?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Varicose veins are a common pathology that can be treated by endovenous thermal procedures like radiofrequency ablation (RFA). Such catheter-based techniques consist in raising the temperature of the vein wall to 70 to 120 °C to induce vein wall coagulation. Although effective, this treatment option is not suited for all types of veins and can be technically challenging.
Materials and methods
In this study, we used High-Intensity Focused Ultrasound (HIFU) as a non-invasive thermal ablation procedure to treat varicose veins and we assessed the long-term efficacy and safety of the procedure in a sheep model. In vivo experiments were first conducted on two saphenous veins to measure the temperature rise induced at the vein wall during HIFU ablation and were compared with reported RFA-induced thermal rise. Thermocouples were inserted in situ to perform 20 measurements during 8-s ultrasound pulses at 3 MHz. Eighteen saphenous veins of nine anesthetized sheep (2–2.5 % Isoflurane) were then exposed to similar pulses (85 W acoustic, 8 s). After treatments, animals recovered from anesthesia and were followed up 30, 60 and 90 days post-treatment (n = 3 animals per group). At the end of the follow-up, vein segments and perivenous tissues were harvested and histologically examined.
Results
Temperatures induced by HIFU pulses were found to be comparable to reported RFA treatments. Likewise, histological findings were similar to the ones reported after RFA and laser-based coagulation necrosis of the vein wall, thrombotic occlusions and vein wall fibrosis.
Conclusion
These results support strongly the effectiveness and safety of HIFU for ablating non-invasively veins.
Introduction
Varicose veins of lower extremities are a frequent disease defined as enlarged veins of 3 mm or more in diameter in the upright position [Citation1,Citation2]. The origins of this pathology can be divided into different categories that may overlap like increased deep venous pressure, primary valvular incompetence, secondary valvular insufficiency and hereditary factors like vein wall weakness [Citation3]. If left untreated, complications may arise and include pathological skin changes and venous ulcers.
Treatments of varicose veins aim at eliminating the pathologic vein from the venous circulation. This can be achieved by surgical removal of the vein or, less invasively, by chemical (sclerotherapy) or thermal occlusion of the vein. The two most frequently used thermal procedures include endovenous laser treatment (EVLT) [Citation4] and radiofrequency ablation (RFA) [Citation5]. They both consist in introducing an optical fiber or a catheter into the target vein and heating the vein to cause vein wall collagen contraction, endothelium destruction, vein wall inflammatory reaction and finally fibrosis [Citation6]. Laser-based interventions have been reported to be better tolerated than RF [Citation7]. The long-term goal of these thermal treatments is to induce complete shrinking or to occlude the pathologic vein.
To ensure a successful treatment, the RF devices used for the treatment of varicose veins expose vein walls to peak temperatures between 70 and 120 °C [Citation8–10]. Both RFA and EVLT have been reported to be safe and effective methods to treat varicose veins [Citation4,Citation11–13]. However, these catheter-based procedures are physician-dependent, are not suitable for all types of veins [Citation14,Citation15] and require skin incisions.
In comparison to endovenous thermal therapies, High-intensity focused ultrasound (HIFU) can heat tissues noninvasively [Citation16]. The use of HIFU as a noninvasive alternative to endovenous treatments of varicose veins has already been investigated in a number of preclinical studies [Citation17–22] and the ability to occlude small veins in vivo noninvasively with thermal HIFU has been demonstrated [Citation18,Citation22]. However, the efficacy and safety of the ablative procedures were demonstrated in the short term only, at most two weeks after treatments [Citation22]. One study conducted by Delon-Martin et al. [Citation18] examined HIFU-mediated vascular occlusion with a longer follow-up and reported that a vein was successfully occluded 2 days post-HIFU but recanalized 28 days after treatment. Permanent vascular occlusion resulting from post-inflammatory processes arises in the weeks after venous thermal injury [Citation17,Citation20,Citation23–25]. Long-term survival studies are thus required to assess whether fibrotic occlusion can be achieved. Furthermore, before translation into humans, the absence of treatment-related complications like vein perforations and embolisms needs to be assessed.
In this study, we investigated the long-term efficacy and safety of a HIFU procedure (85 W acoustic, 8 s long sonication) for the management of varicose veins in a sheep model.
Temperatures induced at the vein wall were first measured in vivo during the HIFU exposures of two saphenous veins. Eighteen saphenous veins were then exposed to similar pulses in nine sheep. The animals were anesthetized during the experiments (Isoflurane 2–2.5%) and were divided into three groups. The tissue changes were evaluated macroscopically and microscopically in the short and long-term. Sheep saphenous veins were selected because of their anatomical resemblance with human’s veins: they lie under superficial tissues, have several tributaries, are surrounded by a fascia and their diameter is close to the human saphenous veins (∼4 mm). Furthermore, these healthy veins are part of the superficial venous system, which allows chronic studies to be performed without the risk of limb necrosis.
Materials and methods
Ultrasound therapy equipment
An Echopulse® HIFU system (Theraclion, Malakoff, France) was used for all experiments. The device encompasses a motorized treatment head including a single-element piezocomposite therapy transducer and an imaging probe for treatments monitoring (). The ultrasound imaging probe is a linear array comprising 128 elements and operating at high frequencies, in the 5 to 12 MHz frequency range. The therapy transducer, driven at 3 MHz, has a spherical geometry with a 38 mm radius of curvature and a 56 mm outer diameter. The −6 dB beam size in the focal region is equal to 0.5 mm in the transverse direction and 2.2 mm in the axial direction. A balloon filled with coupling liquid was fixed to the transducer to ensure acoustic coupling between the transducer and the target. The coupling liquid was circulated and cooled during all the procedure to avoid transducer overheating and skin burns.
Animal experiments
Ten female sheep underwent HIFU ablation of lateral saphenous veins. One sheep (17 months old and 55 kg weight) was used for the thermometry study and nine sheep (21 ± 6 months old and 55 ± 8 kg weight) were used for the efficacy and safety study. Treatments are detailed in the following sections.
