Abstract
Radiofrequency ablation (RFA) has been clinically used as a minimally invasive procedure for the treatment of many solid tumors. However, the current imaging techniques have some shortages in RFA guidance, especially for the assessment of the margin of ablation. Herein, we developed a novel optical imaging platform to guide RFA utilizing fluorescence resonance energy transfer from a thermally sensitive fluorescent protein conjugated to a near-infrared fluorescent dye. Additionally, attaching receptor-targeting ligands further equipped the system with high specificity to tumors overexpressing the targeted receptor.
Introduction
Radiofrequency ablation (RFA) is a minimally invasive procedure that has been used as a standard treatment in many solid tumors [Citation1,Citation2]. RFA irreversibly destroys tumor tissues by employing an alternating current to generate frictional heat and induces coagulation necrosis within the target region [Citation3]. However, the complete destruction of tumors by ablation has several difficulties, such as poor detection of the tumor borders and the heat sink effect of blood vessels [Citation4–6]. Therefore, currently, the tumor and at least 5 mm of surrounding normal tissue are required to be included in the ablation zone [Citation7]. Therefore, it is very important to distinguish the ablation margin during the RFA procedure. Although some current imaging techniques, such as contrast-enhanced computed tomography (CT), contrast-enhanced magnetic resonance (MR) and contrast-enhanced ultrasound (CEUS), have been applied for RFA guidance, distinguishing the ablated tumor margin from the surrounding healthy tissues remains a challenge to determine whether the ablation margin is sufficient. Therefore, insufficient RFA is common in current clinical operations and might result in rapid progression of the residual tumor—one of the major concerns in the clinical applications of RFA [Citation8–10]. Therefore, to achieve the goal of ablation margin assessment, we developed a novel fluorescence resonance energy transfer (FRET)-based optical imaging system that comprises a thermally sensitive fluorescent protein and a near-infrared (NIR) fluorescent dye. Once heated to 60 °C or above, the fluorescent protein is bleached quickly due to heat-induced protein denaturation, which subsequently results in decreasing the fluorescence emission of the NIR dye.
With recent advances in optical imaging techniques, fluorescence imaging using fluorophore-incorporated tumor-specific ligands has exhibited great potential in tumor detection due to good sensitivity and spatial resolution [Citation11–13]. For example, fluorescence imaging has been clinically used to guide surgical operations by the spatiotemporal visualization of targeted tissue and margins [Citation14–16]. Inspired by this fluorescence-guided surgery, we proposed the development of a fluorescence imaging system to guide the RFA procedure that would require rapid and significant fluorescent changes in response to RFA treatment. The thermally sensitive fluorophore can be utilized to fulfill the following tasks: (1) visualization of the targeted tumor margins before RFA via direct imaging of the fluorophore and (2) assessment of RFA efficaciousness on the ablation margin by determining the fluorescence decrease during the RFA procedure. Fluorescent proteins possess appropriate heat sensitivity due to the same heat-induced protein denature mechanism utilized by RFA therapy, but they normally suffer from relatively less tissue penetration and worse scattering compared to the commonly used NIR fluorescent dyes. On the other hand, the NIR fluorescent dyes usually do not have sufficient sensitivity to heat, whereas cancer cell protein denaturation occurs very fast once heated to ≥60 °C. To take advantage of NIR fluorescent dyes and fluorescent proteins, we designed and prepared the following thermally sensitive fluorescence imaging platform to guide the RFA procedure utilizing FRET from fluorescent proteins to NIR fluorescent dyes.
Materials and methods
Materials
All solvents and reagents were used as supplied without further purification. 2-Chlorotrityl chloride resin was purchased from Nankai Hecheng S&T Co. (Tianjin, China). Fmoc-l-Asp-OAll, Fmoc-Gly-OH, Fmoc-l-Arg(Pbf)-OH, Fmoc-l-Lys(Dde)-OH, Fmoc-d-Tyr(tBu)-OH, and Fmoc-l-Lys(Boc)-OH were purchased from Kaijie Peptide Company (Chengdu, China). Fmoc-NH-PEG4-CH2CH2COOH was purchased from Biomatrik, Inc. (Jiaxing, China). Cyanine 5 NHS ester (Cy5-NHS) was purchased from Lumiprobe Corp. (Hunt Valley, MA, USA). R-Phycoerythrin (R-PE) was purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). DAPI was purchased from Beyotime Biotechnology (Shanghai, China). ProLong Gold antifade reagent was purchased from Life Technologies (Carlsbad, CA, USA). The anti-integrin αvβ3 antibody was purchased from Abcam (Cambridge, UK). DMEM, MEM, fetal bovine serum (FBS), and penicillin–streptomycin were all purchased from Gibco (Waltham, MA, USA).
