Abstract
Radiation necrosis is a well described complication after radiosurgical treatment of intracranial pathologies – best recognized after the treatment of patients with arteriovenous malformations and brain metastases but possibly also affecting patients treated with radiosurgery for meningioma. The pathophysiology of radiation necrosis is still not well understood but is most likely a secondary local tissue inflammatory response to brain tissue injured by radiation. Radiation necrosis in brain metastases patients may present radiographically and behave clinically like recurrent tumor. Differentiation between radiation necrosis and recurrent tumor has been difficult based on radiographic changes alone. Biopsy or craniotomy therefore remains the gold standard method of diagnosis. For symptomatic patients, corticosteroids are first-line therapy, but patients may fail medical management due to intolerance of chronic steroids or persistence of symptoms. In these cases, open surgical resection has been shown to be successful in management of surgically amenable lesions but may be suboptimal in patients with deep-seated lesions or extensive prior cranial surgical history, both carrying high risk for peri-operative morbidity. Laser interstitial thermal therapy has emerged as a viable, alternative surgical option. In addition to allowing access to tissue for diagnosis, thermal treatment of the lesion can also be delivered precisely and accurately under real-time imaging guidance. This review highlights the pertinent studies that have shaped the impetus for use of laser interstitial thermal therapy in the treatment of radiation necrosis, reviewing indications, outcomes, and nuances toward successful application of this technology in patients with suspected radiation necrosis.
Introduction
The indications for use of radiosurgery have increased exponentially over the past decade with the demonstration of successful long term control of brain metastases without the toxicities of whole brain radiation therapy [Citation1,Citation2]. The most common complication of radiosurgery however remains cerebral radiation necrosis (RN), which is defined as a self-perpetuating inflammatory response within the irradiated field, most commonly occurring 6–18 months after radiosurgical treatment [Citation1–3]. Although its reported incidence varies widely throughout the literature, rates of RN are believed to be about 5–10% in brain metastases patients [Citation4] but can be as high as 30% in patients with arteriovenous malformations treated with SRS [Citation5] and 10–15% in patients with meningiomas treated with SRS [Citation6,Citation7]. Higher rates of RN have been reported to be associated with increasing radiation dose per fraction, increasing lesion size, repeat SRS and use of concurrent immunotherapy [Citation8–11]. Although the exact mechanisms remain unclear, the pathophysiology of RN has been proposed to be related to radiation-induced disruption of the blood brain barrier through direct endothelial cell injury, pro-inflammatory cytokine and chemokine response, and leaky neovasculature from angiogenesis, all leading to increased perilesional edema [Citation12–14]. Histologically, RN appears as a central area of coagulative necrosis surrounded by a rim of reactive astrocytosis, demyelination, hyalinized vascularity and perivascular and intraparenchymal infiltrates of macrophages and lymphocytes [Citation15,Citation16].
Clinically, RN can grow in size like tumor and cause similar neurological problems related to its location. Symptoms may range from global signs of elevated intracranial pressure to focal neurological deficits affecting eloquent functions such as motor, sensory, visual, speech, and coordination functions. Radiographically, it is also often difficult to distinguish these lesions from recurrent tumor, as both may appear on routine computed tomography (CT) and magnetic resonance imaging (MRI) as contrast-enhancing lesions, with significant perilesional edema reflecting local blood brain barrier disruption. While various imaging adjuncts exist that may help differentiate between recurrent tumor and RN, such as perfusion-weighted MRI and MR-spectroscopy [Citation17–19], biopsy remains the gold standard, in which absence of viable tumor cells within the biopsy specimen signifies a diagnosis of RN [Citation20,Citation21].