For each animal, lower rear limbs were first shaved with a hair trimmer and then with a commercial hair-removing cream (Klorane, Laboratoires Klorane, France) to ensure good ultrasound transmission through the skin and prevent skin burns.
All in vivo experiments were approved by the institutional ethics committee for animal experiments (agreement number CE16/2017022116542356) and were carried out according to the guidelines of the French National Committee for animal trials C.N.R.E.E.A (Comité national de réflexion éthique sur l’expérimentation animale).
Prior to treatments, animals were anesthetized with an intramuscular injection of a mixture of ketamine (5 mg/kg, Ketamine 1000, Virbac, France) and Diazepam (0.5–1 mg/kg, Diazepam TVM5, TVM Lab, France). Animals were intubated and ventilated with Isoflurane (2–2.5%, Vetflurane, Virbac, France) to be kept under anesthesia throughout the experiments.
Electrocardiography, arterial blood pressure, heart rate, body core temperature, oxygen saturation, end-tidal CO2, respiratory rates and pattern were continuously monitored throughout experiments.
Thermometry study
Thermocouples were first implanted in the wall of two saphenous veins to estimate the in situ thermal rise and to model the spatial profile of the thermal damage with a general Arrhenius equation.
The purposes of the thermometry experiments were to quantitatively measure the temperature produced at the vein wall by HIFU exposures and to estimate the extent of thermal damage along the vein.
For these purposes, thermocouples were implanted in the wall of the two lateral saphenous veins of one sheep and the in situ thermal rise was measured during ablation of both lateral saphenous veins on one sheep (17 months old and 55 kg weight).
Twenty sets of measurement were performed and compared to the thermal rise reported in the literature for RF ablation [Citation8–10].
Prior to HIFU exposures, a small skin incision was performed on the lower rear limb to introduce thermocouples in the target vein. The vein (mean diameter of 5 mm) was then catheterized with a 14 G catheter (BD Angiocath™, BD, USA), inferior to the treatment site, and two K-type thermocouples (outer diameters of 0.2 mm and 0.075 mm/Accuracy of 1.5 ± 0.25%) (identified as TC1 and TC2) were introduced into the catheter. The distance between the tips of the thermocouples was 2.5 mm.
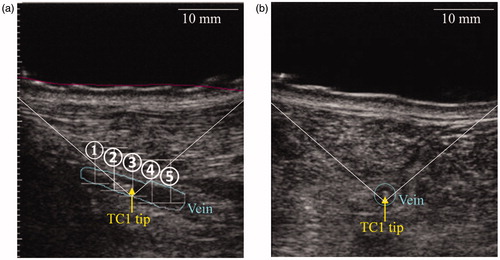
The transducer was positioned in the region of interest to target the lateral saphenous veins. Thermocouples were visualized under ultrasound guidance. Five HIFU procedures were delivered in each hind limb. Each procedure comprises a sequence of 8-s pulses distributed in a row of 5 spots placed every 3.5 mm along the vein. This spacing was chosen as a starting point because it has been demonstrated to induce a continuous ablation in liver tissues [Citation26]. Thermocouples were positioned within the vein in such a way as to have the tip of TC1 in the middle of the targeted venous segment, at spot #3 (). Pulses were applied at 85 W and a minimal pause time of 30 s was observed between subsequent sonications. Before sonication delivery, the fluid pressure within the balloon mounted on the transducer was increased to mechanically compress the vein to ensure a good contact between the thermocouple and the tunica intima. Temperature recording during sonications was achieved by an acquisition board (TC-08, Pico Technology, Cambridgeshire, UK) connected to a computer.
Figure 2. Longitudinal (a) and transverse (b) views of the positioning of the thermocouple TC1 in the vein. Spots are referred by their numbers.
To estimate the vascular thermal damage, the temperature profile induced by a HIFU pulse along the vein is needed. For feasibility reasons, inserting more than two thermocouples in the targeted veins was impossible. Therefore, thermocouples were kept at a fix location and the HIFU pulses were delivered at five different positions along the vein (). We assumed each pulse to be independent from each other so that one thermocouple measuring the temperature during five pulses can be interpreted as five thermocouples measuring the temperature during one pulse ().
Figure 3. Illustration of the change of frame of reference with one thermocouple for sake of clarity. Delivering five pulses (①, ②, ③, ④, ⑤) at given positions (symbolized by crosses) and measuring the temperature with one thermocouple into the vein at fixed locations is considered equivalent to the virtual situation where one pulse would be delivered and five thermocouples would acquire temperatures at the same positions. On the right, TCY with Y ∈ [Citation1–5] represents a virtual thermocouple that measures the temperature rise during pulse Y.
![Figure 3. Illustration of the change of frame of reference with one thermocouple for sake of clarity. Delivering five pulses (①, ②, ③, ④, ⑤) at given positions (symbolized by crosses) and measuring the temperature with one thermocouple into the vein at fixed locations is considered equivalent to the virtual situation where one pulse would be delivered and five thermocouples would acquire temperatures at the same positions. On the right, TCY with Y ∈ [Citation1–5] represents a virtual thermocouple that measures the temperature rise during pulse Y.](/cms/asset/385b1d90-ff9c-4eaf-a0f2-ff67626efd56/ihyt_a_1734672_f0003_c.jpg)
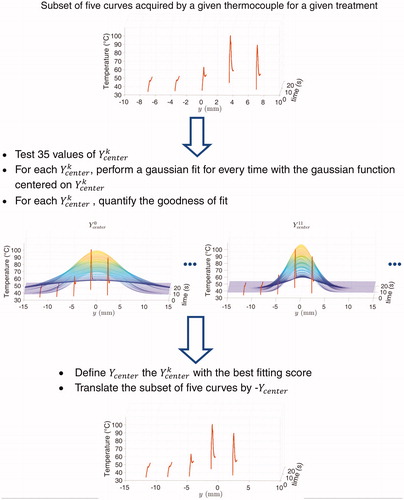
Since the distance between the two thermocouples was subject to little variations during insertion into the catheter, the two sets of temperature profiles were considered independently. For each treatment, the temperature measured with the thermocouples was synchronized with HIFU pulse delivery. Then, for each pulse, a temperature curve was extracted from the beginning of the pulse to 15 s after the end of the pulse. Since five pulses were delivered for each treatment and five treatments per vein were performed with temperature acquisitions by two thermocouples, the whole dataset was considered equivalent to having delivered one pulse with 100 recording thermocouples.
