Abstract
Purpose
The present systematic review and meta-analysis was designed to evaluate the efficacy and safety of microwave ablation (MWA) treatment for secondary hyperparathyroidism (SHPT).
Materials and methods
The study authors systematically searched the Web of Science, Cochrane Library, PubMed, Embase and Ovid databases for studies published in English prior to 7October 2019. All studies included in the meta-analysis measured levels of parathyroid hormone (PTH), calcium and phosphorus, and included data related to complications following MWA treatment for SHPT.
Results
The meta-analysis ultimately included 233 patients from two retrospective cohort studies and six retrospective self-control studies. Compared to PTH level measurements obtained after MWA, measurements obtained at one day (weighted mean differences (WMD): 890.314, 95% confidence interval (CI): 767.121–1013.506, p < 0.01) , one week (WMD: 860.298, 95% CI: 759.401–961.194, p < 0.01), one month (WMD: 800.846, 95% CI: 687.709–913.983, p < 0.01) and six months (WMD: 860.847, 95% CI: 745.214–976.480, p < 0.01) after MWA were significantly lower. Calcium and phosphorus levels at one day and one week after MWA were also significantly lower than those measured before MWA. After MWA, the incidence of nerve injury was 1.2% (3/233; effect size (ES): 0.022, 95% CI: −0.003–0.048, p < 0.01). After MWA, the incidence of hypocalcemia was 15.8% (37/233; ES: 0.449, 95% CI: 0.341–0.556, p < 0.01).
Conclusion
The preliminary results of this meta-analysis indicate that MWA may be effective and safe in treating patients with SHPT, and that future prospective research and randomized controlled trials (RCT) are necessary.
Introduction
Chronic kidney disease (CKD) has increased in prevalence over the past decade [Citation1,Citation2]. CKD has become an important public-health problem worldwide [Citation3]. One common complication of CKD is secondary hyperparathyroidism (SHPT), which starts during the early stage of renal insufficiency as an adaptive response to maintain mineral homeostasis [Citation4]. The features of SHPT include chronic elevations in parathyroid hormone (PTH) levels and parathyroid hyperplasia. The presence of SHPT in CKD patients can increase morbidity and mortality, especially cardiovascular-related mortality [Citation5]. So, a proper treatment method is particularly important in patients with SHPT.
At present, treatment for SHPT mainly comprises a dietary phosphate restriction [Citation6,Citation7], the use of medicines [Citation8,Citation9] and parathyroidectomy [Citation10,Citation11]. However, medical therapy is often not successful in achieving adequate control of SHPT [Citation12]. Oral medications have limitations as well as side effects [Citation13,Citation14]. Patients may suffer from perioperative injury, hypoparathyroidism, or the recurrence of hyperparathyroidism after parathyroidectomy [Citation15]. Moreover, some patients are not suitable candidates for surgery. Microwave ablation (MWA), as a newly developed method for treating tumors, has developed in recent years [Citation16–18]. Compared to classical surgical treatment, MWA has many advantages, such as being minimally invasive, relieving pain, reducing costs, shortening the duration of hospitalization and decreasing recovery time. Thus, MWA may become a valuable alternative treatment to treat SHPT in patients with enlarged parathyroid glands on ultrasonography.
However, studies have reported inconsistent conclusions regarding the efficacy and safety of MWA. Numerous studies have been conducted to evaluate the efficacy of MWA for the treatment of SHPT and associated complications. This systematic review and meta-analysis were designed to evaluate the efficacy and safety of MWA in the treatment of SHPT.
Materials and methods
Database search
The Web of Science, Ovid, Embase, PubMed and Cochrane Library databases were searched systematically for studies in English published before or on 7October 2019. The search terms used were: ‘secondary hyperparathyroidism*’ OR ‘SHPT’ OR ‘hyperplastic parathyroid gland*’, AND ‘microwave ablation’ OR ‘MWA’ OR ‘MW Ablation’ OR ‘thermal ablation’. The study authors manually checked the articles cited in all studies included in the meta-analysis to identify other related articles.