In patients with asymptomatic lesion regrowth demonstrated on MRI alone, observation with serial imaging is often performed given that the majority of these lesions will resolve again spontaneously [Citation3,Citation22]. First-line treatment for patients with symptomatic RN however has been corticosteroid therapy, which acts to abrogate the inflammatory response and reduce symptomatic perilesional edema [Citation23]. The efficacy of steroid therapy can sometimes be transient and may not lead to long-term resolution of symptoms. In addition, chronic steroid therapy carries considerable risk for medical co-morbidities, including uncontrolled diabetes, myopathy, gastrointestinal bleeding, poor wound healing, pneumonia and adrenal insufficiency, among others. Second-line medical therapies include bevacizumab [Citation24,Citation25], hyperbaric oxygen therapy [Citation26], high-dose vitamin E [Citation27], pentoxifylline [Citation27], and anticoagulants, which have been shown to have varying degrees of efficacy [Citation28]. The details of these therapies are outside the scope of this review, but to date the gold standard for both diagnosis and cure of RN remains surgical resection of the lesion [Citation2,Citation21]. In patients with symptomatic lesions in surgically accessible areas, resection affords rapid relief of symptoms and can be curative [Citation29]. However, for lesions that are unable to resected without significant added peri-operative morbidity or in patients who are not surgical candidates for other reasons such as difficulty with wound healing and prior infections, alternative options are limited.
Laser interstitial thermal therapy (LITT) has emerged as a viable alternative surgical option for patients with suspected RN, utilizing latest technologies in stereotaxis and MRI guidance to allow minimally invasive surgical access for diagnostic lesion biopsy and therapeutic thermal ablation. As with RN, given the lack of animal models, it is also unclear how thermal ablation corrects coagulative necrosis, but we propose that the success of this treatment is really more dependent upon thermal ablation of the inflammatory rim around the necrosis.
Surgical technique and case illustration
A 62 year-old male with recently diagnosed lung adenocarcinoma underwent gamma knife radiosurgery prescribed 20 Gy to the 50% isodose line to two asymptomatic intracranial metastatic lesions, located in the right parietal and right occipital lobes. Nine months later, he re-presented with complaints of several weeks of significant headaches and several episodes throughout the day of transient vision loss. Repeat brain MRI demonstrated a growing enhancing lesion in the right occipital lobe with significant surrounding edema on T2-weighted FLAIR images (), in stark contrast to near resolution of the other radiated lesion in the parietal lobe. Although his headaches improved with steroid therapy, he could not be weaned off of steroids without return of symptoms, prompting discussion for surgical intervention. LITT was chosen as an alternative to open surgical intervention, based on patient need for vision preservation given his occupation as a chauffeur.
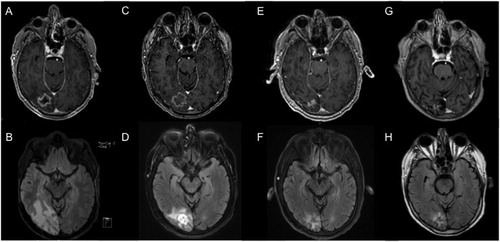
Figure 1. Serial MRI of case illustration patient undergoing LITT. Pre-operative (A) T1-weighted post-contrast MRI demonstrating a contrast enhancing lesion within the right occipital lobe with perilesional edema on (B) T2-weighted FLAIR MRI. Two weeks after LITT, the lesion showed (C) stable enhancing size but (D) decreased surrounding edema, accompanied by further decreases at 6-month follow-up in both (E) lesion size and (F) perilesional edema. At last follow-up 43 months after LITT, the ablated lesion showed (G) minimal nodular enhancement and (H) near-resolution of surrounding edema.
A detailed description of our surgical technique has been previously described [Citation30]. Briefly, after induction of general anesthesia, a fiducial-based MRI is acquired utilizing an intraoperative 3.0 T MR scanner (IMRIS, Mannitoba, Canada) to plan a trajectory to the targeted lesion with the Brainlab system (Brainlab, Winchester, IL, USA). Trajectory is then optimized using the ClearPoint navigation system (ClearPoint Neuro, Irvine, CA, USA). Through a stab skin incision in the skin, a twist drill hole is made along the trajectory through the ClearPoint platform. A stereotactic biopsy is usually performed at this time. The laser probe is then advanced to the target center and its placement is confirmed by MRI. Heat application then proceeds under visual guidance using Monteris software (Monteris Medical, Minneapolis, USA) showing thermal damage threshold lines calculated from serially obtained gradient echo sequences. Laser ablation is stopped once the zone of thermal ablation has sufficiently encompassed the lesion or target-temperature limits near critical structures have been exceeded. A final MRI is obtained to verify ablation volume, and after removal of the laser probe, the skin is closed typically only with a single suture layer.
