Abstract
Purpose
The aim of this paper is to discuss the current evidence for Laser Interstitial Thermal Therapy (LITT) in the treatment of brain metastases, our current recommendations for patient selection and the future perspectives for this therapy. We have also touched upon the possible complications and role of systemic therapy coupled with LITT for the treatment of brain metastases
Material and Methods
Two authors carried out the literature search using two databases independently, including PubMed, and Web of Science. The review included prospective and retrospective studies using LITT to treat brain metastases.
Results
Twenty-two original articles were analyzed in this review, particularly clinical outcomes and complications. We have also provided our institutional experience in the use of LITT to treat brain metastases and addressed future perspectives for the use of this technology.
Conclusions
The current literature supports LITT as a safe and effective therapy for patients with brain metastases that have failed SRS. Larger studies are still required to better evaluate the use of systemic therapy in concomitance with LITT. New images modalities may enable optimized treatment and outcomes.
Introduction
Metastases are the most common intracranial tumor, occurring in approximately a quarter of patients with systemic malignancy [Citation1]. Historically, large tumors (>3–4cm), or with mass effect are surgically resected [Citation2]. Stereotactic radiosurgery (SRS) has increasingly been used for patients with smaller less surgically accessible lesions or in cases with oligo -metastases (between 2 and 4 lesions) [Citation3]. Advances in systemic therapies for a number of primary cancers have drastically improved survival. This phenomenon has led to a pattern of disease progression where the incidence of brain metastases is increasing, due to poor drug penetration in the central nervous system, while extra-cranial disease is controlled [Citation4]. Despite being a noninvasive, SRS is not devoid of complication. Local progression and radiation effects such as radiation necrosis (RN) can be observed in up to 26% and 24% respectively [Citation5,Citation6]. In patients with disease progression after SRS treatment options include resection or additional radiotherapy. For patients with RN, medical treatment with steroids and anti-angiogenic drugs comprise the first line therapy, which have a limited role. For patients with deep-seated lesions, poor candidates for open surgical resection and in case of RN, that have not responded to medical treatment, a minimally invasive cytoreductive therapy would be indicated.
Laser interstitial thermal therapy (LITT) is a minimally invasive technique that relies upon stereotactic guidance to precisely ablate intracranial targets. LITT can be coupled with magnetic resonance (MR) thermography allowing for accurate, real-time temperature feedback in the ablation radius throughout the procedure. Over the last two decades, a number of studies have explored the safety and efficacy of LITT for various neurosurgical disorders, including malignancy, epilepsy, and radiation necrosis following SRS [Citation7]. Since 2008, several retrospective cohort studies have emerged indicating safe delivery of LITT to various intracranial metastases with acceptable rates of local control [Citation8,Citation9]. In this review, we will discuss the current evidence for LITT in the treatment of brain metastases, our current recommendations for patient selection and the future perspectives for this therapy.
Effects of hyperthermia from laser interstitial thermal therapy
The use of interstitially directed laser radiation to destroy a localized lesion was first described by Bown [Citation10]. Photobiological effects of laser irradiation can be divided into three main categories: photochemical, photothermal and photomechanical [Citation11]. Photothermal effect is responsible for direct tissue destruction caused by LITT. It results from the transformation of absorbed light energy to heat, which in turn, leads to target tissue coagulation and destruction at certain temperatures. The extent of photothermal effects are governed by laser-tissue interaction and are dependent on intrinsic tissue properties such as distribution of light within tissues and their thermal properties, and extrinsic factors such as temperature and exposure time to a certain temperature [Citation11]. Distribution of light depends on wavelength and beam diameter. The Nd:YAG laser, operating at 1,064nm has been used due to its good light penetration [Citation12]. Once the absorbed light is converted to heat, the resultant thermal injury will be dependent on the tissue temperature, exposure time, and the interval between exposures. At temperatures of around 40–45 °C, irreversible cell damages occurs only after prolonged exposure (30–60 min), whereas at temperatures above 60 °C, rapid protein denaturation occurs almost immediately, leading to cytotoxicity and coagulative necrosis [Citation13]. There is an exponential relationship between temperature and exposure time. At temperatures above 60 °C the time required for irreversible tissue damage decreases substantially. This relationship between time and temperature can be used to calculate a thermal dose. By mathematically describing the change in temperature as function of time, it is possible to calculate the equivalent time at any chosen reference temperature [Citation14]. The temperature of 43 °C has been arbitrarily chosen to create an equivalent-minutes at this temperature. A computer program is then used to calculate the accumulated exposure providing real-time accumulated thermal dose with the input of temperature values during treatments.