To correct the spatial errors in the positions of the thermocouples relatively to the focal spot, the curves were spatially registered. The spacing between two adjacent sites did not vary and was set to 3.5 mm. A Gaussian distribution of temperature was assumed. The spatial registration thus consisted in applying a spatial gaussian fit to each set of 5 measurements acquired with a 3.5 mm spacing. The Gaussian fit was performed for every time step of the thermocouple data. For the gaussian curves to be spatially consistent, a series of optimizations (see Appendix) was performed for all subsets of five curves.
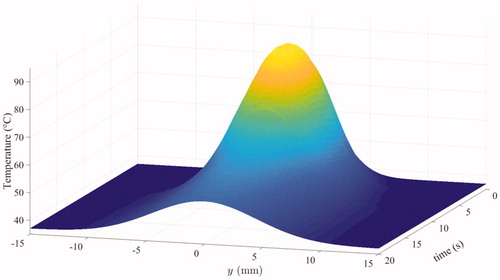
Once the 20 sets of 5 curves were spatially registered, the same process of Gaussian fit was applied on the resulting set of 100 temperature curves. This resulted in the final estimated spatiotemporal temperature distribution induced by a HIFU pulse along the vein.
To estimate the extent of thermal damage along the vein, the spatiotemporal temperature distribution was coupled to the metric of vascular thermal damage described by Agah et al. [Citation27]. Theoretical modeling of vascular thermal damage suggests that vessel damages correlate with thermal denaturation of collagen fibers which begins between 62 and 67 °C [Citation28,Citation29].
By considering the denaturation of collagen as a first-order reaction, vascular damage was modeled using a general Arrhenius equation and is appraised by the parameter
(1)
(1)
with
the degree of vessel damage along the axis of the vein
and
are the concentrations in undamaged collagen molecules respectively before sonications and at a time
The final time
was set to 23 s, that is, 15 s after the end of the 8-s pulse.
is the frequency factor,
is the activation energy,
is the universal gas constant and
is the temperature.
and
were set to
s−1and
kJ.mol−1 according to Agah et al. [Citation27].
To model an extensive damage to the vein, the threshold of vessel damage was set to which formally corresponds to denaturation of 99% of the native collagen fibers.
Efficacy and safety study of the thermal ablation method
HIFU protocol
In these experiments, nine female sheep were used. Animals were split into three groups of three animals each and were followed up 30, 60 and 90 days after treatments.
Each animal had both lateral saphenous veins treated with HIFU. The sheep saphenous vein was selected as the model for this study because it presents similarities to the human’s saphenous vein as it lies under superficial tissues and is surrounded by a fascia. Moreover, saphenous vein is a medium-sized vein of the superficial venous system (average 3 mm diameter), which makes chronic experiments feasible.
Sheep were installed on the surgical table in lateral decubitus position to access the targeted vein. Ultrasound examinations including both B-mode and Duplex imaging were performed to identify the portion of the vein to be treated and to evaluate post-sonications venous changes.
Eighteen saphenous veins with a mean diameter of 3.2 ± 1.0 mm were exposed to HIFU. Each vein was treated with a sequence of HIFU sonications distributed in a row of multiple spots along the vein with a 3.5 mm spacing. Continuous HIFU sonications were applied for 8 s with a mean acoustic power of 84 ± 6 W, according to the power used in a preliminary study performed on rabbit saphenous veins [Citation22]. The corresponding spatial-peak intensity estimated from linear extrapolation from low power hydrophone measurement in water is 28 kW.cm−2. As the mean diameter of the rabbit veins (2.0 ± 0.6 mm) was lower than the sheep veins; two sonications were repeated at each spot. A minimum cooling time of 30 s was set between sonications, for the skin to cool down. During each treatment, the hind limb of interest was slightly elevated to reduce vein caliber and maximize thermal damage of the vein wall. The vein was compressed before each exposure to stop blood flow and limit its heat sink effect and thus help temperature elevation of the vein wall to reach the desired vascular coagulation.
The effect of the HIFU treatments was evaluated by post-treatment ultrasound imaging. Sheep were then recovered from anesthesia and were returned to their pen. From day 1 to the day of euthanasia, the sheep were closely observed and regular physical examinations were performed by the veterinary staff who kept record of a list of subjective and objective observations () to detect possible post-treatment side effects. Common clinical signs like food and drink consumption, behavior and posture were systematically assessed and documented for each animal. Prior to sacrifice, sheep were anesthetized as described above and ultrasound images were performed to assess clinically the targeted venous segment. Animals were then euthanized by intravenous injection of 25 ml of Pentobarbital 18% (Dolethal, Vetoquinol).
Table 1. Clinical signs assessed after HIFU treatments.
Histological examination
Venous tissues along with samples of superficial perivenous muscle tissue and deep perivenous muscle tissue were extracted, fixed in 4% formalin and sent for histologic processing and histopathological analysis. Subsections of 3 to 5 µm thick were then cut and deposited on glass slides. Slides were stained with Hematoxylin and Eosin (H&E) and Gomori’s Elastin Trichrome (GET). Thermal changes and subsequent healing changes were pathologically assessed by an experienced pathologist (ACVP board certified). For vein tissues, the pathologist was explicitly asked to look for possible vein mural injury, vein thrombosis, vein fibrosis and potential occurrence of vein perforation and blood extravasation.
Results
Thermometry experiments
The maximum temperature rise recorded by each thermocouple for each treatment is reported in . The mean temperature rise recorded during all sonications was 90.3 ± 6.5 °C.