The illustrated patient underwent LITT without complications () with intra-operative biopsy showing necrosis, reactive astrocytosis, and absence of viable tumor cells, consistent with a diagnosis of RN. The patient recovered uneventfully from surgery and was discharged within 24 h to home. He was able to be tapered off of steroids by 2 weeks post-operatively and exhibited complete resolution of headaches and visual symptoms at 6-week follow-up without detectable visual field deficits on exam. Follow-up MRI at 2-weeks after LITT demonstrated stable T1-enhancing lesion size but significant decrease in perilesional edema on T2-weighted FLAIR () with further decreases at 6-months after LITT (). At last follow-up 43 months after LITT, MRI showed a small residual T1-weighted post-contrast lesion and minimal surrounding T2 signal change (). Although the patient demonstrated durable local control of his ablated lesion without return of symptoms, he ultimately succumbed to systemic disease progression 52 months after surgery.
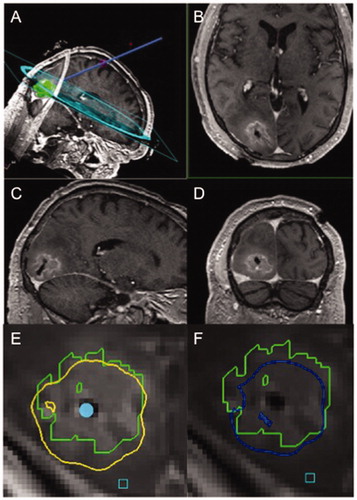
Figure 2. Representative intra-operative MRI of case illustration. (A) A planned LITT trajectory is shown, followed by T1-weighted post-contrast (B) axial, (C) sagittal, and (D) coronal cuts showing catheter placement within the center of the lesion post-ablation. Representative thermal damage threshold lines are shown for the outlined lesion in green at a given depth for (E) protein denaturation in yellow and (F) cell death in blue, obtained during continuously acquired thermal MR imaging that guides the extent of heat application.
Review of the literature
Early clinical data for application of LITT for suspected RN came from Carpentier et al. in a pilot clinical trial conducted in 2008 [Citation31], followed by an update three years later [Citation32], the latter of which included 7 patients with 15 total re-growing lesions who were previously treated with a combination of chemotherapy, whole brain radiation therapy (WBRT), and SRS for brain metastases and who were otherwise not fit for open surgical intervention (). The follow-up period ranged up to 30 months after LITT. Overall, the results were promising with only one patient expiring from local progression of disease, while progression-free survival (PFS) was correlated with degree of coverage of the lesion by thermal ablation as partially covered lesions showed median PFS of 6 months, compared to totally covered lesions with PFS of 15 months. However, biopsies were not performed in this study and as such, recurrent tumor could not be differentiated from RN.
Table 1. Summary of studies on laser interstitial thermal therapy for the treatment of cerebral radiation necrosis.
Subsequently, several groups published anecdotal institutional experience demonstrating success treating biopsy-proven RN with LITT. Rahmathulla et al. described the first case of biopsy-proven RN treated with LITT () [Citation33]. A 74 year-old male had previously undergone SRS for a left centrum semiovale brain metastasis from known non-small cell lung cancer. This lesion subsequently regrew causing dysphasia. Imaging findings, including PET-CT, were consistent with RN that was refractory to prolonged, high-dose steroid therapy. Medical therapies for RN were contraindicated due to his significant co-morbidities which included renal dysfunction, hypertension, and coronary artery disease requiring antiplatelet medications. Surgical resection was also felt to be high risk given patient comorbidities as well as the lesion’s deep location and that standard surgical access would transgress eloquent cortex and white matter. The patient was therefore offered LITT. Intra-operative biopsy showed histopathological findings of RN without evidence of viable tumor cells. The patient was discharged within 48 h of surgery, experienced symptom improvement, and was successfully weaned off of steroids within 2 weeks. Two-month follow-up MRI findings demonstrated mild increase in enhancing lesion size but almost complete resolution of perilesional edema.