Mitochondrial dysfunction and changes to cell membrane integrity are considered the main mechanism of direct hyperthermia-induced cell death. Indirect or delayed cellular damage also occurs after thermal ablation. Several mechanisms have been proposed as the cause of this delayed heat-induced injury, including apoptosis, vascular damage leading to ischemia, ischemia-reperfusion injury, cytokine release by invading granulocytes during tumor necrosis with lysosomal contents release, and inflammatory cascade with further immune response stimulation caused by release of lysosomal contents [Citation15].
Magnetic resonance-guided laser thermal therapy
A central problem in interstitial heating is the difficulty to predict the extent of ablation due to tissue characteristics and proximity to heat sinks, such as large vessels, ventricles and the sulcal interfaces as they contain cerebrospinal fluid in the brain [Citation16,Citation17]. The compatibility of LITT with Magnetic Resonance Imaging (MRI) techniques has allowed the visualization of energy deposition in near real-time. Based on calculated temperature values, the software uses the time and temperature history data from each voxel to evaluate tissue damage. Common population-based thermal dose models are derived from isothermal heating experiments [Citation18,Citation19]. The most prevalent dose models assume that the rate of tissue damage exhibits Arrhenius behavior [Citation19,Citation20]. This model uses the dependency of time and temperature to denature protein. Voxels that surpass the empirically determined tissue damage threshold are considered destroyed. Cumulative equivalent minutes at 43 °C (CEM43) [Citation18,Citation21] is a similar concept that translates all time-temperature histories to a single number representing thermal dose or tissue damage. There are different displays for presumed destroyed tissue on commercially available systems. In the Visualase System (Medtronic), an Arrhenius model is assumed and the ablation area is displayed in orange [Citation9]. In the NeuroBlate System (Monteris Medical, Minneapolis) the software uses the CEM43 model. Thermal damage thresholds (TDT) lines denote the thermal dose volumes [Citation22]. There are three TDT lines (‘yellow’, ‘blue’, and ‘white’) each defined by tissue exposition to a certain amount of time to the thermal equivalent of 43 °C. The yellow TDT line encircles the area of tissue that has been heated for at least 2 min, blue TDT for at least 10 min and the white TDT for at least 60 min to 43 °C or heated to a higher temperature for a shorter interval [Citation21]. is an example of the display in the Monteris system with thermography and a blue TDT line.
Figure 1. Display interface of the NeuroBlade System during ablation of deep-seated occipital brain metastases. Using this interface it is possible to assess the extent of ablation in three axial levels perpendicular to the laser probe (upper panels). On the bottom panels, it is possible to observe the sagittal and coronal planes, parallel to the probe. Note use of DTI-tractography (in white) to generate the optic radiations.
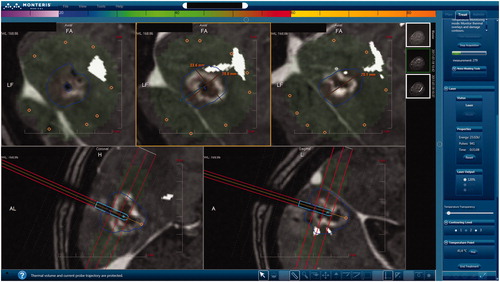
The use of LITT to treat brain metastases
In 2008, Carpentier et al. [Citation9] were the first to publish a study utilizing LITT coupled with magnetic resonance thermal imaging with both qualitative and quantitative visualization of laser heating, as well as thermal ablation zones, in patients with recurrent brain metastases after radiotherapy failure. Four patients with six lesions were treated and at 3 months follow-up, no recurrence was observed. In 2011, the final results of this pilot clinical trial was reported in a second study with fifteen metastatic tumors treated in 7 patients with a imaging follow-up of 30 months [Citation23]. The authors reported no tumor recurrence within thermal ablation zones and median survival of 19.8 months. No biopsy was performed before treatment; therefore, the authors were not able to differentiate between recurrent tumor and RN. No complications were reported.