Synthesis of RGD-Cy5-R-PE
The cyclic RGD peptide [(Arg-Gly-Asp-d-Tyr-Lys); c(RGDyk)] was synthesized as previously reported [Citation17]. Then, Fmoc-NH-PEG4-CH2CH2COOH, Fmoc-l-Lys(Boc)-OH, and Cy5-NHS were sequentially coupled with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetra-fluoroborate (HBTU) (3 equiv) and N-diisopropylethylamine (DIEA) (10 equiv) in DMF. The Kaiser test was performed to verify the completeness of coupling. The Fmoc group was removed with 20% piperidine/DMF. Cleavage of the polypeptide [c(RGDyk)-(PEG4)-K-(Cy5)] from the resin was performed by using a trifluoroacetic acid (TFA) (95%)/1,2-ethanedithiol (EDT) (2%)/triisopropylsilane (TIS) (2%)/H2O (1%) mixture in the dark for 2 h. The purification and analysis of the crude product were carried out on a high-performance liquid chromatography (HPLC) system (Shimadzu, Japan) using a gradient eluent from 70% buffer A (0.1% TFA in water) and 30% buffer B (0.1% TFA in acetonitrile) to 25% buffer A and 75% buffer B over 15 min. The polypeptide was then collected, lyophilized. The identification of the polypeptide was confirmed by MS (the result is shown in SI Figure 1) and stored in the dark at −20 °C until use.
The polypeptide dissolved in PBS (pH 7.4) was mixed with glutaraldehyde at room temperature for 30 min. R-PE was dissolved in PBS (pH 7.4) and then added to the above solution dropwise in the dark at room temperature. After 4 h, the mixture was dialyzed in the dark at 4 °C for 48 h to remove excess reactants. The remaining solution was lyophilized to obtain the titled probe. The purity of RGD-Cy5-R-PE was confirmed by HPLC (the result is shown in SI Figure 2).
Measurements and analysis
To determine how many RGD-Cy5 were conjugated to R-PE, an ultraviolet–visible spectrophotometry-based method was developed and applied. Briefly, the absorbance spectrum was measured using ultraviolet–visible spectrophotometer. The concentration of Cy5 [c(Cy5)] was first calculated using the UV absorbance at 646 nm and the following formula: c(Cy5)=A646nm/ε(Cy5, 646 nm). The concentration of R-PE [c(R-PE)] was calculated using the UV absorbance at 490 nm and the following formula: c(R-PE)= [A490nm-A646nm × ε(Cy5, 490 nm)/ε(Cy5, 646 nm)]/ε(R-PE, 490 nm). The number of RGD-Cy5 conjugated to R-PE (n) was then calculated to be 5 using the following formula: n=c(Cy5)/c(R-PE).
Cell culture and animal models
The human pancreatic cancer cell line PANC-1, the hepatocellular carcinoma cell line HepG2 and the breast cancer cell line MDA-MB-231 were all purchased from Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences. PANC-1 and MDA–MB-231 cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin. HepG2 cells were cultured in MEM containing 10% FBS and 1% penicillin–streptomycin. All cells were cultured in an incubator with a constant temperature of 37 °C and a humidified atmosphere of 5% CO2.
All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University School of Medicine. The xenograft tumor mouse models of hepatic cancer were induced in 6-week-old male BALB/c nude mice by subcutaneously injecting 5 × 106 HepG2 cells suspended in 200 μL of PBS.
Thermal treatment and fluorescent imaging in vitro
PANC-1, HepG2, and MDA-MB-231 cells were seeded into 24-well plates and treated with 5 μM of the titled fluorescent probe at 37 °C for 1 h. After incubation, the cells were washed three times with PBS and fixed with neutral formalin for 20 min at room temperature. The cell nuclei were stained with DAPI for 5 min at room temperature. Cells were then mounted with ProLong Gold antifade reagent and imaged using a Leica TCS SP8 confocal fluorescence microscope (Leica, Wetzlar, Germany) equipped with Cy5 (excitation/emission: 461–531/630–730 nm) and DAPI (excitation/emission: 335–383/407–461 nm) filters. Subsequently, slides were heated with an IKA C-MAG HS4 digital heating magnetic stirrer (IKA, Schwarzwald, Germany) at 60 °C for 30 min followed by imaging again. Finally, slides were heated at 90 °C for 30 min and imaged once more. To compare the fluorescence under thermal change, images were acquired using the same settings of exposure time and black balance.