Study selection
First, the study authors screened articles based on their titles and abstracts. The full text of an article was reviewed when a title or abstract was considered ambiguous and when an article was considered as suitable for inclusion in the meta-analysis. The criteria for inclusion of a study in the meta-analysis were as follows: (1) retrospective study; (2) outcomes that included the following indicators: PTH, calcium and phosphorus; (4) outcomes included data related to complications, such as hypocalcemia, hoarseness, hematoma, pain, bucking and skin burnand (5) follow-up for ≥3 months. The following forms of publications were excluded: (1) case reports, editorials, reviews, conference abstracts and prospective studies; (2) primary hyperparathyroidism; (3) studies based on animal experiments and (4) studies based on pediatric populations. The studies included were selected independently by two investigators. Any disagreement was resolved by consensus among the study authors.
Assessment of quality and extraction of data
The Newcastle-Ottawa Scale (NOS) was used to evaluate study quality. The NOS is an effective tool for evaluating non-randomized studies based on three criteria: patient selection (four points), study group comparability (two points) and outcome evaluation (three points). Each study considered to be a suitable candidate for inclusion in the meta-analysis was awarded a ‘star’. A high-quality study was defined as a study with five or more stars in total.
Two reviewers independently extracted data from relevant studies, and the results were compared to achieve consensus. The extracted data included: (1) country and publication year; (2) number of patients; (3) age; (4) number of target ablation lesions and lesion size; (5) levels of PTH, serum calcium and phosphorus concentrations before versus after ablation; (6) the duration of follow-upand (7) number of patients with complications.
Statistical analysis
All data were analyzed using STATA software version 14.0 (StataCorp, College Station, TX), with p < 0.05 considered statistically significant. In the meta-analysis, weighted mean differences (WMD) with 95% confidence intervals (CI) were evaluated for continuous variables. Effect size (ES) of each single proportion with 95% CIs were evaluated for categorical variables [Citation19,Citation20]. Heterogeneity among studies was assessed by calculating Q statistics or I2 index. Significant heterogeneity was recorded when I2 > 50% and p < 0.10. Non-significantly heterogeneous data were analyzed with a fixed-effect model. Heterogeneity data were analyzed with a random-effect model [Citation21,Citation22]. Publication bias was assessed with Egger’s test [Citation23,Citation24]. Sensitivity analysis was performed to identify the influence of an individual study on pooled estimates by removing one study at a time [Citation22,Citation25].
Results
Study search and characteristics
presents the literature-screening process and final study results. Using the search strategy described above, 77 records were identified from the electronic database; 38 records were discarded because of duplication. After screening the titles and abstracts, 19 studies were excluded. After full text review, 11 studies were excluded. A total of eight studies were included in the final analysis. The baseline characteristics of the patients included in these studies are listed in . The studies covered a period ≥8 years, from 2009 to 2017. Two were retrospective cohort studies [Citation26,Citation27], and six were retrospective self-control studies [Citation28–33]. The mean follow-up period ranged from six to 23 months, and the sample size ranged from 9–56 patients. These eight studies included a total of 233 patients. The NOS assessments are listed in . All eight studies had NOS scores > 5 stars, which is considered to indicate high quality.
Table 1. Baseline characteristics of patients included in the meta-analysis.
Table 2. Quality assessment for the studies included in the meta-analysis.
Meta-analysis results
The change of PTH
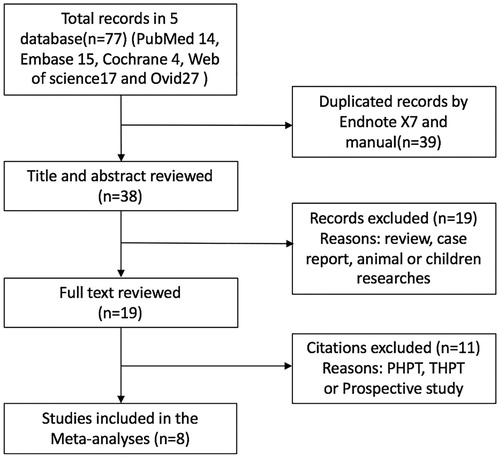
All studies included in the meta-analysis reported PTH measurements obtained before MWA. Five articles reported PTH measurements obtained one day after MWA [Citation27,Citation28,Citation30–32]. The heterogeneity among studies was not significant (p = 0.552, I2 = 0.000%), so the fixed-effects model was used for the analysis. The PTH levels at one day after MWA were significantly lower than those measured before MWA (WMD: 890.314, 95% CI: 767.121–1013.506, p < 0.01; ). Four articles reported PTH levels at one week [Citation26,Citation27,Citation29,Citation30] and one month [Citation26,Citation27,Citation29,Citation31] after MWA, respectively. The heterogeneity among studies was not significant (p = 0.879, I2 = 0.0%; p = 0.365, I2 = 5.6%), so the fixed-effects model was used for the analysis. The PTH levels at one week and one month after MWA were significantly lower than those before MWA (WMD: 860.298, 95% CI: 759.401–961.194, p < 0.01; WMD: 800.846, 95% CI: 687.709–913.983, p < 0.01; ). Two articles reported PTH measurements obtained 6 months after MWA [Citation29,Citation33]. The heterogeneity among studies was not significant (p = 0.287, I2 = 11.9%), so the fixed-effects model was used for the analysis. The PTH levels measured at the sixth month after MWA were significantly lower than those measured before MWA (WMD: 860.847, 95% CI: 745.214–976.480, p < 0.01; ).