From 2011 to 2015, several case reports or small patients’ series have reported the use of LITT to treat lesion recurrence after radiotherapy, mostly looking at feasibility and addressing safety concerns [Citation22,Citation24–31]. These studies included patients with biopsy proven radiation necrosis (RN), tumor recurrence and a mixture of both with no biopsy confirmation. Up to 40% complication rate was reported in these studies. Overall, these studies concluded that LITT was safe treatment option for patients with BM recurrence after radiation therapy.
During this period, Rao et al. [Citation32] in a study with 14 patients with 15 lesions treated with LITT with no biopsy during the procedure, reported a local recurrence rate of 24.2% with a median progression free survival of 9 months and a complication rate of 14%. Patel el al. [Citation33], in a large cohort of patients treated with LITT for different intracranial tumors, among which, 37 were BM, reported the differences between the first 20 patients and the following 82 patients. According to the authors, with experience, the laser catheters were better positioned and delivery of thermal therapy was optimized leading to reduced laser-on time. Nonetheless, the complication rate increased from 5% to 16% and according to the authors, this was due to the lower complexity of cases with their initial experience, however as they became more proficient in the technology they treated more complex cases, which in-turn increased the complication rates observed.
From 2016 and on, series with larger number of patients were published (). Most studies started focusing on not only feasibility and safety of the procedure, but also on the response to LITT and local control rates. Beechar et al. [Citation35] analyzed the volumetric responses over a period of one year in 36 patients with BM treated with LITT. They concluded that pretreatment tumor volume plays a significant role in determining response to LITT, with smaller tumor volumes responding better than larger volumes tumors. In another study, when describing their experience with LITT in a large single-institutional cohort, Kamath et al. [Citation36] reported that in 25 patients with biopsy proven BM recurrence, the median OS was 17.2 months and the median PFS could not been reached. They hypothesized that these patients tended to succumb to their systemic cancer rather than intracranial metastasis.
Table 1. Large published cohorts on the use of LITT treat brain metastases.
The importance of the extent of ablation for the PFS was demonstrated in a study with 23 patients that had previously failed radiation therapy and were treated with LITT () [Citation34]. The local recurrence rate at one year was 35% but only patients with incomplete ablated lesions recurred. The complication rate in this cohort was 21% and no biopsy was performed.
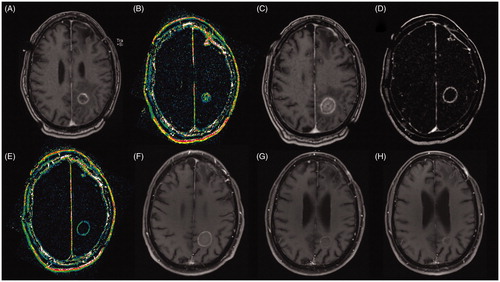
Figure 2. Images of 67-year-old male patient with a brain metastases recurrence after SRS from non-small cell lung cancer. (A) Pre-ablation axial T1C + MRI showing a deep seated ring enhancement in the left parietal lobe, compatible with recurrent tumor; (B) Pre-ablation Dynamic Contrast-Enhanced k-trans MR showing a high signal intensity inside the lesion suggesting high vascularization; (C) Post-ablation axial T1C + MRI showing the classic eggshell enhancement in the borders of the ablation; (D) Post-ablation MRI subtraction compatible with complete ablation; (E) Post-ablation axial Dynamic Contrast-Enhanced k-trans MR showing low signal intensity compatible with no viable tissue within the ablation zone; (F) One month post-ablation axial T1C + MRI showing eggshell enhancement and no signs of local recurrence; (G) Three month post-ablation axial T1C + MRI without local recurrence; (H) Six month post-ablation axial T1C + MRI with only remnant signs of ablation.