Quantitative fluorescence intensity of RGD-Cy5-R-PE in vitro
PANC-1, HepG2, and MDA-MB-231 cells were seeded into 96-well optical plates and incubated with 5 μM of the titled fluorescent probe at 37 °C for 1 h. Then, the cells were washed twice with cell culture medium. A Molecular Devices SpectraMax i3 Multi-Mode Microplate Reader (Silicon Valley, CA, USA) was used to read fluorescence at 490/665 nm (excitation/emission). After the reading, plates were heated at 60 °C for 30 min, and the fluorescence emission was measured again. Once more, plates were heated at 90 °C for 30 min, and fluorescence was measured. Subsequently, the cells were fixed with neutral formalin for 20 min, and then DAPI was added for 5 min at room temperature. After rinsing twice with PBS, fluorescence at 364/454 nm (excitation/emission) was recorded for cell number quantification. The fluorescence emission recorded was normalized by dividing each point by the cell number (data are expressed in terms of relative fluorescence units, RFU).
Tumor ablation and fluorescent imaging in vivo
After two weeks of tumor cells growth after implantation, when tumors reached approximately 1 cm in diameter, a total of ten mice were randomly divided into two groups (n = 5 for each group). Each mouse was injected intratumorally with 10 nmol of the titled fluorescent probe dissolved in 100 μL saline under general anesthesia with 2.5% isoflurane. Images were captured pre- and post-injection immediately under a Xenogen IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA, USA). The imaging parameters were as follows: excitation filter, 490 nm; emission filter, 665 nm; exposure time, 5 s; binning, small; field of view, 22.2; f/stop, 2; open filter. Images were analyzed using Living Image 4.5 software (PerkinElmer, Waltham, MA, USA). The signal intensity was displayed as radiant efficiency ([photons/s/cm2/sr]/[μW/cm2]). To determine tumor contrast, the radiant efficiency of the tumor area at the right flank of the animal (T) and normal tissue at the left flank (N) were calculated by the region of interest (ROI) function in the Living Image software. Dividing T by N yielded the contrast between the tumor and normal tissue.
After imaging, all mice underwent ablation immediately by using an RFA generator (RITA Medical Systems, Inc, Silicon Valley, CA, USA). To form the RF circuit, the mouse was placed on an electricity-conducting pad. An 18-gauge electrode needle was inserted into the center of the tumor. RFA was performed with a power output of 15 W for 1.5 min, and the temperature of the needle tips reached 60 °C for the first group and 90 °C for the other group. After ablation, the mice were imaged again. The imaging procedure and image processing method were the same as described above. Finally, all tumors were excised and stained with H&E.
Statistical analysis
Statistical analysis was carried out with SPSS software, version 23. All data are given as the mean ± standard deviation (SD) of n independent measurements. Fluorescence intensity at different temperatures of thermal treatment in vitro and fluorescence intensity pre- and post-RFA in vivo were compared using paired-samples Student’s t test. A p value <.05 was considered statistically significant.