This report was followed by a single-institution retrospective study conducted by Smith et al. of 25 patients treated with LITT for intra-operative biopsy-proven RN after prior SRS to primary and metastatic brain tumors [Citation34], comprised of 8 WHO grade IV gliomas, 5 WHO grade III gliomas, 5 WHO grade II gliomas, and 7 brain metastases (). LITT was chosen as a salvage therapy in these patients, all of whom had imaging findings of increasing enhancing lesion size and/or perilesional edema, despite prior histories of open surgical resection, adjuvant chemotherapy and radiation, and further bevacizumab treatment in select cases. Mean progression free survival (PFS) after LITT was 8.5 and 9.1 months for WHO grade III/IV glioma patients and 11.4 months for brain metastases patients. Unlike the results from Rahmathulla et al. [Citation33] not all lesions decreased in size on follow-up imaging. In patients who survived 12 months after LITT, however, the authors did show that quality of life metrics were favorable after LITT, including significant improvements in KPS scores and SF-36 scores at 12-months compared to pre-operative metrics. Regarding follow-up imaging findings in this select group of patients, 5/9 patients showed decreased enhancing lesion size at 1-year follow-up and in 5/6 patients, these findings persisted at 2-year follow-up. Five patients required open surgical resection of the treated lesion at an average time after LITT of 5.15 months because of progressive lesion growth and edema on serial imaging, but of note, in four cases there was evidence of recurrent WHO IV glioma. Among the 7 patients requiring steroids prior to LITT, 3 patients underwent successful progressive taper, while 3 stayed at the same dose, and 1 patient required increased dosing prior to death. Another group reported a similar safety profile in 10 patients with biopsy-confirmed RN, comprised of 4 previous diagnoses of high-grade glioma and 6 cases of metastatic lesions and also reported transient increases in lesion volume after LITT at 1-2 weeks before decreasing below original volumes at 6-month follow-up () [Citation35]. Together, the findings from these studies showed that LITT can be a viable treatment option for RN after SRS for both primary and secondary brain tumors. Local control of the ablated lesions was largely similar despite varying histologies between WHO III/IV gliomas and brain metastases. The accuracy of intraoperative biopsy was also brought into question in the patients with gliomas.
The two largest multicenter studies published on the topic of LITT for RN were reported by Chaunzwa et al. and Ahluwalia et al. [Citation36,Citation37] In the retrospective study by Chaunzwa, 30 patients across 4 institutions underwent LITT for recurrent lesions after prior SRS for brain metastases () [Citation36]. Among these patients, 19 (79%) had biopsy proven RN. Outcomes however were not divided by pathology. For both RN and tumors cases together, imaging findings following LITT were similar to prior reports with increases in T1-weighted post-contrast volumes but decreased edema on T2-weighted MRI within weeks after LITT. The majority of patients (73.3%) were weaned off of steroids with a median time to cessation of 4.5 weeks. Only 48% of the patients however showed improvement of their pre-operative symptoms after LITT. As expected, both successful weaning off of steroids and obtainment of symptomatic relief were correlated with greater reductions in perilesional edema on T2-weighted MRI but it was not determined if RN diagnosis contributed to improvement. RN pathology did not correlate with improved Karnofsky Performance Status (KPS) at follow-up or overall survival (OS) when compared with tumor. However, of the five patients who required salvage open surgical resection of their LITT-treated lesion, four exhibited recurrent tumor on their initial biopsy at time of LITT (with information unavailable for the fifth patient), while none of the patients whose biopsies showed RN required further surgical intervention. Survival after LITT was dependent upon pre-operative functional status, as measured by KPS, and related to control of systemic disease and whether cancer treatment was being administered at time of LITT. The study concluded that LITT was a well-tolerated and viable alternative intervention to open surgery for rapid relief of pre-operative symptoms in select patients, typically within 2 weeks of surgery, that correlated with successful steroid wean and significant reduction of perilesional edema on MRI T2 FLAIR. LITT may be better suited for patients with higher pre-operative KPS and as such, should be considered earlier prior to potential further clinical deterioration. Although LITT for patients with RN did not result in improved OS or KPS compared to those with recurrent tumor, local control appears to be superior for RN.