Dynamic contrast-enhanced MR imaging (DCE-MRI), a dynamic T1-weighted perfusion imaging technique, can be used to characterize the vasculature of tissue from which many quantifiable physiologic and non-physiologic variables can be extracted. The initial area under the time-to-signal intensity curve at 60 s (iAUC60) is one such quantitative parameter that has been evaluated to assess the presence of viable tissue after LITT [Citation43]. Higher iAUC60 values were associated with shorter time to local recurrence due to a unique profile of contrast enhancement in tumor tissue and associated vasculature ().
Figure 3. Images of 66-year-old female patient with metastatic breast cancer to the brain with a tumor recurrence after treatment with SRS. (A) Pre-ablation axial T1C + MRI showing a heterogeneous ring-enhancing lesion in the left frontoparietal region, compatible with recurrent tumor, note the large caliber vessel in the anterior third of the lesion (red arrow); (B) Pre-ablation sagittal T1C + MRI showing the large caliber vessel crossing the lesion; (C) ) Pre-ablation Dynamic Contrast-Enhanced k-trans MR showing a high signal intensity in the lesion’s margins compatible high vascularization; (D) Post-ablation axial T1C + MRI showing the classic eggshell enhancement in the margins of the ablation, notice in the anterior margin a solid enhancement compatible with unablated tissue (yellow arrow), it is possible that the large vessel worked as a heat sink preventing the complete ablation of the lesion; (E) Post-ablation MRI subtraction showing increased enhancement in the anterior margin of the ablation zone (yellow arrow); (F) Post-ablation axial Dynamic Contrast-Enhanced k-trans MR showing increased signal in the anterior margin of the ablation zone (yellow arrow); (G) One month post-ablation axial T1C + MRI showing eggshell enhancement a remnant unablated tumor in the anterior margin of the ablation zone; Three months post-ablation axial T1C + MRI showing tumor recurrence in the anterior part of the ablation zone.
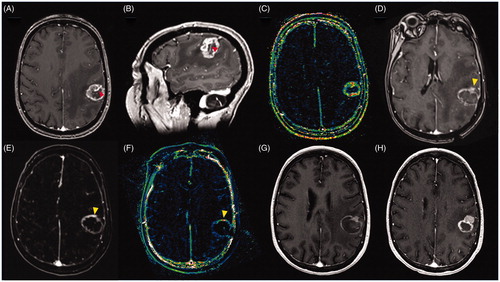
Ahluwalia et al. [Citation37] published the first study that recognized the difference between RN and recurrent tumor. Forty-two patients were included in this study and all but three patients had a conclusive diagnosis of either RN or recurrent tumor. The authors recognized that extent of ablation was important in predicting PFS. Patients with complete ablation had a good local control overall, but more importantly, local control in patients with RN was almost 100% regardless the extent of ablation, whereas local control rate in patients with recurrent tumor was 75% for completely ablated lesions and 37.5% for incompletely ablated lesions. Due to this finding, the authors recommended biopsy at the time of LITT and, in case of, recurrent tumor, evaluate the use of additional therapies such as radiation and systemic therapy postoperatively.
The first multicenter study using LITT for the treatment of metastases that failed radiosurgery included 30 patients across four different centers [Citation38]. The study showed a 73.3% steroid cessation, with a median time to cessation of 4.5 weeks. The study also reports that LITT was more effective or less morbid in patients with larger lesions and a higher pre-LITT KPS. Therefore, patients should be treated earlier before KPS declines. Hernandez et al. [Citation39], in large cohort of patients, also advocates for early intervention with LITT as soon as progression is determined to avoid the use of high-dose steroids that is associated with increased complication rates.
Salehi et al. [Citation40] in a study with 25 patients with BM treated with LITT established a cutoff volume of 5.62 cm3 where patients with equal or smaller lesions had longer PFS. On a univariate analysis, the authors also reported that patients with coverage equal to 97% or lower had a significantly shorter PFS when compared to patients with greater than 97% of lesion coverage. These results were not significant in a multivariate analysis, but this was probably due to the small number of patients, according to the authors. The main criticism to this study was the lack of distinction between recurrent tumor and RN either radiologically or using biopsy.