Results
Design and synthesis of RGD-Cy5-R-PE
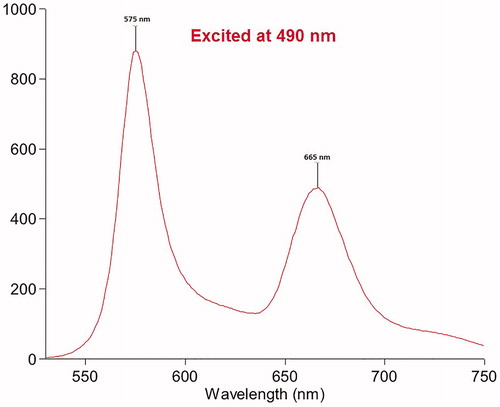
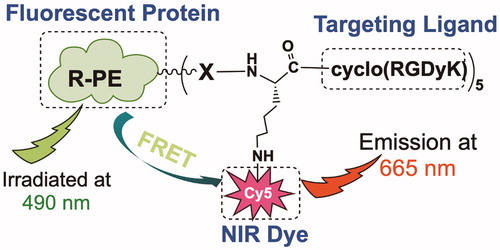
As shown in Scheme 1, the designed system comprises three major components: (1) a fluorescent protein that emits strong red fluorescence in the visible region and denatures upon heating, subsequently losing fluorescent properties; (2) an NIR fluorescent dye that absorbs the fluorescence emitted by the conjugated fluorescent protein, subsequently emitting NIR fluorescence (FRET); and (3) a tumor receptor-targeting ligand that promotes tumor-specific delivery of the designed fluorescence imaging probe. In our proof-of-principle study, we prepared an RGD-Cy5-R-PE (R-phycoerythrin) conjugate for RFA guidance; herein, RGD is a peptide ligand targeting integrin αvβ3 (an extensively investigated tumor biomarker); Cy5 is a widely used NIR fluorescent dye with strong fluorescence emission at 665 nm and broad excitation at 560–660 nm, while R-phycoerythrin is a thermally sensitive protein with maximal excitation at 490/546/566 nm and maximal emission at 576 nm that can be absorbed by Cy5 via the FRET mechanism. As shown in , when the RGD-Cy5-R-PE conjugate was excited at 490 nm, strong fluorescence emission at 665 nm was recorded, indicating highly efficient FRET between R-PE and Cy5.
Thermally sensitive properties of RGD-Cy5-R-PE
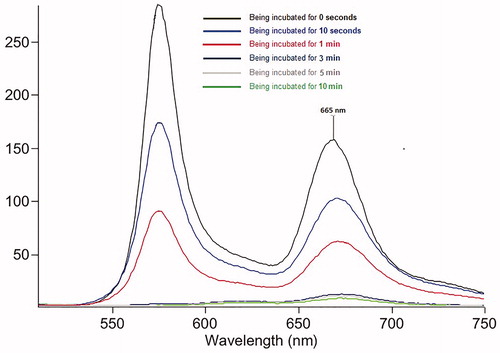
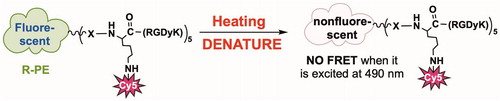
Then, we investigated the RGD-Cy5-R-PE conjugate response to heat in phosphate-buffered saline (PBS). As shown in Scheme 2, once heated to 80 °C, R-PE (the fluorescent protein) denatured very fast and immediately lost fluorescence emission at 576 nm, which resulted in a rapid decrease in fluorescence intensity at 665 nm. After being incubated for 10 s and 1 min at 80 °C, we observed an approximate 30% and 65% intensity loss, respectively. After the incubation time was extended to 3 min, a 95% fluorescence emission decrease was observed at 665 nm (). These results validated the feasibility of using the prepared RGD-Cy5-R-PE to assess the ablation margin.
Thermal treatment and fluorescent imaging in vitro
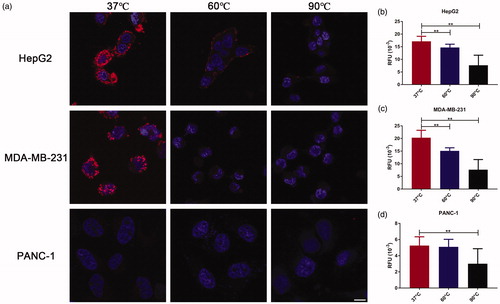
Then, confocal fluorescence microscopy was used to assess its in vitro performance regarding tumor-targeting specificity and fluorescence sensitivity toward heating. Herein, in vitro uptake of the prepared RGD-Cy5-R-PE was measured in three fixed human cancer cell lines, HepG2, MDA-MB-231, and PANC-1, which express the targeted integrin αvβ3 receptor at different levels, as assessed by flow cytometry (the results are shown in SI Figure 3). The initial confocal fluorescence imaging was performed at 37 °C. Then, the fixed cells were incubated at an elevated temperature and reimaged by confocal fluorescence microscopy to monitor in vitro heat-induced denaturation of the cell-bound RGD-Cy5-R-PE. The results are summarized in with 490 nm excitation at 37 °C, we could clearly observe strong fluorescence emission at 665 nm on HepG2 and MDA-MB-231 cells that overexpress high levels of integrin αvβ3, where the uptake was mainly localized on the cell membrane, while PANC-1 cells, with low/moderate αvβ3 expression, exhibited only very weak fluorescence emission at 665 nm. These results indicated that the cellular uptake of the RGD-Cy5-R-PE conjugate was integrin αvβ3 receptor-specific, and such uptake could be visualized via FRET if excited at 490 nm. Upon further investigation at an elevated temperature, we found that the RGD-Cy5-R-PE bound to the cell membrane lost fluorescence emission quickly in response to heating, indicating successful fluorescence turn-off through the thermal denaturation of R-PE.