The change of calcium
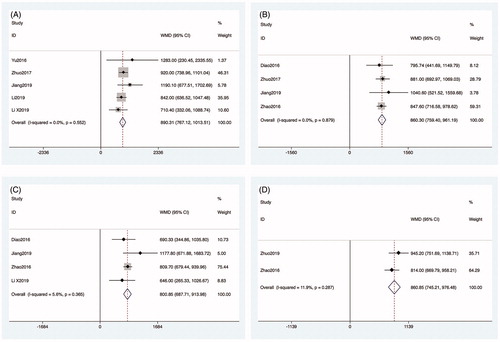
All studies included in the meta-analysis reported calcium measurements obtained before MWA. Five articles reported calcium levels as measured one day after MWA [Citation27,Citation28,Citation30–32]. The heterogeneity among studies was not significant (p = 0.914, I2 = 0.0%), so the fixed-effects model was used for the analysis. The calcium levels at one day after MWA were significantly lower than those measured before MWA (WMD: 0.370, 95% CI: 0.302–0.438, p < 0.01; ). Four articles reported the calcium levels at one week [Citation26,Citation27,Citation29,Citation30] and one month [Citation26,Citation27,Citation29,Citation31] after MWA, respectively. The heterogeneity among studies was significant, so the random-effects model was used for the analysis (p < 0.01, I2 = 92.7%; p < 0.01, I2 = 92.5%). The calcium measurements obtained at one week and one month after MWA were significantly lower than measurements obtained before MWA (WMD: 0.227, 95% CI: 0.024–0.429, p = 0.028; WMD: 0.294, 95% CI: 0.043–0.546, p = 0.022; ). Two articles reported the calcium levels at six months after MWA [Citation29,Citation33]. The heterogeneity among studies was significant (p < 0.01, I2 = 94.4%), so the random-effects model was used for the analysis. However, the calcium measurements obtained six months after MWA were not significantly different from those obtained before MWA (WMD: 0.181, 95% CI: −0.172–0.533, p = 0.316; ).
The change of phosphorus
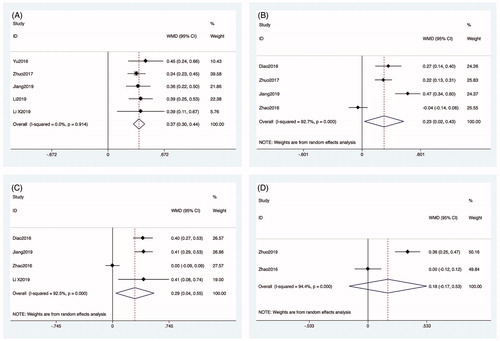
Data about phosphorus measurements obtained before MWA were reported in all articles. Five articles reported phosphorus measurements obtained one day after MWA [Citation27,Citation28,Citation30–32]. The heterogeneity among studies was not significant (p = 0.242, I2 = 26.9%), so the fixed-effects model was used for the analysis. The phosphorus measurements obtained one day after MWA were significantly lower than those obtained before MWA (WMD: 0.289, 95% CI: 0.163–0.415, p < 0.01) (). Four articles reported phosphorus measurements obtained at one week [Citation26,Citation27,Citation29,Citation30] and one month [Citation26,Citation27,Citation29,Citation31], respectively, after MWA. The heterogeneity among studies was significant, so the random-effects model was used for the analysis (p < 0.01, I2 = 86.5%; p < 0.01, I2 = 85.3%). Phosphorus measurements obtained at one week after MWA were significantly lower than those obtained before MWA (WMD: 0.255, 95% CI: 0.152–0.358, p < 0.01; ). However, the phosphorus measurements obtained at one month after MWA were not significantly different from those obtained before MWA (WMD: 0.307, 95% CI: −0.066–0.679, p = 0.106; ). Two articles reported phosphorus measurements obtained 6 months after MWA [Citation29,Citation33]. The heterogeneity among the studies was not significant (p = 0.17, I2 = 44.6%), so the fixed-effects model was used for the analysis. However, the phosphorus measurements obtained at six months after MWA were not significantly different from those obtained before MWA (WMD: 0.218, 95% CI: −0.084–0.520, p = 0.157; ).