Table 2. Maximum temperatures recorded by each thermocouple during each treatment performed in both left and right hind limb.
represents the aggregated temperature profiles for the right treated vein (a) and shows an example of a Gaussian fit of the temperatures acquired by the thermocouple TC1 during the treatment #3 delivered in the right saphenous vein (b). The mean spatial correction over the 20 sets of 5 curves was 1.8 ± 1.6 mm.
Figure 4. Illustration of temperature profiles measured by the thermocouple TC1 during the treatment #3 in the right vein for each of the y-locations before spatial registration (a) and example of spatial registration of the data acquired by the thermocouple TC1 during the treatment #3 (b).
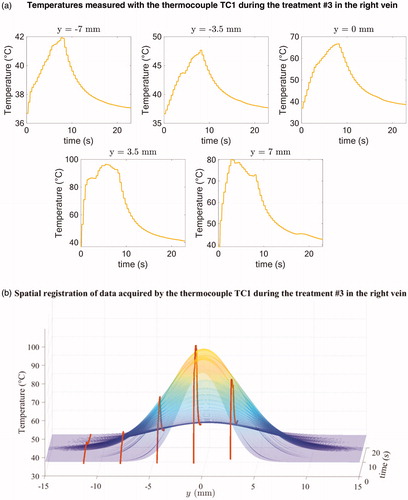
The final estimated spatiotemporal distribution is represented in .
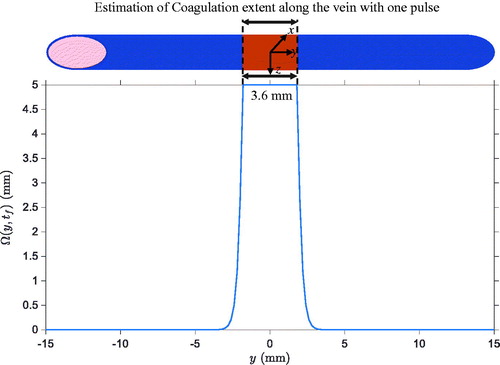
Based on the estimated spatiotemporal temperature distribution represented in , the spatial profile of thermal damages was computed. It is displayed in . The result demonstrates that the collagen is expected to be denatured over a length of 3.6 mm along the vein.
Macroscopic and microscopic findings
Immediately following HIFU exposures, hyperechoic marks and vein lumen reduction were observed in all treated veins as compared to before treatment. shows a typical ultrasound image of a saphenous vein before () and immediately after exposure . After the procedure, all targeted vein segments showed sonographically blurred vascular contours as well as vein wall constriction. illustrates a typical vein shrinkage observed after the procedure.
Figure 7. Ultrasound images of the lateral saphenous vein (pointed by a white arrow) of a sheep before exposure (a) and immediately after exposure (b).The exposed vein has a reduced lumen and a hyper-echoic mark (pointed by a yellow arrowhead) is visible.
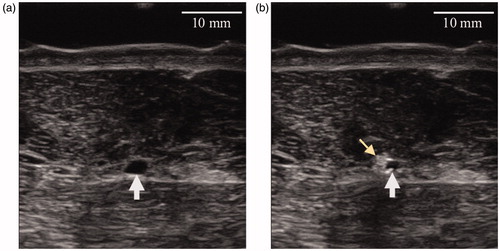
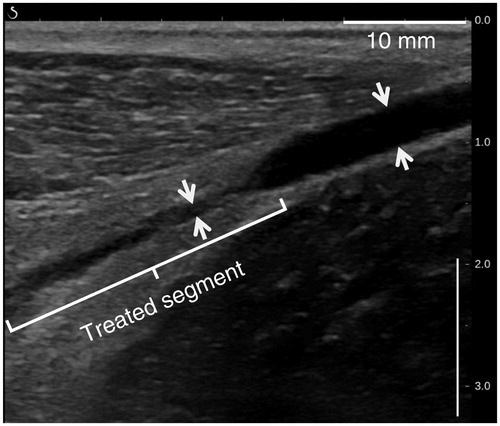
Figure 8. B-mode image after HIFU procedure showing a vein diameter constriction and blurred vascular contours.
Before sacrifice, occlusion was also observed 30, 60 and 90 days post-treatment, as evidenced by the incompressibility of the vein with ultrasound probe pressure (), the interruption of color Doppler signals () or the presence of a hyperechoic cord filling the lumen (). In total, 83% of the veins showed before sacrifice interruption or reduction of the blood flow as judged by the color Doppler ultrasound. summarizes the imaging results of the treated sheep saphenous veins.
Figure 9. Ultrasound images showing a non-compressible treated vein at 30 days post-treatment (left column) and a compressible untreated vein (right column). Veins are pointed by white arrows. (a) and (b) represent respectively the sheep veins before and after external ultrasound compression.
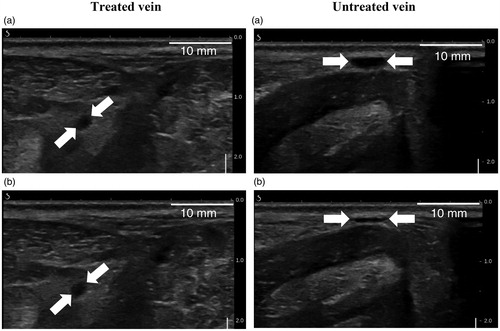
Figure 10. Color Doppler ultrasound image showing the absence of flow in a sheep vein (white arrow) 60 days after treatments.
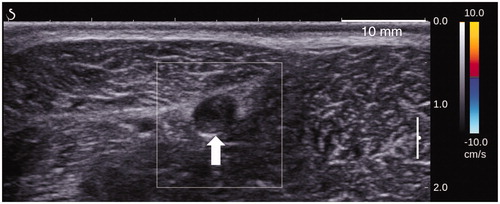
Figure 11. B-mode image of a sheep vein (white arrow) taken 90 days after sonications and showing complete fibrosis of the treated segment.