Quantitative fluorescence intensity of RGD-Cy5-R-PE in vitro
To quantify the fluorescence intensity changes in response to heating, a fluorescence plate reader was applied to measure fluorescence intensities after fixed cells were incubated at different temperatures. As shown in , at 37 °C, a remarkably higher fluorescent signal was observed in HepG2 and MDA-MB-231 cells than in PANC-1 cells. After incubation at 60 and 90 °C, the fluorescent signal decreased significantly in all three cell lines: HepG2, 17.2 ± 2.0 at 37 °C, 14.7 ± 1.3 at 60 °C (p < 0.01), and 7.7 ± 4.0 at 90 °C (p < 0.01); MDA-MB-231, 20.3 ± 2.9 at 37 °C, 15.1 ± 1.2 at 60 °C (p < 0.01), and 7.7 ± 4.0 at 90 °C (p < 0.01); PANC-1 cells, 5.3 ± 1.1 at 37 °C, 5.1 ± 0.9 at 60 °C, and 3.0 ± 1.9 at 90 °C (p < 0.01). The decrease in fluorescence emission (at 665 nm) was correlated with the expression of integrin αvβ3 receptor. These in vitro results showed excellent thermal sensitivity of the prepared RGD-Cy5-R-PE probe.
Tumor ablation and optical imaging in vivo
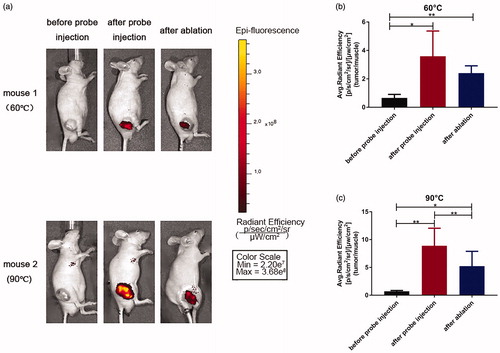
Encouraged by the promising results obtained from the above in vitro evaluation, we further prepared an RGD-Cy5-R-PE probe to investigate in vivo in the HepG2 human hepatic cancer xenograft mouse model. All the tumors exhibited a strong FRET fluorescence signal with high contrast () immediately after direct injection of the probe into the tumor. After the start of RFA, as the local temperature of the tumor gradually increased, the fluorescence intensity in the tumor gradually decreased. When the temperature reached 60 °C, the fluorescence intensity of the same part of the local area decreased by a 33% percentage compared with that before the ablation (3.6 ± 1.8 vs 2.4 ± 0.5, p = 0.27). When the temperature rose further, the local fluorescence intensity decreased more significantly, with an approximate 2-fold reduction in signal contrast (8.9 ± 3.2 vs 5.2 ± 2.7, p < 0.01) (). These results are consistent with those obtained from the in vitro studies and further confirmed the thermal sensitivity of our designed probe.
Figure 4. Fluorescence imaging in xenograft tumor mouse models before probe injection, after probe injection and after ablation. (a) Mice images. All images are displayed at the same scale. (b,c) Tumor contrast enhancement (T/N signal ratio) profiles. T represents the fluorescence intensity of tumor at the right flank, and N represents the fluorescence intensity of normal tissue at the left flank. (*p < 0.05, **p < 0.01).