Complications
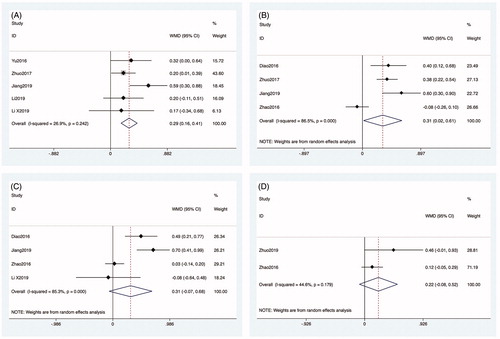
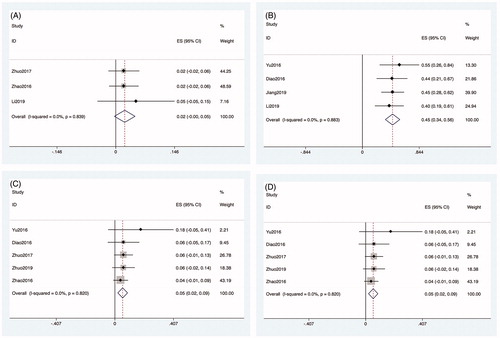
Data about the incidence of complications after MWA were reported in all articles. Data about the incidence of nerve injury were reported in three articles. The heterogeneity among studies was not significant (p = 0.839, I2 = 0.0%), so the fixed-effects model was used for the analysis. The incidence of nerve injury after MWA was 1.2% (3/233; ES: 0.022, 95% CI: −0.003–0.048, p < 0.01; ). The complications of nerve injury included one case of injury to the vagus nerve and two cases of injury to the recurrent laryngeal nerve. Four studies reported data about the incidence of hypocalcemia. The heterogeneity among studies was not significant (p = 0.883, I2 = 0.0%), so the fixed-effects model was used for the analysis. The incidence of hypocalcemia after MWA was 15.8% (37/233; ES: 0.449, 95% CI: 0.341–0.556, p < 0.01; ). Five studies reported data about the incidence of temporary hoarseness. The heterogeneity among studies was not significant (p = 0.820, I2 = 0.0%), so the fixed-effects model was used for the analysis. The incidence of temporary hoarseness after MWA was 4.2% (10/233; ES: 0.054, 95% CI: 0.020–0.088, p < 0.01; ). Four studies reported data about the incidence of hematoma. The heterogeneity among the studies was not significant (p = 0.682, I2 = 0.0%), so the fixed-effects model was used for the analysis. The incidence of hematoma after MWA was 1.7% (4/233; ES: 0.029, 95% CI: −0.005–0.064, p = 0.095; ).
Publication bias and sensitivity analyses
Publication bias was assessed with Egger’s test. No obvious asymmetry or p > 0.05 was detected, which suggested there was no evidence of publication bias. A sensitivity analysis was used to assess the reliability of the results. When one study was deleted at a time, no such deletion had any significant impact on the results.
Discussion
SHPT is a common complication in CKD [Citation4]. Currently, various treatment options are available, including vitamin D receptor activators, cinacalcet hydrochloride, parathyroidectomy and ablation techniques [Citation9,Citation11,Citation30]. The Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline recommends that parathyroidectomy should be performed for SHPT patients in whom drug treatment has failed [Citation34]. However, some patients are not suitable candidates for surgery. In recent years, thermal ablation (e.g., MWA, radiofrequency, laser and high-intensity focused ultrasound) has emerged as a new method for the treatment of neck tumors (e.g., thyroid nodules, neck metastatic lymph nodes and hyperparathyroidism) [Citation35–37]. Compared with other thermal ablation techniques, MWA may translate into larger and more consistent ablation zones in less time. At present, no definite evidence has proven the efficacy and safety of MWA in SHPT. A comprehensive search for studies to appraise the efficacy and safety of MWA in SHPT was carried out for this systematic review and meta-analysis.
