Abstract
Objective
To evaluate the impact of ultrasound-guided high-intensity focused ultrasound (USgHIFU) for uterine fibroids on ovarian reserve in women of reproductive age.
Materials and methods
From September 2015 to September 2017, 84 patients with uterine fibroids in reproductive age were enrolled from Chongqing Haifu Hospital, Three Gorges Central Hospital of Chongqing and Aegisroen obstetric gynecology Clinic of Seoul, Korea. Blood was collected before HIFU treatment and 6 months after USgHIFU treatment. The enzyme-linked immune analysis was used for assay of circulating anti-Mullerian hormone (AMH).
Results
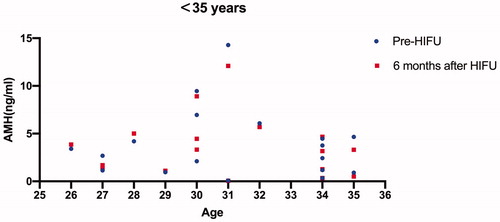
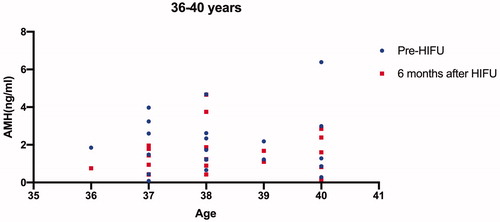
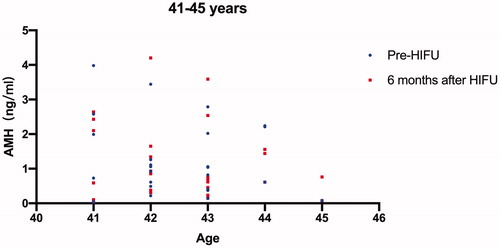
All the 84 patients were successfully treated with USgHIFU and 67 patients completed the follow-up. The median age of the 67 patients was 38 years at the time of treatment. The median AMH value before and 6 months after treatment was 1.26 ng/mL and 1.27 ng/mL, respectively. Patients who completed AMH measurements 6 months after treatment were further classified into three groups based on age, including younger than 35 years, 36 to 40 years, and older than 40 years. The median AMH values before treatment were 3.04 ng/mL, 1.73 ng/mL and 0.87 ng/mL, and the corresponding values 6 months after treatment were 3.24, 1.44 and 0.75, respectively. A significant difference in AMH level was observed in the group of patients at the age between 36 and 40 years (p < 0.05), but no significant difference in AMH levels was observed in the other two groups (p > 0.05).
Conclusion
Based on our results, we conclude that USgHIFU has no influence on ovarian reserve.
Introduction
Uterine fibroids, also known as myomas, are the most prevalent benign gynecologic tumors in reproductive age women. Although inherently benign, with symptoms such as heavy bleeding and painful menstruation, anemia, frequent urination, and constipation, the disease can result in a serious influence on women’s health and quality of life [Citation1]. The management of uterine fibroids currently is still based mainly on surgical treatment. However, because other treatment options for uterine fibroids are available, the treatment choice depends on the size, location, and number of fibroids; the patient’s age; and whether she wishes to preserve the uterus. According to a survey from the United States of America, almost 50% of respondents expressed a wish to preserve the uterus, and most patients preferred noninvasive treatments for uterine fibroids [Citation2]. Therefore, clinicians have been always working toward providing less invasive treatments for patients with uterine fibroids.
As a noninvasive treatment, high-intensity focused ultrasound (HIFU) has been widely used in the treatment of uterine fibroids [Citation3–6]. HIFU treatment is performed under the guidance of either ultrasound or magnetic resonance imaging. The ultrasound beams can be precisely focused on fibroids and cause coagulative tumor necrosis without damage to the surrounding normal uterine tissue and adjacent organs. Over the last decade, many studies from different groups have demonstrated that HIFU is safe and effective in the treatment of uterine fibroids [Citation7–10]. However, available data on the effects of HIFU on the ovarian reserve are scarce. Many patients seek less invasive treatment options for uterine fibroids because of their concern about ovarian function. Therefore, the aim of this study was to evaluate the influence of ultrasound guided HIFU (USgHIFU) treatment for uterine fibroids on ovarian reserve.
Materials and methods
This prospective observational open-label multicenter study was approved by the ethics committees at the hospitals mentioned above. Every patient signed an informed consent before each procedure.