The largest study to date analyzing the predictive factors of local control in patients with brain metastases treated with LITT was published by Bastos et al. [Citation8]. It is a single center study with 61 patients comprising 82 lesions. Lesions were separated into radiological changes favoring RN vs. recurrent tumor. Using a receiver operating characteristic (ROC) analysis, a volume cutoff of 6 cc was identified with the optimal lesion volume that would predict complete ablation. Univariate analysis showed that complete ablation, tumor volume <6cc, RN, systemic therapy before and after LITT was predictors of improved local control. A multivariate Cox-regression model including these variables and controlled for confounding factors showed that incomplete ablation had a hazard ratio (HR) for local recurrence of 4.85, followed by radiological changes favoring recurrent tumor (HR of 3.43) and no systemic therapy within 3 months after LITT (HR of 2.56). Whereas a logistic regression showed that volumes >6cc had a higher rate of incomplete ablation. The complication rate for this cohort was 26.2% and the local recurrence rate in the first year was 59.4% overall.
Hong et al. [Citation41] compared LITT to craniotomy for the treatment of radiation necrosis or recurrent tumor in 75 patients with brain metastasis failing radiosurgery. The authors concluded that LITT is as efficacious as craniotomy in achieving local control and steroid taper. Craniotomy appears to be more advantageous for providing symptoms relief in patients that presented with pre-operative symptoms related to mass effects. Nonetheless, LITT appeared to be as efficacious as craniotomy in achieving desirable functional outcomes.
Indications and advantages of LITT
Surgical resection followed by adjuvant radiotherapy is still the paradigm for initial treatment of large (>3cm) accessible single or oligo brain metastases [Citation1–4,Citation44]. For patients with smaller lesions and/or multiple scattered lesions, radiosurgery or external beam radiation is indicated [Citation4]. LITT is mainly indicated to treat metastatic brain lesions refractory to standard treatment (failing radiation or craniotomy), which are deeply seated or surgically inaccessible, especially in patients too frail to tolerate major surgical stress.
One of the great advantages of LITT is the theoretical possibility to retreat patients multiple times since cumulative dose is not a concern in this scenario and is the the main constrain for repeat radiotherapy. Although studies that demonstrate the feasibility and safety of treating patients multiple times with LITT are still pending, it is our experience that patients who have developed local recurrence after LITT can be safely retreated (unpublished data). Another advantage of LITT is that patients with low clinical performance scores benefit from shorter hospitalizations and less surgical stress due to LITT’s less invasive nature, when compared with craniotomy [Citation33]. This can be particularly important for patients that need to resume systemic therapy as soon as possible in a scenario of progressive systemic disease. For deep-seated lesions in eloquent regions, diffusion tensor imaging (DTI) tractography can be incorporated to guide the extent of ablation and help preserve important tract. LITT can also be used as a diagnostic (biopsy during the procedure) and therapeutic tool in patients with symptomatic progression after radiation, refractory to medical therapy, where a definitive diagnosis could not be achieved by noninvasive methods requiring tissue sampling.
Complications
The complication rate for LITT in published series varies from 4% to 44% depending on the categorization utilized [Citation8,Citation32–39,Citation41,Citation42]. Hernandez et al. [Citation39] reports a complication rate of 25%, with only 3.4% permanent neurological deficits. The most common complications reported are new neurological deficits, increased seizure frequency and cerebral edema. Tumor location plays a major role in neurological outcomes for patients treated with LITT [Citation35]. Thermal damage to eloquent white matter tracts is the major cause of neurological deficits [Citation45]. For lesions with large volume and important mass effect pre-operatively, it is important to be aware of potential symptomatic cerebral edema [Citation46]. The attending physicians should have a high suspicion for this potential complication and a low threshold to start treatment with high dose steroids and occasionally bevacizumab. Other potential important adverse outcomes include bleeding in the ablation zone that could potentially require evacuation in cases of with considerable mass effect; incomplete ablation coverage caused by heat sinkers such as ventricles, blood vessels or sulci; intraventricular hemorrhage; and operative site infection [Citation47]. Overall, LITT is considered a safe procedure of acceptable complication rates when compared to salvage therapies available for patients with recurrent brain metastases.