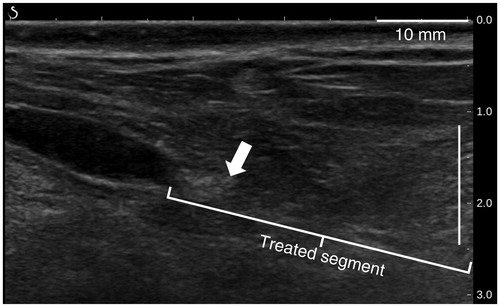
Table 3. Imaging findings synthesis.
During tissue samples harvesting, HIFU-treated regions were observable as whitened and indurated zones. Neither perforation nor charring were reported for any of the targeted veins.
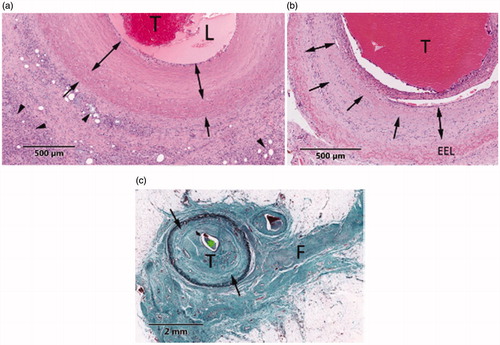
Histological evaluation of venous tissues harvested 30, 60 or 90 days post treatment showed typical changes of thermal injury as can be seen on . Microscopic changes were characterized at 30 days by coagulative necrosis of the vein wall (). Smooth muscle cells showed coagulation necrosis and the mural, adventitial and perivascular collagen showed thermal denaturation characterized by a loss in microfibrillar structure and collagen hyalinization. Vein thrombosis and occlusion were observed. In addition, histiocytes and foreign body giant cells were found in the HIFU-affected vascular tissues. At 60 days after the treatment, vein thrombosis and occlusion associated with vein wall fibrosis were observed. Residual hyalinization was also found in the tunica media (). Treatment-induced vein changes at 90 days presented occlusive thrombosis, characterized by organized fibrocellular thrombus, and vein wall fibrosis (). No vein lacerations and no subsequent blood extravasation were observed in any of the evaluated veins.
Figure 12. Representative illustrations of HIFU lesions in vascular tissue. (a) H&E-section of a vein extracted 30 days after treatment. HIFU lesion shows vein wall hyalinization (arrows), thrombus (T) and necrotic perivascular adipose tissue (arrowheads). (b) H&E-section of a vein extracted at 60 days. Thrombus (T), fibroblast infiltration (double arrows) and residual media hyalinization (arrows) are observed. (c) GET-section of a vein extracted at 90 days and presenting fibrocellular thrombus (T), media fibrosis (arrows) and perivascular fibrosis (F).
Microscopic evidence of thermal damage was also found in perivenous tissues. At 30 days, perivascular fat necrosis and limited muscle necrosis in the superficial perivenous skeletal muscle were observed. Perivascular fat necrosis was detected in the form of local and demarcated steatonecrosis with the presence of macrophages and giant cells into the necrotic tissue (). Muscle necrosis was observed as delimited areas of coagulative necrosis with fibrous healing (). Perivenous collateral changes at 60 days post-treatment included perivascular fibrosis and confined muscle necrosis that was limited to the superficial perivenous muscle exclusively. Muscle-affected tissues presented regenerating myofibers and muscle fibrosis (). No fat necrosis was observed at 60 days. Surrounding tissue changes at 90 days after HIFU treatment were primarily found in perivenous connective tissue and were characterized by perivascular fibrosis (). No fat necrosis neither residual necrotic adipose tissue was observed at 90 days post-treatment. Histological findings are summarized in .
Figure 13. Typical HIFU lesions in perivenous collateral tissues. (a) GET-section of a superficial perivenous muscle extracted at 30 days; the area under the dotted line presents focal muscle necrosis with fibrosis (arrowhead) and residual necrotic muscle tissue (asterisk). (b) GET-section of a superficial perivenous muscle harvested 60 days post-treatment (GET); the area above the dotted line shows necrosis and regeneration with fibrosis in the muscle.
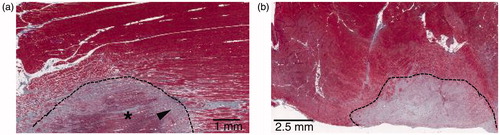
Table 4. Histopathological findings synthesis.
No adverse events occurred during exposures and all animals recovered well after HIFU treatments (). None of the sheep showed complications during the follow-up. Sheep continued their normal pattern of feeding and lived normally until the end of the scheduled sacrifice.
Discussion
Based on the temperatures recorded by the thermocouples, the mean peak temperature achieved at the intima was 90 ± 7 °C. Using the metric of vascular thermal damage developed by Agah et al. [Citation27], the veins were estimated to have been heated sufficiently to cause irreversible thermal damage of the vein wall.
Other studies have measured the temperature during thermal ablation of veins and have correlated vascular effects with the temperature elevation. In a laser study, Martinot et al. [Citation30] reported the vascular changes observed for different peak temperatures generated at the vein during irradiation. No effects were observed when the measured temperature was below 54 °C. However, successful vascular welding was reported when temperatures reached 77 °C on average, during 3–4 s irradiation. The accumulated thermal damage () was calculated for these exposure parameters based on the formula expressed in (1) and is reported in . For the irradiations where a peak temperature of 54 °C was measured, the estimated accumulated damage value was below the threshold reported for first noticeable irreversible damage. On the contrary, the predicted accumulated thermal damage for the exposures where the reported temperatures reached 77 °C was above the threshold for an irreversible thermal damage. Observations agree with the theoretical modeling of vascular thermal damage suggested by Agah et al. [Citation27].
Table 5. Vascular thermal damage computed for different exposure parameters reported in the studies conducted by Martinot el al. [Citation25] and Fujiwara et al. [Citation27].