Patients
From September 2015 to September 2017, 84 patients with uterine fibroids were enrolled. Among them, 23 were from Chongqing Haifu Hospital, 36 were from Chongqing Three Gorges Central Hospital, and 25 were from Aegisroen Obstetric Gynecology Clinic of Seoul, Korea. The diagnosis of uterine fibroids was made by medical history, physical examination, ultrasound and magnetic resonance imaging.
The inclusion criteria were as follows: (1) all patients were older than 18 years and younger than 45 years; (2) all patients had symptoms; (3) patients could communicate with the nurse or physician during HIFU procedure; (4) patients agreed to take pre- and post-HIFU anti-Müllerian hormone (AMH) tests.
The exclusion criteria were as follows: (1) patients had ovarian diseases, endocrine diseases, or severe pelvic endometriosis; (2) patients suspected to have extensive pelvic adhesions; (3) had a history of ovarian surgery, chemotherapy, or pelvic radiotherapy prior to HIFU treatment; (4) patients who cannot complete HIFU treatment or the follow-up after HIFU were also excluded.
USgHIFU treatment
The procedure of USgHIFU treatment has been described in previous publications [Citation10]. Briefly, the patients were requested to have a specific bowel preparation, including ingesting semi-liquid and liquid food for two days, followed by a 12-h fasting period and an enema the morning before the procedure of USgHIFU. The skin of the lower abdominal wall was shaved, degreased with 70% alcohol, and degassed with a vacuum device. A catheter was inserted to control bladder volume. HIFU treatment was performed using the Model JC or JC200 focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co. Ltd., Chongqing, China). The patient was positioned prone and the procedure was performed under intravenous conscious sedation (midazolam and fentanyl for sedation and pain control) with the guidance of real-time ultrasound. The adequacy of USgHIFU ablation was controlled and determined by the patients’ response to the treatment and the degree of grayscale changes of the target lesion on ultrasound. Contrast-enhanced ultrasound with SonoVue was used to evaluate the ablation volume and effect. Uterine volume, fibroid volume, and non-perfused volume were calculated by measuring longitudinal (D1), anteroposterior (D2), and axial lengths (D3) and using the following formula: V = 0.5233 × D1 × D2 × D3. The nonperfused volume ratio was calculated using the following formula: R = V1/V2 × 100%, where V1 indicates nonperfused volume and V2 indicates fibroid volume immediately after treatment.
AMH test
Blood samples of 10 mL were obtained before HIFU treatment and 6 months after HIFU treatment. Blood samples were centrifuged and the serum was extracted and stored at –20 °C for an enzyme-linked immune sorbent assay.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software. Continuous variables with normal distribution are presented as mean ± standard deviation (±SD) and compared using paired t-test. Continuous variables with skewed distribution were presented as median (interquartile range) and compared using paired Wilcoxon’s test. Statistical significance was defined as p < 0.05.
Results
Patients and lesions
All 84 patients were successfully treated with USgHIFU. The median age of the patients was 38 years (range: 24 to 45 years) at the time of USgHIFU treatment. Thirty-eight of the 84 patients had multiple fibroids and 46 had a solitary fibroid. The median uterine volume was 198.56 cm3 (interquartile range: 137.26 to 295.26 cm3). The median fibroid volume was 71.95 cm3 (interquartile range: 42.44 to 126.41 cm3) ().
Table 1. Baseline characteristics of the 84 enrolled patients.
Peri-procedural evaluation
The treatment power was controlled between 300 and 400 W. The median sonication time, sonication energy, and non-perfused volume ratio were 728.5 s (interquartile range: 497.8 to 1231.3 s), 261,421.0 Joules (interquartile range: 178,241.3 to 468,311.8 Joules), and 80.0% (interquartile range: 70.0 to 90.0%), respectively ().
Table 2. Treatment results of uterine fibroids treated by HIFU.
AMH levels assessment
Among the 84 patients, 67 patients completed the AMH test 6 months after treatment. The median AMH values in the patients who completed the AMH test 6 months after treatment were 1.26 ng/mL before and 1.27 ng/mL after USgHIFU, respectively. We further classified these 67 patients into three groups based on the patient’s age (<35 years, 36–40 years, >40 years); the pre- and post-HIFU AMH values for each patient were shown on –Citation3; the median AMH values before HIFU treatment in the three groups were 3.04 ng/mL, 1.73 ng/mL and 0.87 ng/mL, and the corresponding values 6 months after treatment were 3.24 ng/mL, 1.44 ng/mL and 0.75 ng/mL, respectively. A significant difference in AMH level was observed in the group of patients with ages between 36 and 40 years, but no significant difference in AMH levels was observed in the other two groups (p > 0.05) (). Four patients were pregnant and no patients had amenorrhea or perimenopausal symptoms during the follow-up period.