Systemic therapy and LITT
The role of systemic therapy in the treatment of brain metastases has been revisited in recent years particularly in consequence of studies illustrating improved survival in patients with brain metastases who received systemic therapy compared to those who did not [Citation48]. The blood brain barrier (BBB) is still a challenge to effective systemic therapy in these patients. Using dynamic contrast-enhancement brain MRI to calculate the vascular transfer constant (Ktrans) in the peritumoral region as direct measures of BBB permeability before and after laser ablation, Leuthardt et al. [Citation49] published a pilot study showing that disruption of the peritumoral BBB was induced by hyperthermia. The peak of high permeability occurred within 1–2 weeks after laser ablation and resolving by 4–6 weeks. The authors concluded that this provides a therapeutic window of opportunity where delivery of BBB-impermeant therapeutic agents may be enhanced. In a multivariate analysis of predictive factors for local recurrence after LITT for the treatment of BM, the use of systemic therapy within 3 months after LITT was found to be a protective factor [Citation8]. Though, the authors did not specified the details of systemic therapy used the results demonstrate a potential role for LITT in enhancing local delivery of therapeutic agents, something that deserves further exploration in well-designed prospective trials.
Future perspectives
Several studies [Citation8] have identified predictive factors associated with local recurrence after LITT. These indicators should be further validated in multi-site analysis of large-scale patient cohorts such as the ongoing LAANTERN trial [Citation47]. Large-scale image analysis will require a high-quality relational database of all longitudinal imaging data pre- and post- LITT as well as clinical and demographic metadata. It is important that the appropriate IRB is in place such that the data is collected in a centralized structured database to query information across all sites.
Anticipating that imaging data will be collected at multiple sites; it is important that the image intensity values acquired on potentially different vendor platforms (GE, Siemens, Philips, etc.) be normalized using this properly controlled methodology. MR images across study sites will need to be normalized to a population model of internal reference intensity values of surrounding healthy tissue. This intensity normalization step is important to harmonize the data across geographic sites with potentially different imaging protocols. This will effectively reduce potential bias of imaging biomarker extraction and subsequent analysis.
Accurate image registration of the longitudinal data to a common time point is important to access treatment change-effects. An important step in this image registration step is skull stripping to isolate the brain for image analysis [Citation50]. In a large patient cohort with multiple time points, manual skull stripping is time intensive. Automated image segmentation methods will be needed to automate skull stripping. Segmentation errors are likely in the lower resolution data and efficient semi-automatic tools will be needs for cleaning up the contours. Further, the target lesion is typically manually contoured in the neuronavigation software used to guide the LITT procedure. Lesions with clearly defined boundaries can be expected to have low interobserver variability. However, lesions with less defined boundaries have a higher interobserver variability that can affect the analysis from differences in the lesion labeling. Again, automated segmentations tools [Citation51] may be used for lesion contouring to increase both the efficiency and repeatability of the image analysis pipeline. Machine learning methods to enable automatic segmentation will required training data to incorporate spatial heterogeneities of the gray matter, white matter, cerebrospinal fluid, edema, and tumor.
‘Radiomics’ [Citation52] approaches may also be applied with the contoured lesions to develop and validate additional imaging biomarkers for quantitatively assessing thermal dose with respect to the lesion response observed. Radiomic image features, such as the lack of a clearly defined T2 mass lesion [Citation53] and high edema to enhancing lesion volume ratio [Citation54], may also be extracted from image segmentation methods. Radiomics is a systematic process of extracting high-dimensional quantitative data from radiographic images. An extensive body of literature [Citation55] exists that explores the radiomic and clinical variable data space to mine correlations with treatment outcomes for improved decision support. The overarching hypothesis is that peri-treatment quantitative image features may aid in characterizing the underlying treatment response. Quantitative correlations between the radiographic signature and the treatment outcome may be established. The methodology shares much in common with the computer aided diagnosis and quantitative imaging communities and represent the natural progression of traditional handcrafted imaging biomarkers. These procedures for finding a combination of features correlated with outcome are analogous to the development of biomarker assays [Citation56].