Following this study, results were reported from Laser Ablation After Stereotactic Radiosurgery (LAASR), a multicenter prospective phase 2 clinical trial of LITT in patients with radiographic progression after SRS for brain metastases () [Citation37]. This study comprised 42 patients, including 19 cases of biopsy proven RN with the remainder made up of 20 with recurrent tumor and 3 with no diagnosis. Validating previous retrospective data, LITT for either diagnosis was tolerated well with a median hospital stay of 2 days after surgery. Peri-procedural complications occurred in 12% of patients, related to worsened neurological deficits related to proximity to nearby eloquent brain areas, while one patient experienced new intracerebral hemorrhage without clinical consequence that was managed expectantly. OS among all patients was 86.5% at 12 weeks and 72.2% at 26 weeks. PFS and OS were significantly improved for patients with RN (PFS: 100% at 12 weeks, OS: 82.1% at 26 weeks) versus those with recurrent tumor (PFS: 54% at 12 weeks, OS: 64.5% at 26 weeks). In addition, while complete radiographic response was seen when total ablation was achieved in both RN and tumor cases, incomplete ablation still resulted in decrease or stabilization of lesion size when the pathology was RN but much more commonly resulted in tumor recurrence when the pathology was tumor. Given the small study cohort size however, RN diagnosis was not noted to impact KPS, quality of life, or neurocognitive outcomes. The study concluded that for patients with few options for further salvage treatment, LITT was a safe therapy, effective in stabilizing KPS, preserving quality of life, and effective in weaning pre-operative steroid dependence. Both PFS and OS were superior for patients with biopsy-confirmed RN at time of LITT versus those with recurrent tumor. Additionally, local control of histopathologically confirmed RN was excellent, even in cases of incomplete thermal ablation coverage. These last findings have resulted in two main changes in practice at our institution. Firstly intra-operative biopsy at time of LITT is essential to guide intra-operative and further follow-up care, including making total thermal coverage during LITT a goal, as well as post-operative adjunctive treatment in the form of radiation or systemic therapies for those with tumor. Indeed, this approach seems supported by a recent single-institution study of recurrent lesions after previous SRS for brain metastases, comprising 51 brain metastases and 31 cases of RN, which revealed poorer progression-free survival in incompletely ablated lesions, for recurrent tumor pathology, and for patients not receiving systemic therapy after LITT () [Citation38]. Secondly, given that best results are obtained when complete ablation is achieved for either pathology, we encourage our patients to undergo LITT for radiographically progressing lesions when they are small prior to development of symptomatology.
With the firm establishment of LITT as an efficacious tool for the treatment of RN, we sought to compare outcomes after LITT versus craniotomy (i.e. open surgical resection), based on our single institutional experience () [Citation29]. Our cohort was comprised of 33 (44%) cases of RN and 42 (56%) cases of recurrent tumor. There were no differences in patient demographics or disease specifics (i.e. systemic disease status between patient cohorts, based on modality of surgical intervention (LITT versus craniotomy) or pathology (RN versus recurrent tumor). Likewise, mean length of hospital stay, rates of neurological symptom improvement, and ability to wean off steroids at 1-month follow-up were similar among all groups. In both pathology groups, PFS and OS were significantly dependent upon pathology with patients with recurrent tumor having worse OS and PFS than those with RN. Treatment modality, however, did not have any significant effect on PFS or OS, even when adjusted for a size bias with larger lesions tending to undergo craniotomy. We did find that craniotomy was more effective for providing complete relief of pre-operative symptoms, regardless of pathology. Although limited by its single-institution nature and relatively small sample size, this study demonstrated equivalence of efficacy for LITT compared to open resection for the treatment of RN, as well as recurrent tumor after previous SRS for brain metastases.
