IL-6 secretion in medium of R3230 cells that were heated to 43 °C for 5 min followed by incubation of 3 h or 8 h at 37 °C. (B) Western blot assay show an increase in phosphorylation of STAT3 following moderate hyperthermia (dense bands on gel electrophoresis at 80-kDa level as expected, after β-actin standardization). R3230 cells were heated to 43 °C for 5 or 10 min and incubated thereafter at 37 °C for 3 h. Bar charts indicate a significant increase in STAT3 phosphorylation in cells that were heated for 5 min. (C) Western blot assay show increase in c-Met receptor protein following moderate hyperthermia seen as dense bands on gel electrophoresis at 50-kDa level, where c-Met receptor α subunit is expected, after β-actin standardization. Bar charts indicate a significant increase in c-Met receptor protein in R3230 cells that were heated both 5 and 10 min at 43 °C and incubated thereafter at 37 °C for 8 h.
Moderate hyperthermia promotes induction of pro-tumorigenic factors over a range of human cancer cell lines
For MCF7 breast adenocarcinoma, cell survival showed 85–70% viability following 45 °C × 5–20 min heating. mRNA peak expression of IL-6, TNFα, and HSP70 occurred at 8 h following heating to 45 °C × 10 min (p < 0.001) (). HGF mRNA levels were also elevated, but at the earlier 3 h time point following 45°Cx10min heating and continued to increase to maximum at 8 h (10.8 ± 2.0 fold increase; p < 0.001). STAT3 expression showed little if any upregulation at 3 h.
In contrast, the Huh7 HCC cell line (88–73% viability) showed more variable expression of key makers following hyperthermia. An increase of IL-6 mRNA levels were observed at 3 h following 45°Cx5min (p = 0.06), which peaked at 8 h (7.0 ± 0.6 fold increase; p < 0.001) (). However, HSP70 and STAT3 expression did not increase at 3 h and 8 h following 45 °C heating. TNFα mRNA levels were also elevated at 3 h following 45 °C × 5 min (p < 0.001). HGF mRNA levels peaked 8 h following 45°Cx5min (p = 0.02). By contrast, only TNFα mRNA was increased (8.7 ± 1.4 fold induction) 3 h following 45°Cx5min heating of HepG2 tumor cells (76–73% viability). Other studied genes did not show increased activation from heating these cells (p = 0.02).
Colon adenocarcinoma HT-29 had more variable and heat sensitive cell survival decreasing from 87 to 57% viability following 45 °C for 5–20 min heating. mRNA expression levels of HSP70 doubled expression at 3 h following 43 °C × 10min heating. HGF expression levels also rose following both 43 °C and 45 °C ×10 min heating and 3 h incubation (p = 0.1). TNFα mRNA levels peaked at 3 h following 43 °C × 10 min heating (p = 0.01). Yet, IL-6 mRNA expression was not detected at any studied thermal dose or incubation. For 8 h incubation post 45 °C heating, no mRNA upregulation was noted. For SW480 cell survival showed 82–64% viability following 45 °C × 5–20 min heating. At 43 °C, STAT3 was the only gene with elevated expression at 3 h (8.0 ± 0.0 fold; p < 0.001). At 45 °C × 10 min, IL-6 transcript was detected at 3 h incubation, but not in control samples.
Cancer cells subject to moderate hyperthermia can promote their own accelerated cell growth
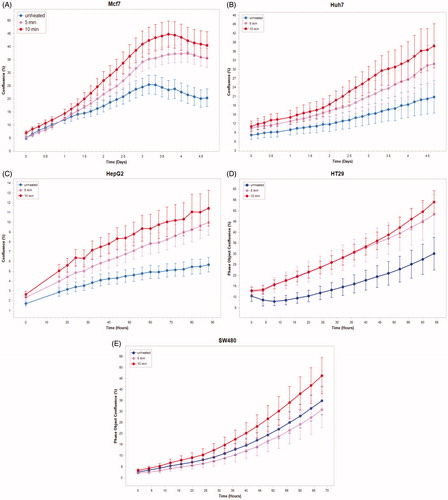
Medium derived from R3230 cells heated to 43°Cx5min followed by incubation for 8 h at 37 °C lead to increased growth when introduced into the media of unheated R3230 cells. Following the addition of this primed media, significant increased cell growth was observed from 2 h to 15 h (, p < 0.001). Likewise, medium harvested from heated HepG2 cells doubled the cell growth of naïve-cells compared to non-heated cell media (). Huh7 cells incubated with heated Huh7 medium (45 °C × 5 min and 10 min) also significantly increased cell growth (). For HT-29, MCF7 heated cell medium (45 °C × 5min and 10 min; 3 h/37 °C) () likewise enhanced cell growth of HT-29 cells immediately after the addition of the condition medium (). Similarly, SW480 heated medium (45 °C × 10 min; 3 h/37 °C) enhanced cell growth ().