Incompletely ablated lesions are among the dominant indicators for recurrent disease. A-priori mathematical model predictions [Citation17] may optimize treatment planning for more complete tumor coverage during delivery. Nonlinear models of bioheat transfer with homogeneous temperature dependent perfusion and optical properties are likely to provide a practical methodology in planning thermal damage. A population model derived from thermal imaging data of the heating may be used calibrate the parameters needed for patient specific prediction. Delta-P models of predicting the laser fluency and subsequent heating may also be considered to model increase photon depth [Citation57].
Conclusion
Overall, the current literature supports LITT as a safe and effective therapy for patients with brain metastases that have failed SRS. In order to prevent local recurrence, particular care should be taken to provide full coverage of lesion where recurrent tumor is suspected. For patients with RN, studies have demonstrated that even incomplete ablation could suffice to halt the disease progression. Larger prospective studies are required to better evaluate the role of systemic therapy with LITT. Incorporating new imaging modalities such as radiomics may enable optimized treatment plans and better outcomes.
Author contribution
All authors contributed equally to the manuscript writing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lassman AB, DeAngelis LM. Brain metastases. Neurologic Clinics. 2003;21(1):1–23.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
- Soliman H, Das S, Larson DA, et al. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7(11):12318–12330.
- Fecci PE, Champion CD, Hoj J, et al. The evolving modern management of brain metastasis. Clin Cancer Res. 2019;25(22):6570–6580.
- Bennett EE, Angelov L, Vogelbaum MA, et al. The prognostic role of tumor volume in the outcome of patients with single brain metastasis after stereotactic radiosurgery. World Neurosurgery. 2017;104:229–238.
- Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6(1):48.
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus. 2015;38(3):E13.
- Bastos DCA, Rao G, Oliva ICG, et al. Predictors of local control of brain metastasis treated with laser interstitial thermal therapy. Neurosurgery. 2019;3:161–169.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(suppl_1):ONS21–ONS28. Discussion ONS8-9.
- Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700–709.
- Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol. 1991;53(6):825–835.
- Wyman DR, Schatz SW, Maguire JA. Comparison of 810 nm and 1064 nm wavelengths for interstitial laser photocoagulation in rabbit brain. Lasers Surg Med. 1997;21(1):50–58.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800.
- Fajardo LF, Egbert B, Marmor J, et al. Effects of hyperthermia in a malignant tumor. Cancer. 1980;45(3):613–623.
- Fahrenholtz SJ, Moon TY, Franco M, et al. A model evaluation study for treatment planning of laser-induced thermal therapy. Int J Hyperther. 2015;31(7):705–714.
- Mitchell D, Fahrenholtz S, MacLellan C, et al. A heterogeneous tissue model for treatment planning for magnetic resonance-guided laser interstitial thermal therapy. Int J Hyperther. 2018;34(7):943–952.
- Stoll AM, Greene LC. Relationship between pain and tissue damage due to thermal radiation. J App Physiol. 1959;14(3):373–382.
- Henriques F, Jr, Moritz A. Studies of thermal injury: I. the conduction of heat to and through skin and the temperatures attained therein. A theoretical and an experimental investigation. Am J Pathol. 1947;23(4):530.
- McNichols RJ, Gowda A, Kangasniemi M, et al. MR thermometry‐based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med. 2004;34(1):48–55.
- Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the neuroblate system first-in-humans phase I clinical trial for recurrent glioblastoma. J. Neurosurg. 2013;118(6):1202–1219.
- Hawasli AH, Ray WZ, Murphy RK, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery. 2012;70(2):332–337. Discussion 8.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950.
- Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 Suppl Operative):133–144; 44–5.
- Rahmathulla G, Recinos PF, Valerio JE, et al. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200.
- Hawasli AH, Bagade S, Shimony JS, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017.