Table 3. AMH levels in different age groups assessed before and 6 months after HIFU.
Discussion
AMH is a reliable marker to evaluate ovarian reserve and it has been widely accepted that serum AMH level presents a negative correlation with age in women after puberty. Unlike other markers, serum AMH level is independent of the menstrual cycle and is not influenced by administration of hormones. Also, the decrease of serum AMH level can occur before changes in follicle-stimulating hormone (FSH) and estradiol (E2) levels. The previous study reported that AMH levels decreased 2 days after surgery [Citation11], indicating that changes in ovarian function can be observed by the AMH level within a short time. Therefore, serum AMH level is considered to be a more sensitive and more reliable reflection of ovarian function.
The possible disturbance of the ovarian function resulting from the surgical procedure is always a main concern. Gökgözoğlu et al. performed a study to evaluate the effects of total abdominal hysterectomy on ovarian function and the results showed that the AMH serum level decreased significantly in the first month after surgery [Citation12]. In another study, the patients either had total laparoscopic hysterectomy or laparoscopic supracervical hysterectomy, and the serum AMH level in these patients also significantly decreased in measurements made 1 month and 4 months after surgery [Citation13]. Wang et al. tested serum AMH values before and 2 days after the patients had hysterectomy or myomectomy; they found that the serum AMH values 2 days following either surgical procedure were significantly lower than the value before surgery [Citation11]. The reduced ovarian reserve after hysterectomy and myomectomy may be explained by the ligation or clamping of uterine blood vessels and electrocoagulation for hemostasis during the surgical procedure that may affect blood flow to the ovaries. Because the blood supply to the ovaries is partially from the uterine arteries, uterine artery embolization (UAE) for uterine fibroids can also affect the collateral blood supply to the ovaries and has a potential risk of unintended embolization to the ovarian arteries [Citation14,Citation15]. Many studies have shown that the AMH values of patients with uterine fibroids who received UAE were significantly lower after the procedure compared to the expected AMH level [Citation16,Citation17]. The AMH levels in patients who had UAE were significantly lower than those in patients who had laparoscopic myomectomy (0.78 vs. 2.17 ng/mL) [Citation17].
As a noninvasive treatment, HIFU can be used to treat fibroids selectively without damaging to the surrounding structures (). Cheung et al. tested the AMH values of 12 patients with uterine fibroids before and after HIFU treatment. In that study, the mean age of the 12 patients was 46.3 ± 3.3 years [Citation18]. The median AMH value was 0.3 ng/mL before treatment, 0.47 ng/mL 1 month after treatment, 0.205 ng/mL 3 months after treatment, 0.26 ng/mL 6 months after treatment, and 0.06 ng/mL 12 months after treatment. Statistical analysis results showed that there was no significant difference between AMH levels before and levels at any follow-up time point after HIFU treatment. Since the number of enrolled patients was small and the average age of the patients was over 45 years old, the results were limited. Lee et al. treated 79 patients with adenomyosis or uterine fibroids using HIFU and evaluated the AMH level of the patients. The mean AMH value was 2.11 ± 2.66 ng/mL before treatment and 1.84 ± 2.57 ng/mL 6 months after treatment. The results showed no significant decrease in AMH level after HIFU treatment [Citation19]. In our study, all 84 patients were successfully treated with USgHIFU. The median non-perfused volume ratio of the uterine fibroids after USgHIFU was 80.0% (interquartile range: 70.0 to 90.0%) and the mean AMH value was similar to the results from Lee’s study and there was no significant difference between the circulating level of AMH before and 6 months after USgHIFU. Since the AMH level is related to the age of patients, as shown in , we further classified the patients into three groups based on age, and the only significant difference between AMH levels before treatment and levels 6 months after treatment was observed in the group of patients with ages between 36 and 40 years, but no significant difference was observed in the groups covering other ages (). Many studies have shown that the ovarian reserve declines with age in women of reproductive age, with a significant decline at the age of 37.5 years and then a slow decrease after 40 years [Citation20,Citation21]. Therefore, the significant decrease in AMH level 6 months after HIFU treatment in the group of patients aged from 36 to 40 years is considered to be related to age, not to HIFU treatment.