Figure 4. R3230 cells subject to moderate hyperthermia can promote their own accelerated cell growth. Medium of R3230 cells heated at 43 °C for 5 min followed by 8 h incubation was added to naïve R3230 cell culture and enhanced growth of unheated cells compared to the control (C) medium-unheated condition medium. (‘cell index’ refers to confluency of the well.).
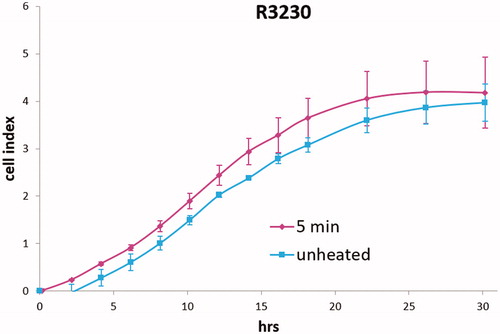
Figure 5. Cancer cells subject to moderate hyperthermia can promote their own accelerated cell growth. Medium of human cancer cells heated at 45 °C for 5 min or 10 min followed by 3 h incubation was added to naïve cell culture and enhanced growth of naïve unheated cells compared to unheated media. Media from human breast MCF7 cells (A), human hepatocellular carcinoma Huh7 (B) and HepG2 cells (C), and human colorectal HT-29 (D) and cells SW480 (E).
Protein expression in human cell lines
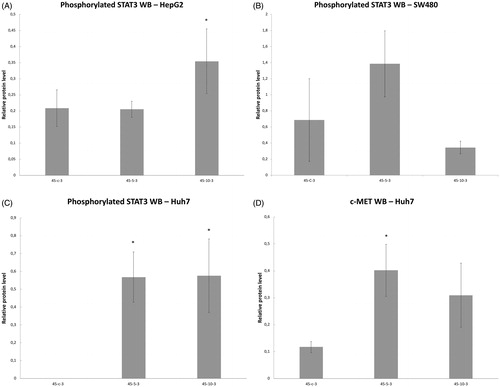
Variable line-to-line changes in protein profiles were observed. Substantial increases in increases of phosphorylation of STAT3 at 3 h were seen for HepG2 heated for 45°Cx5min (41 ± 10% increase over baseline, p < 0.05) () and SW480 heated for 45°Cx5min (50 ± 10%) (p < 0.05) (). Moreover, Huh7 showed p-STAT only when exposed to heat (45 °C × 5 min and 10 min) (). Only Huh7 a significant increase in c-Met (70 ± 10%, p < 0.05) was also observed at 3 h following 45 °C × 5 min heating compared to unheated controls (). Despite increased cellular growth, other lines did not show changes in p-STAT or c-met expression. IL-6 secretion was not detected in any of the human cell lines.
Figure 6. Moderate hyperthermia induces pro-tumorigenic factors in Human cell lines. Western blot assays show an increase in phosphorylation of STAT3 and c-MET receptor α subunit following moderate hyperthermia. cells were heated to 45 °C for 5 or 10 min and incubated thereafter at 37 °C for 3 h. Bar charts indicate an increase in STAT3 phosphorylation or c-MET in cells that were heated. (A) HepG2 cells show an increase in pSTAT3 10 min post heating. (B) SW480 cells show an increase in pSTAT3 5 min post heating. (C) Huh7 cells show an increase in pSTAT3 5 and 10 min post heating. (D) Huh7 cells show an increase in c-MET receptor α subunit 5 and 10 min post heating.
Discussion
Key cytokine, growth factor, and transcription factor mediators (i.e., IL-6/IL-6R, HGF/c-Met, STAT3, and VEGF/VEGFR) are upregulated in the periablational rim and serum after hepatic thermal RF and MW ablation and contribute to downstream ablation-induced tumor growth stimulation in in vivo rodent tumor ablation models [Citation8,Citation31]. Yet, it is essential to characterize cellular sources of pro-tumorigenic mediators produced in vivo particularly given the multiple cell populations subjected to the ablation process and the host of responses to heating. This includes native cells such as hepatocytes which will always be present in the required periablational margin of normal liver needed to insure complete ablation [Citation32,Citation33], and infiltrating inflammatory cells as part of an inflammatory wound-healing response. In addition, residual tumor cells, either in an incompletely ablated margin or in the form of micro or macrometastases elsewhere, could potentially produce a wide range of potentially tumorigenic cytokines when activated directly by moderate hyperthermia and/or by general inflammation. Indeed, here we show that multiple rodent and human tumor cell types react in vitro to heating at near ablative hyperthermic temperatures by increasing transcription, secretion, protein production and activation of factors known to induce increased post-ablation tumorigenesis in particularly the IL-6 and HGF/c-Met signaling pathway.