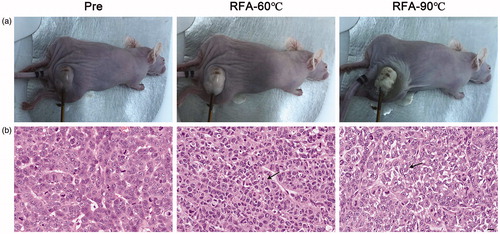
Furthermore, the pathological changes of thermal ablation on xenograft tumors were also investigated. The macro images of tumors pre- and post-RFA are shown in . The whole tumor became pale, while the adjacent tissue remained unchanged under ablation at 60 °C. When the temperature reached 90 °C, the whole tumor became paler and shriveled, while the surrounding tissues were also damaged. Coagulation necrosis with nuclear fragmentation and that the normal morphology of the tumor cells could not be distinguished was observed in both tumors that were ablated at 60 °C and 90 °C, as shown in the hematoxylin and eosin (H&E) stains in .
Figure 5. Representative histological images of tumors pre and post RFA. (a) Macro images. (b) H&E staining images. Arrows showing representative areas of coagulation necrosis with nuclear fragmentation and that the normal morphology of the tumor cells could not be distinguished. Scale bars = 50 μM.
Discussion
Optical imaging techniques, especially fluorescence imaging, exhibit unique advantages for detecting tumors and guiding surgeries [Citation18,Citation19], which can delineate tumor margins in real-time and non-invasively, but few studies had focused on ablation treatment guiding with optical imaging [Citation20]. To our knowledge, this is the first study to investigate the potential value of fluorescence imaging in the guidance and assessment of radiofrequency ablation in tumors using an integrin αvβ3-targeted thermally sensitive fluorescent probe.
Integrin αvβ3 is frequently used as a targeting molecule in fluorescence imaging, which overexpressed in a variety of tumors, such as breast cancer, pancreatic cancer, and ovarian cancer [Citation21,Citation22]. In this study, using three human cancer cell lines, which express the targeting integrin αvβ3 receptor at different levels, we demonstrated that this fluorescent probe bound to αvβ3 specifically in vitro. It is more difficult to test the binding specificity in xenograft tumor mouse models in vivo. This is likely due to the expression of αvβ3 in these cell lines at a medium level. For another, the monomeric cRGD peptide (cRGDyk) displays moderate integrin binding affinity, which c(RGDyk) may impair tumor uptake [Citation23]. While the dimeric and tetrameric cRGD peptides or analogs have provided a viable approach to improve the binding affinity and in vivo imaging [Citation24,Citation25]. Moreover, the excitation wavelength of 490 nm is absorbed by hemoglobin and scattered in tissue, which also impaired in vivo performance. NIR light (650–900 nm) is currently adopted in vivo imaging, owing to the low tissue absorption, scattering and autofluorescence, which can penetrate deeper biological tissues [Citation26,Citation27]. NIR fluorescent probe is promising in tumor diagnosis and intraoperative guidance.
Furthermore, our study demonstrated that fluorescence imaging using this thermally sensitive fluorescent probe can be used to assess response to thermal treatment of tumors effectively and quantitatively. The fluorescence signal progressively diminished as the temperature of thermal treatment increasing in vitro and in vivo. Especially when the temperature reached 90 °C (sufficient ablation), the fluorescence signal diminished significantly. Macro images and H&E staining images confirmed the effectiveness of RFA. All the above results demonstrated that RFA can immediately change fluorescence signal and that such change correlates with the effectiveness of ablation. These provide support on the potential of this thermally sensitive fluorescent probe as a sensitive and useful fluorescence imaging agent for guiding and assessing ablation therapy.
We acknowledge that further improvement is required for our study. On the one hand, multiple targeted fluorescence imaging probes, including integrin αvβ3-targeted, have been evaluated for tumor detecting and surgery guiding, however, all these probes are limited by the lack of broad cancer detection capability across diverse cancer phenotypes, as well as the expression heterogeneity of targeting molecule inter- and intra-tumors over normal tissues, resulting in decreased accuracy in delineating tumor margins. Thus, a cancer-specific biomarker is still needed to be explored. On the other hand, our thermally sensitive probe, together with previously reported ones, preliminary assess the temperature changes, could not quantify the temperature in the ablation area [Citation28–30]. Considering the temperature dependent characteristic of RFA and nonuniformly distributed temperature in the ablation area [Citation3], it is critical to precisely assess the ablation temperature covering the tumor zone. Thereby, it is possible to determine the safety ablation margins, improving the effectiveness of thermal therapy.