In the published literature, the response of blood vessels to the HIFU-induced temperature rise was also investigated [Citation31–33]. Among these studies, Fujiwara et al. [Citation32] measured the local temperature induced for different sonication exposure parameters. Investigators reported a successful vascular occlusion at peak temperatures reaching 97 °C during the delivery of a 5-s pulse but not when the temperature achieved 46 °C at the end of the pulse. No occlusion was reported for a measured peak temperature of 48 °C during a longer sonication (10 s). Again, by translating the exposure parameters into values (), the observations agree with theoretical modeling of vascular thermal damage.
In the formulation of the vascular thermal damage, the accumulated damage is exponentially related to the temperature and linearly related to heating time. The temperature reached during heating is therefore a key factor for generating thermal injury to the vein wall almost immediately.
In a previous study, the immediate treatment success of the proposed HIFU procedure was estimated by measuring the vein diameter constriction after treatment [Citation34].
The assessment of the success of the treatments was based here on the histological examination of tissues that was performed 30, 60 and 90 days post-treatment. Microscopic evaluation of HIFU-treated veins revealed evidence of thermal damages as demonstrated by coagulation necrosis of the vein wall, venous thrombosis and fibrosis.
At 30 days, thermal coagulation was primarily associated with media hyalinization, thermal denaturation of collagen fibers, collagen hyalinization and fibrin thrombus. Similar histologic changes consecutive to high temperature elevation have been reported on veins treated by laser or RF and examined at 21 to 42 days following treatment [Citation35–39].
At 60 days, vascular wall still showed residual vein wall hyalinization but exhibited fibroblasts infiltration in addition. Likewise, fibrin thrombus was observed at 60 days but fibrous organized thrombi were also found. These changes are characteristic of gradual and ongoing fibrous healing, as evidenced by the presence of fibroblasts reportedly engaged in tissue repair [Citation40]. Injured tissues were gradually replaced by granulation and fibrous connective tissues.
At 90 days, treated veins showed complete healing with transformation of heat-affected sections to scar tissues. Fibrous healing and vein damages were considered stable at 90 days after HIFU treatment. The desired therapeutic effect has thus been achieved: vein fibrotic closure and the ultrasound setting used in this study is effective. To the best of our knowledge, no other study reported fibrotic vein occlusion after extracorporeal HIFU application on non-injured medium-sized veins.
We additionally observed here changes in the perivascular tissues and the collateral muscles. They were primarily characterized by localized areas of coagulation necrosis. Surrounding connective tissues showed fat necrosis with chronic inflammatory response at 30 days post-treatment exclusively. Healing reaction to adipose tissue necrosis as evidenced by inflammatory cells infiltration that resolved over time since perivascular fat necrosis (steatonecrosis) was no longer observed at 60 nor 90 days. From a pathology standpoint, steatonecrosis is thus not considered to be a safety concern as it fully heals and resorbs over time. Surrounding muscles also showed evidences of prior thermal injury. At 30 days, it was evidenced by local necrotic areas with gradual fibrous healing confined exclusively to the superficial perivenous muscle. One can also note here that the presence of hyperechoic marks at focus resulting from boiling could have been impeded the propagation of the HIFU beam beyond focus. At 60 days, evidences of prior thermal injury were also confined to superficial perivenous muscle and were characterized by demarcated areas of coagulation, myofiber regeneration and fibrous healing. Substantial muscle regeneration was observed at 90 days and healing was complete at 90 days.
Based on these results, thermal collateral damages to perivenous tissues was limited and completely healed over time. The treatment setup used in this study thus appears to be safe regarding perivascular tissues. No hemorrhage was observed following HIFU exposures contrarily to previous studies [Citation31,Citation41–44]. This supports the choice of the ultrasound parameters used in this paper in terms of safety and efficiency for treating medium-sized veins.
Microscopic evaluation of treated veins and surrounding tissues helps understanding clinical findings. Thermal collagen contraction and denaturation have been reported to be responsible for heat-induced vein shrinkage by different studies [Citation45,Citation46]. We also observed here microscopically thermal denaturation of collagen after HIFU treatment. We hypothesize that the HIFU-induced vein shrinkage was attributed to contraction and denaturation of intramural collagen fibers. Besides, Shaw et al. [Citation25] related the contribution of collagen hyalinization to tissue stiffening [Citation25]. Thermal collagen denaturation could thus explain why treated vein segments were stiffer than untreated ones. Vein occlusion assessed by ultrasound imaging could also be explained by the histologic findings: the transformation of the treated vein segment into a hyperechoic cord visible on the ultrasound images could be attributed to fibrotic sealing of the veins. Fibrotic scars are indeed mainly composed of collagen, as determined by histologic examinations. The collagen content in fibrotic tissues is known to increase the acoustic impedance and increase echogenicity [Citation47].
Macroscopic and microscopic features reported here inside and around the vein after HIFU treatment have also been observed following thermoablation procedures like RFA or laser: primary changes after radiofrequency and laser treatments include vein shrinkage, vein induration, medial and adventitial lesions, vein thrombosis and perivenous tissue damages [Citation36–38,Citation48–56]. Tissue repair response has also been reported in RF or laser chronic studies and were characterized by gradual replacement of injured tissue components by granulation and fibrous connective tissue [Citation35,Citation49,Citation57,Citation58]. HIFU-induced vascular and perivascular changes, as well as subsequent healing changes, are hence similar to that reported after endovenous thermotherapies. In particular, according to the analysis of the experienced pathologist in the evaluation of RF-affected veins, HIFU and RF create lesions that display identical features, to the extent that HIFU lesions were deemed indistinguishable from the lesions that can be induced by radiofrequency.
In conclusion, the ultrasound exposure parameters used in this study have been demonstrated to be both safe and efficient in vivo for medium-sized veins. For larger veins (> 3 mm) as might be human varicose veins [Citation2], the exposure parameters should be adjusted. With the current HIFU device, multiple sonications, for example, can be considered in order to adequately sonicate the entire vein wall circumference. To our knowledge, no previously published work demonstrated noninvasive fibrotic closure in medium-sized veins, which is the ultimate desired effect for varicose veins treatment. Ongoing work is investigating the possibility to treat different human pathologic leg veins like great saphenous veins, stumps and perforators.