Our results reinforce the notion that non-ablative heating of tumor cells (i.e., incomplete ablation of a tumor) may be potentially sufficient to initiate pro-tumorigenic effects [Citation8]. Moreover, we show that there are not only increases in mRNA expression directing tumor cells to produce tumorigenic cytokines, but that such heating can cause secretion of some of these factors (i.e., IL-6 for R3230) and activation of pro-tumorigenic proteins (i.e., c-met and phosphorylation of STAT3 observed in R3230 and some, but not all human cancer cell lines). We note that an increase in total STAT3 protein was observed only after 8 h, possibly indicating early phosphorylation of STAT3 already stored within the cell as an immediate response to stress, and later translation of additional STAT3 as a secondary response. HSP70 and TNFa mRNA were also upregulated in all lines studies denoting cellular reactions to heating including inflammation signaling due to this stress [Citation22].
Although our findings suggest that there is sufficient activation by tumor cells to potentially trigger a cytokinetic cascade, it is still not known if this is sufficient to induce tumorigenesis by itself or whether the other inflammatory cells also play an essential role. Indeed, we note that although HGF mRNA is upregulated in R3230 cells, HGF secretion was not detected in the medium and VEGF mRNA was not elevated within the time frame studied. However, the heat-induced c-Met protein observed in the rodent breast line and one human hepatoma line suggests a potential auto-regulatory effect of HGF on these cells. Alternatively, prior experiments showed peak elevation in hepatic HGF and VEGF 3d post-RFA and thus these processes may require further time to develop and contribute to this process [Citation6]. Ultimately, we must also bear in mind the wide variability in gene and protein expression and activation seen which strongly suggests that multiple mechanisms are likely at play in different tumor lines.
Potential clinical relevance of our findings, showing elevated mRNA levels of pro-tumorigenic factors in the R3230 cell line after heating, has been amplified by confirming our findings in human cancer cell lines. Although differences in the extent, thermal dose, and timing of the increases were seen between the rodent and human lines, we note an overall similarity between the cytokines that upregulated in R3230 and MCF7 breast cancer after heating, especially IL-6 and HGF, two cytokines linked to tumorigenesis [Citation34,Citation35] and worse clinical prognosis post-ablation therapy [Citation13]. For human HCC and colorectal cancer cell lines, we likewise show that each of the two tumor cell lines studied had a robust, but different mRNA amplification response profiles to moderate hyperthermia. Thus, given a wide spectrum of genetic alterations found in many tumors types that are commonly ablated [Citation36,Citation37], it will likely be necessary to elucidate additional post-ablation gene and protein profiles for a wider range of tumor types, in order to best adapt treatment to each cancer type in clinical practice. Indeed, other factors and pathways, including HIF-1a and PI3/Akt, have been implicated in hyperthermia and ablation-induced tumorigenic effects [Citation38,Citation39]. Thus, the search for additional pathways is mandated. Hence, future study will include fuller characterization of thermal doses in different cell types to determine which tumor sub-populations are most susceptible to thermal ablation pro-oncogenic effects - potentially using receptor expression as a biomarker to predict responsiveness to pharmacologic targeting of key mediators. In addition, elucidation of tumorigenesis in other relevant organs particularly for kidney tumors, where increased IL-6 and/or HGF have been detected post-RFA, must be another complementary line of inquiry.
We acknowledge that this study has some additional limitations. Although we showed that pro-tumorigenic factors are activated in heat-damaged tumor cells, we have not determined whether other cell populations may be affected by the heat doses in the periablational site. Hence, we readily acknowledge that other native cell populations and recruited cell populations may also play a role in the tumorigenesis pathway. Therefore, it will be necessary in the future to study the activation of signaling pathway and secretion of factors by these cell populations starting with analyzing the parenchymal cells as these cells will always be in the periablational site. In addition, further study is required to assess the effect of other ablation methods and a wider range of thermal heating doses.
In conclusion, the doses of non-lethal hyperthermia commonly encountered in the periablational rim post-thermal ablation induce upregulation of genes associated with cytokines and growth factors including IL-6, STAT3, HGF, and VEGF in a wide range of human cancer cell lines, which may promote their accelerated cell growth. Thus, caution is advised when employing strategies that subject tumor cells to moderate hyperthermic doses routinely encountered in the periablational zone during thermal ablation therapies without achieving total ablation. This would include situations where incomplete or insufficient ablation margins are achieved [Citation16] or cases where palliative debulking is contemplated. Indeed, residual partially injured tumor cells within an ablative zone may themselves be significant drivers of tumorigenic growth locally or in distant tumors. Regardless, further studies to develop strategies that can modulate or inhibit such secondary effects of thermal ablation in residual tumor cells are both warranted and given variability in responses to hyperthermia observed will likely need to be refined on a tumor-by-tumor basis.