This meta-analysis shows that MWA may be an effective treatment for patients with SHPT. PTH levels after MWA represent important indicators related to clinical efficacy. Similar to previous studies about parathyroidectomy [Citation38,Citation39], our review of measurements obtained during follow-up periods of ≥ 3 months in duration showed that PTH levels decreased significantly after MWA. This result demonstrates that MWA effectively destroyed the hyperplastic parathyroid glands revealed by ultrasound. The results also indicate that calcium measurements obtained at one day, one week and one month after MWA were all significantly lower than those obtained before MWA. The phosphorus levels at one day and one week after MWA were also significantly lower than those before MWA. However, phosphorus measurements obtained at one and six months, respectively, after MWA were not significantly different than those obtained before MWA. This result is consistent with previously published results [Citation39] indicating that some patients had elevated serum phosphorus six months after surgery. Montenegro et al. [Citation40] similarly reported that parathyroidectomy may be less effective in decreasing serum phosphorus levels.
This meta-analysis shows that MWA is a safe treatment for patients with SHPT. The incidence of complications was below the threshold considered to indicate a lack of clinical safety. The results of this meta-analysis showed that the incidence of nerve injury was only 1.2%. Among the 233 patients enrolled in eight studies, only three patients with SHPT had serious complications, including one case of injury to the vagus nerve and two cases of injury to the recurrent laryngeal nerve. Hypocalcemia is a common complication after surgery or MWA. Previous studies reported that the incidence of hypocalcemia after parathyroidectomy ranged from 24.3 to 62.1% [Citation39,Citation41]. However, the results of our meta-analysis showed that the incidence of hypocalcemia after MWA was 15.8%. So, MWA may reduce the risk of hypocalcemia. In addition, our meta-analysis revealed that MWA did not reduce the risk of hoarseness; the incidence of temporary hoarseness after MWA was 4.2%. Tominaga et al. [Citation42] reported that the incidence of hoarseness after PTX was 2.9%. There are two main reasons for this result. First, analysis of the eight included studies showed that many patients were not eligible for surgical resection of a recurrence after primary surgery [Citation28,Citation31,Citation33]. These cases are more difficult to perform, so the incidence of complications may be higher. Second, lidocaine in normal saline solution (1:3, lidocaine concentration 0.5%) was injected in the area surrounding the hyperplastic gland to protect adjacent organs and nerves before MWA. Thus, although the incidence of hoarseness after MWA is high, this hoarseness is usually transitory.
This meta-analysis had some limitations. First, at present, there are few prospective studies on secondary hyperparathyroidism, so all the studies included in our analysis were retrospective, which inevitably leads to biases in patient selection. There is therefore a pressing need for well-designed randomized controlled trials. Second, the research on MWA for the treatment of SHPT is relatively new. The number of articles included in this study is small. Although the article set was highly heterogeneous, there was no obvious publication bias. Third, this meta-analysis included only single-center studies, so it is hoped that more centers can actively conduct research in this area in the future.
In conclusion, the preliminary results of the current meta-analysis indicate that MWA may be effective and safe in treating patients with SHPT, and that future prospective research and RCTs are necessary.
Acknowledgements
The authors thank all participants for their support in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bruck K, Jager KJ, Dounousi E, et al. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant. 2016;31(4):680.
- Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet. 2014;2(3):e174–81.
- Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957.
- Bellorin-Font E, Vasquez-Rios G, Martin KJ. Controversies in the management of secondary hyperparathyroidism in chronic kidney disease. Curr Osteoporos Rep. 2019;17(5):333–342.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305(11):1119–1127.
- Kahan S, Manson JE. Nutrition counseling in clinical practice: how clinicians can do better. JAMA. 2017;318(12):1101–1102.
- Bump M. Organic phosphorus versus inorganic phosphorus: empowering adult kidney patients with nutrition education. J Ren Nutr. 2016;26(5):e31–3.
- Rodriguez M, Rodriguez-Ortiz ME. Advances in pharmacotherapy for secondary hyperparathyroidism. Expert Opin Pharmacother. 2015;16(11):1703–1716.
- Rroji M, Spasovski G. Calcimimetics versus parathyroidectomy: what is preferable? Int Urol Nephrol. 2018;50(7):1271–1275.
- Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30(12):2027–2033.
- Eidman KE, Wetmore JB. The role of parathyroidectomy in the management of secondary hyperparathyroidism. Curr Opin Nephrol Hypertens. 2017;26(6):516–522.