Acknowledgments
We thank Alizée Pathology, LLC for the histopathological analysis.
Disclosure statement
Nesrine Barnat, Anthony Grisey, Bjoern Gerold, Sylvain Yon and Jérémie Anquez are employees of Theraclion.
Additional information
Funding
References
- Eklöf B, Rutherford RB, Bergan JJ, et al. Revised CEAP classification for chronic venous disorders: consensus statement. Phlebologie. 2005;34(4):220–225.
- Allegra C, Antignani P-L, Bergan JJ, et al. The ‘C’ of CEAP: suggested definitions and refinements: an international union of phlebology conference of experts. J Vasc Surg. 2003;37(1):129–131.
- Goldman MP, Weiss RA. Sclerotherapy E-book: treatment of varicose and telangiectatic leg veins. Amsterdam: Elsevier Health Sciences; 2016.
- Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Intervent Radiol. 2003;14(8):991–996.
- Weiss RA, Weiss MA. Controlled radiofrequency endovenous occlusion using a unique radiofrequency catheter under duplex guidance to eliminate saphenous varicose vein reflux: A 2-year follow-up. Dermatol Surg. 2002;28(1):38–42.
- Bergan JJ and Shortell CK, editors. Venous ulcers. Amsterdam: Elsevier Inc.; 2007.
- Bozoglan O, Mese B, Eroglu E, et al. Comparison of endovenous laser and radiofrequency ablation in treating varices in the same patient. J Lasers Med Sci. 2017;8(1):13–16.
- Braithwaite B, Hnatek L, Zierau U, et al. Radiofrequency-induced thermal therapy: results of a European multicentre study of resistive ablation of incompetent truncal varicose veins. Phlebology. 2013;1:38–46.
- Lohr J, Kulwicki A. Radiofrequency ablation: evolution of a treatment. Semin Vasc Surg. 2010;23(2):90–100.
- Newman JE, Meecham L, Walker RJ, et al. Optimising treatment parameters for radiofrequency induced thermal therapy (RFiTT): A comparison of the manufacturer’s treatment guidance with a locally developed treatment protocol. Eur J Vasc Endovasc Surg. 2014;47(6):664–669.
- Rasmussen LH, Lawaetz M, Bjoern L, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98(8):1079–1087.
- Van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49(1):230–239.
- Proebstle TM, Alm J, Göckeritz O, et al. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg. 2011;54(1):146–152.
- Nelzén O. Great uncertainty regarding treatment of varicose vein recurrence. Phlebologie. 2014;43(1):13–18.
- Theivacumar NS, Gough MJ. Endovenous Laser Ablation (EVLA) to treat recurrent varicose veins. Eur J Vasc Endovasc Surg. 2011;41(5):691–696.
- Ter Haar G, Coussios C. High intensity focused ultrasound: Physical principles and devices. Int J Hyperther. 2007;23(2):89–104.
- Schultz-Haakh H, Li JK, Welkowitz W, et al. Ultrasonic treatment of varicose veins. Angiology. 1989;40(2):129–137.
- Delon-Martin C, Vogt C, Chignier E, et al. Venous thrombosis generation by means of high-intensity focused ultrasound. Ultrasound Med Biol. 1995;21(1):113–119.
- Pichardo S, Milleret R, Curiel L, et al. In vitro experimental study on the treatment of superficial venous insufficiency with high-intensity focused ultrasound. Ultrasound Med Biol. 2006;32:883–891.
- Hwang JH, Zhou Y, Warren C, et al. Targeted venous occlusion using pulsed high-intensity focused ultrasound. IEEE Trans Biomed Eng. 2010;57(1):37–40.
- Zhou Y, Zia J, Warren C, et al. Targeted long-term venous occlusion using pulsed high-intensity focused ultrasound combined with a pro-inflammatory agent. Ultrasound Med Biol. 2011;37(10):1653–1658.
- Barnat N, Grisey A, Lecuelle B, et al. Noninvasive vascular occlusion with HIFU for venous insufficiency treatment: Preclinical feasibility experience in rabbits. Phys Med Biol. 2019;64(2):12.
- Schmedt C-G, Meissner OA, Hunger K, et al. Evaluation of endovenous radiofrequency ablation and laser therapy with endoluminal optical coherence tomography in an ex vivo model. J Vasc Surg. 2007;45(5):1047–1058.
- Heger M, Van Golen RF, Broekgaarden M, et al. Endovascular laser-tissue interactions and biological responses in relation to endovenous laser therapy. Lasers Med Sci. 2014;29(2):405–422.
- Shaw CJ, Ter Haar GR, Rivens IH, et al. Pathophysiological mechanisms of high- intensity focused ultrasound-mediated vascular occlusion and relevance to non-invasive fetal surgery. J R Soc Interface. 2014;11(95):20140029.
- Grisey A, Yon S, Letort V, et al. Simulation of high-intensity focused ultrasound lesions in presence of boiling. J Ther Ultrasound. 2016;4(1):14.
- Agah R, Pearce JA, Welch AJ, et al. Rate process model for arterial tissue thermal damage: Implications on vessel photocoagulation. Lasers Surg Med. 1994;15(2):176–184.
- Pankhurst K. Incipient Shrinkage of Collagen and Gelatin. Nature. 1947;159(4042):538–538.
- Plaut GWE. Extracellular and supporting structures (Vol 26C). In: Florkin, Marcel Stotz, Elmer H, editors. Comprehensive biochemistry. Amsterdam: Elesevier; 1971.
- Martinot VL, Mordon SR, Mitchell VA, et al. Determination of efficient parameters for argon laser-assisted anastomoses in rats: macroscopic, thermal, and histological evaluation. Lasers Surg Med. 1994;15(2):168–175.