Conclusions
In conclusion, we report the design, preparation, and evaluation of the first thermally sensitive FRET system for tumor radiofrequency ablation guidance. The prepared RGD-Cy5-R-PE probe showed highly efficient FRET and a quick response to heat in vitro and demonstrated decent sensitivity to in vivo radiofrequency ablation. More importantly, the extent of its decrease in fluorescence correlated well with the efficacy of ablation in the tumor xenograft mouse model. Therefore, the reported thermally sensitive FRET system (such as RGD-Cy5-R-PE) may offer a new paradigm for the imaging guidance of RFA.
Acknowledgements
This work was supported by Shanghai Pujiang Program [grant numbers 16PJ1406200] and Shanghai Municipal Key Clinical Specialty [grant numbers shslczdzk06002].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266(2):407–430.
- Venkatesan AM, Wood BJ, Gervais DA. Percutaneous ablation in the kidney. Radiology. 2011;261(2):375–391.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97(5):1253–1262.
- Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23(1):190–197.
- Poch FG, Rieder C, Ballhausen H, et al. The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hypertherm. 2016;32(7):749–756.
- Ahmed M, Solbiati L, Brace CL, et al.; For the International Working Group on Image-guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, and the Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Zhang N, Ma D, Wang L, et al. Insufficient radiofrequency ablation treated hepatocellular carcinoma cells promote metastasis by up-regulation ITGB3. J Cancer. 2017;8(18):3742–3754.
- Zhao Z, Wu J, Liu X, et al. Insufficient radiofrequency ablation promotes proliferation of residual hepatocellular carcinoma via autophagy. Cancer Lett. 2018;421:73–81.
- Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527.
- Gao D, Gao L, Zhang C, et al. A near-infrared phthalocyanine dye-labeled agent for integrin alphavbeta6-targeted theranostics of pancreatic cancer. Biomaterials. 2015;53:229–238.
- Guo X, Ling X, Du F, et al. Molecular imaging of pancreatic duct adenocarcinoma using a type 2 cannabinoid receptor-targeted near-infrared fluorescent probe. Transl Oncol. 2018;11(5):1065–1073.
- Huynh AS, Chung WJ, Cho HI, et al. Novel toll-like receptor 2 ligands for targeted pancreatic cancer imaging and immunotherapy. J Med Chem. 2012;55(22):9751–9762.
- van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17(10):1315–1319.
- Crane LM, Themelis G, Arts HJ, et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: first clinical results. Gynecol Oncol. 2011;120(2):291–295.
- Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med: Soc Nucl Med. 2011;52(11):1778–1785.
- Chen X, Park R, Shahinian AH, et al. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31(1):11–19.
- Schols RM, Connell NJ, Stassen LP. Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg. 2015;39(5):1069–1079.
- Wang C, Wang Z, Zhao T, et al. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials. 2018;157:62–75.
- Au JT, Gonzalez L, Chen CH, et al. Bioluminescence imaging serves as a dynamic marker for guiding and assessing thermal treatment of cancer in a preclinical model. Ann Surg Oncol. 2012;19(9):3116–3122.
- Trajkovic-Arsic M, Mohajerani P, Sarantopoulos A, et al. Multimodal molecular imaging of integrin alphavbeta3 for in vivo detection of pancreatic cancer. J Nucl Med: Soc Nucl Med. 2014;55(3):446–451.
- Taherian A, Li X, Liu Y, et al. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11(1):293.
- Liu Z, Yu L, Wang X, et al. Targeted RGD peptide based probe for cancer optical imaging. CPPS. 2016;17:570–581.
- Cheng Z, Wu Y, Xiong Z, et al. Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice. Bioconjug Chem. 2005;16(6):1433–1441.
- Schottelius M, Laufer B, Kessler H, et al. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res. 2009;42(7):969–980.
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(4):316–317.
- Yuan L, Lin W, Zheng K, et al. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev. 2013;42(2):622–661.
- Deng X, Li K, Cai X, et al. A hollow-structured CuS@Cu2 S@Au nanohybrid: synergistically enhanced photothermal efficiency and photoswitchable targeting effect for cancer theranostics. Adv Mater. 2017;29(36):1701266.
- Sun M, Peng D, Hao H, et al. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl Mater Interfaces. 2017;9:10453–10460.
- Yan F, Wang S, Yang W, et al. Tumor-penetrating peptide-integrated thermally sensitive liposomal doxorubicin enhances efficacy of radiofrequency ablation in liver tumors. Radiology. 2017;285(2):462–471.