Lastly, considering the efficacy of LITT for RN in brain metastases, we propose that given that the pathophysiology of RN is related to high dose radiation rather than underlying pathology, LITT might be successfully used for treatment of RN associated with other pathologies. We therefore recently reported successful cases of LITT for RN in cases of AVM and meningioma previously treated with radiosurgery () [Citation39,Citation40]. Digital subtraction angiography was performed in the former case to ensure complete AVM obliteration prior to LITT, and intra-operative biopsies in both cases demonstrated RN histopathology. Both cases yielded significant radiographic and clinical responses to laser ablation as expected. Further studies exploring LITT for RN in these pathologies will help determine its long-term role in this field.
Discussion
With increasing use of SRS and other forms of radiotherapy in the treatment of brain metastases, proper diagnosis and management of RN will remain paramount in the care of the cancer patient. The aforementioned studies have demonstrated that LITT is well-tolerated with low rates of peri-operative complications (<3–5%), the majority stemming from post-operative peri-lesional edema that can be successfully managed medically with steroid therapy. Patients are typically discharged within 24 to 48 h after surgery and exhibit symptom improvement typically within 2–4 weeks after surgery, accompanied by radiographic evidence of decreased perilesional edema. LITT can achieve robust local control of the targeted lesion, particularly for cases where complete lesion coverage is achieved on thermal mapping and a pathological diagnosis of RN is confirmed. In contrast, the majority of cases of local progression after LITT occurred in lesions confirmed to harbor recurrent tumor, while overall survival was typically measured by expiration from sequelae of systemic disease, rather than local progression of the ablated lesion.
The growing literature on LITT continues to define the optimal indications for this procedure in the patient with suspected cerebral RN. As previously mentioned, many radiographic cases of RN improve on surveillance imaging without need for treatment [Citation3,Citation22], and it remains as yet to be determined when it might be best to intervene in these patients. Most commonly, LITT is chosen in cases where patients have failed medical therapies, particularly for patients who exhibit steroid resistance or dependence, and are otherwise not candidates for an open surgery due to lesion location in a surgically inaccessible area (i.e. deep structures carrying high risk for surgical co-morbidities) or previous history of multiple surgeries that may portend high risk for re-operation. The aforementioned studies have not only demonstrated that LITT could result in the successful weaning off of steroids in the majority of patients, typically within weeks after surgery, accompanied by significant decreases in perilesional edema on follow-up MRI but also that in lesions that are completely ablated, complete resolution of the lesion can be achieved. It could be recommended then that, given its minimally invasive nature, LITT should be considered earlier in the course of lesion regrowth in order to achieve best outcome for local control. This may be particularly relevant for asymptomatic patients with lesions that are growing on serial imaging, who may benefit from simultaneous biopsy to differentiate between RN and recurrent tumor. Taking this concept one step further, given that RN rates are increased by the use of immunotherapy, in our institution, patients who have previously undergone SRS and are receiving immunotherapy are treated even when asymptomatic with LITT within 6–12 weeks of when their lesions start to regrow given both the good control rates of RN with LITT and the ability to completely ablate the lesion when it is small. In most of these patients, the use of steroids is avoided completely.
Additionally, there are instances where the decision for LITT over craniotomy may stem from patient preference, particularly for cases where the advantages of one therapy over the other are equivocal. Based on findings from our single-institution comparison of LITT versus craniotomy [Citation29], the ideal scenario for LITT may be in asymptomatic patients in whom biopsy for diagnosis of a regrowing surgically accessible lesion is required to guide further therapy.