Disclosure statement
S. Nahum Goldberg has unrelated sponsored research and consulting from Angiodynamics and Cosman Company. All other authors have no conflicting interests to report.
Additional information
Funding
References
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Ahmed M, Brace CL, Lee FT Jr, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369.
- Lencioni R, Cioni D, Fau-Crocetti L, Crocetti L, Fau-Franchini C, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term. Radiology. 2005;234:961–967.
- Meloni MF, Andreano A, Laeseke PF, et al. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation–intermediate and long-term survival rates. Radiology. 2009;253:861–869.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968.
- Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-Met inhibition. Radiology. 2016;279:103–117.
- Rozenblum N, Zeira E, Scaiewicz V, et al. Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology. 2015;276:426–432.
- Markezana A, Goldberg SN, Kumar G, et al. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Radiology. Forthcoming.
- Ahmed M, Kumar G, Navarro G, et al. Systemic siRNA nanoparticle-based drugs combined with radiofrequency ablation for cancer therapy. PLoS One. 2015;10:e0128910.
- Erinjeri JP, Thomas CT, Samoilia A, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol. 2013;24:1105–1112.
- Kumar G, Goldberg SN, Gourevitch S, et al. Targeting STAT3 to suppress systemic pro-oncogenic effects from hepatic radiofrequency ablation. Radiology. 2018;286:524–536.
- Rozenblum N, Zeira E, Bulvik B, et al. Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology. 2015;276:416–425.
- Hinz S, Tepel J, Roder C, et al. Profile of serum factors and disseminated tumor cells before and after radiofrequency ablation compared to resection of colorectal liver metastases – a pilot study. Anticancer Res. 2015;35:2961–2967.
- Nakagawa H, Mizukoshi E, Iida N, et al. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol Immunother. 2014;63:347–356.
- Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18:3702–3710.
- Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–959.
- Mertyna P, Dewhirst MW, Halpern E, et al. Radiofrequency ablation: the effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia. 2008;24:550–559.
- Yang W, Ahmed M, Tasawwar B, et al. Radiofrequency ablation combined with liposomal quercetin to increase tumour destruction by modulation of heat shock protein production in a small animal model. Int J Hyperthermia. 2011;27:527–538.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27:320–343.
- Moussa M, Goldberg SN, Kumar G, et al. Effect of thermal dose on heat shock protein expression after radio-frequency ablation with and without adjuvant nanoparticle chemotherapies. Int J Hyperthermia. 2016;32:829–841.
- Solazzo S, Mertyna P, Peddi H, et al. RF ablation with adjuvant therapy: comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia. 2008;24:560–567.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
- Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910.
- Jung IH, Choi JH, Chung YY, et al. Predominant activation of JAK/STAT3 pathway by interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia. 2015;17:586–597.
- Wang X, Wang Y, Xiao G, et al. Hypermethylated in cancer 1(HIC1) suppresses non-small cell lung cancer progression by targeting interleukin-6/Stat3 pathway. Oncotarget. 2016;7:30350–30364.
- Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527.
- Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746.
- Kho D, MacDonald C, Johnson R, et al. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors (Basel). 2015;5:199–222.
- Artymovich K, Appledorn DM. A multiplexed method for kinetic measurements of apoptosis and proliferation using live-content imaging. Methods Mol Biol. 2015;1219:35–42.
- Tang H, Cai Q, Li H, et al. Comparison of droplet digital PCR to real-time PCR for quantification of hepatitis B virus DNA. Biosci Biotechnol Biochem. 2016;80:2159–2164.
- Velez E, Goldberg SN, Kumar G, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281:782–792.
- Teng W, Liu KW, Lin CC, et al. Insufficient ablative margin determined by early computed tomography may predict the recurrence of hepatocellular carcinoma after radiofrequency ablation. Liver Cancer. 2015;4:26–38.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Liska D, Chen CT, Bachleitner-Hofmann T, et al. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res. 2011;17:472–482.
- Sansone P, Storci G, Tavolari S, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002.
- Dhanasekaran R, Nault JC, Roberts LR, et al. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology. 2019;156:492–509.
- Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 6:31.
- Nijkamp MW, van der Bilt JD, de Bruijn MT, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814–823.
- Thompson SM, Callstrom MR, Jondal DE, et al. Heat stress-induced PI3K/mTORC2-dependent AKT signaling is a central mediator of hepatocellular carcinoma survival to thermal ablation induced heat stress. PLoS One. 2016;11:e0162634.