- Hynynen K, Colucci V, Chung A, et al. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med Biol. 1996;22(8):1071–1077.
- Fujiwara R, Sasaki K, Ishikawa T, et al. Arterial blood flow occlusion by high intensity focused ultrasound and histologic evaluation of its effect on arteries and surrounding tissues. J Med Ultrasonics. 2002;29(3):85–90.
- Ishikawa T, Okai T, Sasaki K, et al. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med Biol. 2003;29(10):1471–1477.
- Barnat N, Grisey A, Gerold B, et al. Vein wall shrinkage induced by thermal coagulation with HIFU: numerical modelling and in vivo experiments in sheep, submitted to the International Journal of Hyperthermia. Int J Hyperther.
- Weiss R, Feied C, Weiss M. RF-mediated endovenous occlusion. In: Weiss, Robert A, Feied, Craigand Weiss, Margaret, editors. Vein diagnosis and treatment. USA: McGraw-Hill Medical Publishing Division, 2001. p. 211–221.
- Manfrini S, Gasbarro V, Danielsson G, et al. Endovenous management of saphenous vein reflux. J Vasc Surg. 2000;32(2):330–342.
- Vuylsteke M, Van Dorpe J, Roelens J, et al. Intraluminal fibre-tip centring can improve endovenous laser ablation: a histological study. Eur J Vasc Endovasc Surg. 2010;40(1):110–116.
- Vuylsteke M, Van Dorpe J, Roelens J, et al. Endovenous laser treatment: a morphological study in an animal model. Phlebology. 2009;24(4):166–175.
- Bush RG, Shamma HN, Hammond K. Histological changes occurring after endoluminal ablation with two diode lasers (940 and 1319 nm) from acute changes to 4 months. Lasers Surg Med. 2008;40(10):676–679.
- Delavary BM, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762.
- Rivens IH, Rowland IJ, Denbow M, et al. Vascular occlusion using focused ultrasound surgery for use in fetal medicine. Eur J Ultrasound. 1999;9(1):89–97.
- Denbow ML, Rivens IH, Rowland IJ, et al. Preclinical development of noninvasive vascular occlusion with focused ultrasonic surgery for fetal therapy. Am J Obstet Gynecol. 2000;182(2):387–392.
- Ichihara M, Sasaki K, Umemura S, et al. Blood flow occlusion via ultrasound image-guided high-intensity focused ultrasound and its effect on tissue perfusion. Ultrasound Med Biol. 2007;33:452–459.
- Mahoney K, Martin H, Hynynen K. Focused ultrasound effects on blood vessels in vivo-limits for vascular interventions. IEEE Ultrasonics Symposium Proceedings. 2000;2:1405–1408.
- Gorisch W, Boergen K-P. Heat-induced contraction of blood vessels. Lasers Surg Med. 1982;2(1):1–13.
- Serrone J, Kocaeli H, Mast TD, et al. The potential applications of high-intensity focused ultrasound (HIFU) in vascular neurosurgery. J Clin Neurosci. 2012;19(2):214–221.
- Fields S, Dunn F. Correlation of echographic visualizability of tissue with biological composition and physiological state. J Acoustical Soc Am. 1973;54(3):809–812.
- Bedi H, Calton N, Kwatra K, et al. Histopathological findings of the human great saphenous vein treated with endoluminal radio frequency ablation. Int Surg J. 2014;1(1):3–5.
- Brachmann K, Gütz U. Histologische veränderungen nach endoluminaler varizenbehandlung durch die zirkuläre thermoablation (ClosureFastTM). Phlebologie. 2012;41(2):73–76.
- Schmedt C-G, Sroka R, Steckmeier S, et al. Investigation on radiofrequency and laser (980 nm) effects after endoluminal treatment of saphenous vein insufficiency in an ex-vivo model. Eur J Vasc Endovasc Surg. 2006;32(3):318–325.
- Thomis S, Verbrugghe P, Milleret R, et al. Steam ablation versus radiofrequency and laser ablation: an in vivo histological comparative trial. Eur J Vasc Endovasc Surg. 2013;46(3):378–382.
- Blagova R, Burgmeier C, Steckmeier S, et al. Ex-vivo investigations on endoluminal laser therapy of varicosis – an optimization process. Med Laser Appl. 2008;22(4):242–247.
- Meissner OA, Schmedt CG, Hunger K, et al. Endovascular optical coherence tomography ex vivo: venous wall anatomy and tissue alterations after endovenous therapy. Eur Radiol. 2007;17(9):2384–2393.
- Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28(1):56–61.
- Jelev L, Guirov K, Minkov M, et al. Morphological changes in the wall of great saphenous vein after radiofrequency ablation. Scripta Scientifica Medica. 2013;45:60–62.
- Shaidakov EV, Grigoryan AG, Korzhevskii DE, et al. Morphologic changes in the vein after different numbers of radiofrequency ablation cycles. J Vasc Surg Venous Lymphat Disord. 2015;3(4):358–363.
- Vuylsteke ME, Mordon SR. Endovenous laser ablation: a review of mechanisms of action. Ann Vasc Surg. 2012;26(3):424–433.
- Wang X, Wang X, Su W, et al. Microwave ablation versus laser ablation in occluding lateral veins in goats. J Huazhong Univ Sci Technol [Med Sci]. 2016;36(1):106–110.
Appendix
This appendix provides a description of the process used to register spatially the temperature curves acquired by the thermocouples.
For each subset of 5 temperature curves, a series of optimization was performed as follows:
− 35 values of the coordinate of the center of the Gaussian were tested with a spatial spacing of 0.2 mm:
– Then, for each Ycenterk, a Gaussian curve centered on was fitted for each time step
at the five data points. The fitting error for each time step, denoted
was calculated. Finally, the fitting errors for all time steps,
were then summed:
The best denoted
was selected as the value minimizing the temporal sum of fitting errors
The centers of the corresponding Gaussian profiles were then spatially translated by −
The spatial registration process is depicted on .