- Kuczera P, Adamczak M, Więcek A. Safety and efficiency of treatment with cinacalcet of haemodialysed patients with chronic kidney disease and secondary hyperparathyroidism. Endokrynologia Polska. 2013;64(3):176–181.
- Toussaint ND, Damasiewicz MJ. Do the benefits of using calcitriol and other vitamin D receptor activators in patients with chronic kidney disease outweigh the harms? Nephrology. 2017;22(Suppl 2):51–56.
- Xu L, Wan X, Huang Z, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PloS One. 2013;8(4):e61387.
- Sun X, Zhang X, Lu Y, et al. Risk factors for severe hypocalcemia after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Sci Rep. 2018; 8(1):7743.
- Vogl TJ, Nour-Eldin NA, Albrecht MH, et al. Thermal ablation of lung tumors: focus on microwave ablation. Rofo. 2017;189(9):828–843.
- Vogl TJ, Nour-Eldin NA, Hammerstingl RM, et al. Microwave ablation (mwa): basics, technique and results in primary and metastatic liver neoplasms. Rofo. 2017; 89(11):1055–1066.
- Dou JP, Liang P, Yu J. Microwave ablation for liver tumors. Abdom Radiol. 2016;41(4):650–658.
- Miller JJ. The inverse of the freeman – Tukey double arcsine transformation. Am Stat. 1978;32(4):138–138.
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607–611.
- Borenstein MH, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester, West Sussex: John Wiley & Sons, Ltd; 2009.
- Sutton AA, Jones DR, Sheldon TA, et al. Methods for meta‐analysis in medical research. Chichester, West Sussex: John Wiley & Sons, Ltd;. 2000.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statist Med. 2006;25(20):3443–3457.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Diao ZL, Liu X, Qian LX, et al. Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthe. 2016;32(6):614–622.
- Jiang BH, Wang XY, Yao ZY, et al. Microwave ablation vs. parathyroidectomy for secondary hyperparathyroidism in maintenance hemodialysis patients. Hemodial Int. 2019;23(2):247–253.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperther. 2016;32(2):180–186.
- Zhao J, Qian L, Zu Y, et al. Efficacy of ablation therapy for secondary hyperparathyroidism by ultrasound guided percutaneous thermoablation. Ultrasound Med Biol. 2016; 42(5):1058–1065.
- Zhuo L, Peng L-L, Zhang Y-M, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282(2):576–584.
- Li X, An C, Yu MA, et al. US-guided microwave ablation for secondary hyperparathyroidism in patients after renal transplantation: a pilot study. Int J Hyperther. 2019;36(1):321–327.
- Li X, Wei Y, Shao HZ, et al. Efficacy and safety of microwave ablation for ectopic secondary hyperparathyroidism: a feasibility study. Int J Hyperther. 2019;36(1):646–653.
- Zhuo L, Zhang L, Peng L-L, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperther.. 2019;36(1):29–35.
- Isakova T, Nickolas TL, Denburg M, et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Lang BH, Woo YC, Wong C. High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology. 2017;284(3):897–906.
- Teng D, Ding L, Wang Y, et al. Safety and efficiency of ultrasound-guided low power microwave ablation in the treatment of cervical metastatic lymph node from papillary thyroid carcinoma: a mean of 32 months follow-up study. Endocrine. 2018;62(3):648–654.
- Wan J, Li W, Zhong Y. Parathyroidectomy decreases serum intact parathyroid hormone and calcium levels and prolongs overall survival in elderly hemodialysis patients with severe secondary hyperparathyroidism. J Clin Lab Anal. 2019;33(3):e22696.
- Zhang Y, Lu Y, Feng S, et al. Evaluation of laboratory parameters and symptoms after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Renal Failure. 2019;41(1):921–929.
- Montenegro J, Cornago I, Gallardo I, et al. Efficacy and safety of cinacalcet for the treatment of secondary hyperparathyroidism in patients with advanced chronic kidney disease before initiation of regular dialysis. Nephrology. 2012;17(1):26–31.
- Gong L, Tang W, Lu J, et al. Thermal ablation versus parathyroidectomy for secondary hyperparathyroidism: a meta-analysis. Int J Surg. 2019;70:13–18.
- Tominaga Y, Kakuta T, Yasunaga C, et al. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the Parathyroid Surgeons’ Society of Japan. Ther Apher Dial. 2016; 20(1):6–11.