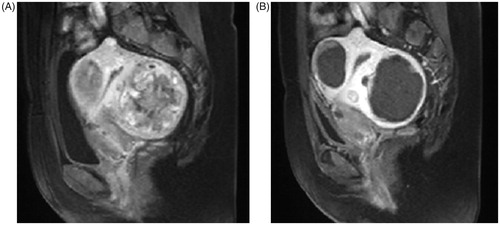
Figure 4. A sagittal plane of contrast enhanced MRI obtained from a 43-year-old patient with multiple uterine fibroids before and 1 day after HIFU treatment. (A) pre-HIFU image showed multiple uterine fibroids with poor to medium blood supply; (B) post-HIFU image showed the multiple uterine fibroids were completely ablated without damaging to the normal structures of the uterus.
This study is limited because the sample size is relatively small and the small number of patients from each center may cause bias. Therefore, it is necessary to perform prospective studies and enroll more patients from multiple centers to validate our findings.
Conclusion
In summary, our study showed that no significant change in AMH level after HIFU treatment was observed in the group of patients younger than 35 years and the group older than 40 years at 6 months. In the group of patients at the age between 36 and 40 years, the significant change in AMH level was related to age, not to HIFU treatment. Based on our results, we conclude that HIFU treatment has no influence on the ovarian reserve.
Disclosure statement
Zhibiao Wang and Lian Zhang are senior consultants to Chongqing Haifu. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Hervé F, Katty A, Isabelle Q, et al. Impact of uterine fibroids on quality of life: a national cross-sectional survey. Eur J Obstet Gynecol Reprod Biol. 2018;229:32–37.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- He M, Jacobson H, Zhang C, et al. A retrospective study of ultrasound-guided high intensity focused ultrasound ablation for multiple uterine fibroids in South Africa. Int J Hyperthermia 2018;34(8):1304–1310.
- Park J, Lee JS, Cho JH, et al. Effects of high-intensity focused ultrasound treatment on benign uterine tumor. J Korean Med Sci. 2016;31(8):1279–1283.
- Chen R, Keserci B, Bi H, et al. The safety and effectiveness of volumetric magnetic resonance-guided high-intensity focused ultrasound treatment of symptomatic uterine fibroids: early clinical experience in China. J Ther Ultrasound. 2016;4:27.
- Zhao WP, Zhang J, Han ZY, et al. A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep. 2017;7(1):10812.
- Zhang C, Jacobson H, Ngobese ZE, et al. Efficacy and safety of ultrasound-guided high intensity focused ultrasound ablation of symptomatic uterine fibroids in Black women: a preliminary study. Int J Obstet Gyenecol. 2017;124 (Suppl 3):12–17.
- Ji Y, Hu K, Zhang Y, et al. High intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181–1188.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia 2018;35(1):56–61.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Gökgözoğlu L, Islimye M, Topçu HO, et al. The effects of total abdominal hysterectomy on ovarian function - Serial changes in serum anti-Müllerian hormone, FSH and estradiol levels. Adv Clin Exp Med. 2014;23(5):821–825.
- Yuan H, Wang C, Wang D, et al. Comparing the effect of laparoscopic supracervical and total hysterectomy for uterine fibroids on ovarian reserve by assessing serum anti-mullerian hormone levels: A prospective cohort study. J Minim Invasive Gynecol. 2015;22(4):637–641.
- Ouyang Z, Liu P, Yu Y, et al. Role of ovarian artery-to-uterine artery anastomosis in uterine artery embolization: Initial anatomic and radiologic studies. Surg Radiol Anat. 2012;34(8):737–741.
- Ryu RK, Chrisman HB, Omary RA, et al. The vascular impact of uterine artery embolization: Prospective sonographic assessment of ovarian arterial circulation. J Vasc Interv Radiol. 2001;12(9):1071–1074.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016;206:172–176.
- Arthur R, Kachura J, Liu G, et al. Laparoscopic myomectomy versus uterine artery embolization: Long-term impact on markers of ovarian reserve. J Obstet Gynaecol Can. 2014;36(3):240–247.
- Cheung VY, Lam TP, Jenkins CR, et al. Ovarian reserve after ultrasound-guided high intensity focused ultrasound for uterine fibroids: preliminary experience. J Obstet Gynaecol Can. 2016;38(4):357–361.
- Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high- intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG: Int J Obstet Gynecol. 2017;124 (Suppl 3):18–22.
- Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346.
- Tehrani FR, Mansournia MA, Solaymani-Dodaran M, et al. Age-specific serum anti-Müllerian hormone levels: estimates from a large population-based sample. Climacteric 2014;17(5):591–597.