- Patel NV, Jethwa PR, Barrese JC, et al. Volumetric trends associated with MRI-guided laser-induced thermal therapy (LITT) for intracranial tumors. Lasers Surg Med. 2013;45(6):362–369.
- Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113(3):495–503.
- Fabiano AJ, Alberico RA. Laser-interstitial thermal therapy for refractory cerebral edema from post-radiosurgery metastasis. World Neurosurgery. 2014;81(3–4):652 e1-4.
- Fabiano AJ, Qiu J. Delayed failure of laser-induced interstitial thermotherapy for postradiosurgery brain metastases. World Neurosurgery. 2014;82(3–4):e559–63.
- Sun XR, Patel NV, Danish SF. Tissue ablation dynamics during magnetic resonance-guided, laser-induced thermal therapy. Neurosurgery. 2015;77(1):51–58. Discussion 8.
- Rao MS, Hargreaves EL, Khan AJ, et al. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74(6):658–667; discussion 67.
- Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurooncol. 2016;125(4):853–860.
- Ali MA, Carroll KT, Rennert RC, et al. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(4):E11.
- Beechar VB, Prabhu SS, Bastos D, et al. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neurooncol. 2018;137(1):57–65.
- Kamath AA, Friedman DD, Hacker CD, et al. MRI-guided interstitial laser ablation for intracranial lesions: a large single-institution experience of 133 cases. Stereotact Funct Neurosurg. 2017;95(6):417–428.
- Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2019;130(3):804–808.
- Chaunzwa TL, Deng D, Leuthardt EC, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63.
- Hernandez RN, Carminucci A, Patel P, et al. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019;85(1):84–90.
- Salehi A, Kamath AA, Leuthardt EC, et al. Management of intracranial metastatic disease with laser interstitial thermal therapy. Front Oncol. 2018;8:499.
- Hong CS, Deng D, Vera A, et al. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142(2):309–317.
- Shah AH, Semonche A, Eichberg DG, et al. The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery. 2019.
- Traylor JI, Bastos DCA, Fuentes D, et al. Dynamic contrast-enhanced mri in patients with brain metastases undergoing laser interstitial thermal therapy: a pilot study. Am J Neuroradiol. 2019;40(9):1451–1457.
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048.
- Sharma M, Habboub G, Behbahani M, et al. Thermal injury to corticospinal tracts and postoperative motor deficits after laser interstitial thermal therapy. Neurosurg Focus. 2016;41(4):E6.
- Maraka S, Asmaro K, Walbert T, et al. Cerebral edema induced by laser interstitial thermal therapy and radiotherapy in close succession in patients with brain tumor. Lasers Surg Med. 2018;50(9):917–923.
- Rennert RC, Khan U, Bartek J, Jr, et al. Laser Ablation of Abnormal Neurological Tissue Using Robotic Neuroblate System (LAANTERN): procedural safety and hospitalization. Neurosurgery. 2019.
- Niwińska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010;116(18):4238–4247.
- Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613.
- Roy S, Butman JA, Pham DL, et al. Robust skull stripping using multiple MR image contrasts insensitive to pathology. NeuroImage. 2017;146:132–147.
- Menze BH, Jakab A, Bauer S, et al. The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging. 2015;34(10):1993–2024.
- Tiwari P, Danish S, Wong S, Madabhushi A, editors. Quantitative evaluation of multi-parametric MR imaging marker changes post-laser interstitial ablation therapy (LITT) for epilepsy. Medical Imaging 2013: image-guided procedures, robotic interventions, and modeling; 2013: International Society for Optics and Photonics.
- Kano H, Kondziolka D, Lobato-Polo J, et al. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010;66(3):486–492.
- Leeman JE, Clump DA, Flickinger JC, et al. Extent of perilesional edema differentiates radionecrosis from tumor recurrence following stereotactic radiosurgery for brain metastases. Neuro-oncology. 2013;15(12):1732–1738.
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577.
- Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16(6):1745–1755.
- Carp SA, Prahl SA, Venugopalan V. Radiative transport in the delta-P1 approximation: accuracy of fluence rate and optical penetration depth predictions in turbid semi-infinite media. J Biomed Opt. 2004;9(3):632–648.