Significant debate remains regarding the need for biopsy prior to LITT. The studies discussed in this review comprise only biopsy-confirmed cases of RN. However, many have argued that recurrent lesions after prior SRS for brain metastases may represent a mixture of both radiation necrosis and recurrent tumor pathologies, subject to local sampling bias and thus minimizing the diagnostic accuracy of intra-operative biopsy [Citation41–43]. While the aforementioned studies suggest LITT may result in similar peri-procedural outcomes in terms of overall symptom improvement and steroid weaning, the underlying pathology has significant implications. Not only does the pathology itself affect both PFS and OS [Citation29,Citation30,Citation36,Citation37], but in incompletely ablated lesions, knowledge of the pathology based on intra-operative biopsy, which remains the gold standard for diagnosis, may impact post-operative management [Citation21,Citation43]. For instance, a diagnosis of RN for patients on immunotherapy for melanoma may necessitate cessation of immunotherapy and initiation of steroid therapy. In contrast, an intra-operative biopsy result of viable tumor may guide more aggressive thermal ablation of the lesion or following the LITT procedure with consolidative radiation therapy or medical therapies, supported by prior evidence that adjunctive treatment after LITT for recurrent tumors may prolong local progression-free and overall survival [Citation38]. Further data are needed to determine the value of lesion biopsy at time of LITT but distinguishing between RN and recurrent tumor at time of surgery may be valuable for both intra-operative decision-making as well as post-operative management of intracranial and systemic disease.
Although most cases of RN have been described in cases of radiation of brain metastases, RN has also been reported extensively in the neurosurgical literature for gliomas [Citation44–46] and arteriovenous malformations [Citation5,Citation47,Citation48]. and likely as peritumoral edema for SRS-treated meningiomas. However, studies for LITT in cases of suspected RN after radiation of these pathologies have been markedly absent and are an area of much needed further study. Our institution has preliminary experience in the treatment of RN with LITT outside of prior radiation to brain metastases, and we suspect LITT may be equally as efficacious in these cases of RN, irrespective of original pathology.
Conclusions
LITT appears to be a viable surgical option for patients with suspected RN after previous radiosurgery for brain metastases with low rates of peri-procedural morbidity, short hospitalization stays. Its use can result in an ability to wean off of steroids with acceptable rates of symptom improvement. LITT can be considered as an equally efficacious alternative surgical therapy to open resection in asymptomatic patients with surgically accessible lesions less than 3 cm diameter, for whom histopathological diagnosis and local control of the lesion are the main goals of surgery. Further data are needed to determine if LITT can be similarly applied in cases of RN after radiation for other pathologies, including primary brain tumors and arteriovenous malformations.
Disclosure statement
Dr. Chiang is a consultant for Monteris Medical Inc. (Minnesota, USA) and speaker for BrainLab, Inc. (Munich, Germany). Otherwise, we have no further disclosures.
References
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–1228.
- Ali FS, Arevalo O, Zorofchian S, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21(8):66.
- Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502.
- Akanda ZZ, Hong W, Nahavandi S, et al. Post-operative stereotactic radiosurgery following excision of brain metastases: a systematic review and meta-analysis. Radiother Oncol. 2019;142:27–35.
- Pollock BE, Link MJ, Branda ME, et al. Incidence and management of late adverse radiation effects after arteriovenous malformation radiosurgery. Neurosurgery. 2017;81(6):928–934. Dec 1
- Novotny J, Jr., Kollova A, Liscak R. Prediction of intracranial edema after radiosurgery of meningiomas. J Neurosurg. 2006;105:120–126.
- Conti A, Pontoriero A, Siddi F, et al. Post-treatment edema after meningioma radiosurgery is a predictable complication. Cureus. 2016;8(5):e605.
- Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. JCO. 2002;20(9):2267–2276.
- Corn BW, Yousem DM, Scott CB, et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02). Cancer. 1994;74(10):2828–2835.
- Sneed PK, Mendez J, Vemer-van den Hoek JG, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. JNS. 2015;123(2):373–386.
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? JNS. 2016;125(1):17–23.
- Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12(11):1965–1969.
- Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–3353.
- Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23 Pt (1):297–330.
- Woodworth GF, Garzon-Muvdi T, Ye X, et al. Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol. 2013;113(3):485–493.
- Tihan T, Barletta J, Parney I, et al. Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol. 2006;37(3):272–282.
- Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99(1):81–88.
- Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112(9):758–765.
- Chuang MT, Liu YS, Tsai YS, et al. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PloS One. 2016;11(1):e0141438.
- Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395.
- Narloch JL, Farber SH, Sammons S, et al. Biopsy of enlarging lesions after stereotactic radiosurgery for brain metastases frequently reveals radiation necrosis. Neuro Oncol. 2017;19(10):1391–1397.
- Glass JP, Hwang TL, Leavens ME, et al. Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer. 1984;54(9):1966–1972.
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180–188.
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495.
- Furuse M, Nonoguchi N, Kuroiwa T, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis(dagger). Neurooncol Pract. 2016;3(4):272–280.
- Woo E, Lam K, Yu YL, et al. Cerebral radionecrosis: is surgery necessary? J Neurol Neurosurg Psychiatry. 1987;50(11):1407–1414.
- Williamson R, Kondziolka D, Kanaan H, et al. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–366.
- Glantz MJ, Burger PC, Friedman AH, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–2027.
- Hong CS, Deng D, Vera A, et al. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142(2):309–317.
- Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113(3):495–503.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(suppl_1):ONS21–28; discussion ONS28-29.
- Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–950.
- Rahmathulla G, Recinos PF, Valerio JE, et al. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200.
- Smith CJ, Myers CS, Chapple KM, et al. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(suppl_1):S59–S72.
- Rammo R, Asmaro K, Schultz L, et al. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neurooncol. 2018;138(3):609–617.
- Chaunzwa TL, Deng D, Leuthardt EC, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63.
- Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2019;130(3):804–811.
- Bastos DCA, Rao G, Oliva ICG, et al. Predictors of local control of brain metastasis treated with laser interstitial thermal therapy. Neurosurgery. 2019. DOI:10.1093/neuros/nyz357
- Hong CS, Cord BJ, Kundishora AJ, et al. MRI-guided laser interstitial thermal therapy for radiation necrosis in previously irradiated brain arteriovenous malformations. Pract Radiat Oncol. 2020. DOI:10.1016/j.prro.2020.02.003
- Hong CS, Beckta JM, Kundishora AJ, et al. Laser Interstitial Thermotherapy for Treatment of Symptomatic Peritumoral Edema After Radiosurgery for Meningioma. World Neurosurg. 2020;136:295–300.
- Torcuator RG, Hulou MM, Chavakula V, et al. Intraoperative real-time MRI-guided stereotactic biopsy followed by laser thermal ablation for progressive brain metastases after radiosurgery. J Clin Neurosci. Feb 2016;24:68–73.
- Hernandez RN, Carminucci A, Patel P, et al. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019;85(1):84–90.
- Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457.
- Strauss SB, Meng A, Ebani EJ, et al. Imaging glioblastoma posttreatment: progression, pseudoprogression, pseudoresponse, radiation necrosis. Radiol Clin North Am. 2019;57(6):1199–1216.
- Ellingson BM, Chung C, Pope WB, et al. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504.
- Delgado-Lopez PD, Rinones-Mena E, Corrales-Garcia EM. Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, pseudoprogression, pseudoresponse and radionecrosis. Clin Transl Oncol. 2018;20(8):939–953.
- Kano H, Flickinger JC, Tonetti D, et al. Estimating the risks of adverse radiation effects after Gamma knife radiosurgery for arteriovenous malformations. Stroke. 2017;48(1):84–90.
- Cohen-Inbar O, Starke RM, Paisan G, et al. Early versus late arteriovenous malformation responders after stereotactic radiosurgery: an international multicenter study. J Neurosurg. 2017;127(3):503–511.
