Abstract
Introduction
High-intensity focused ultrasound (HIFU) for pancreatic cancer is a growing therapeutic field which has been proven to reduce cancer pain and provide a local tumor control additionally to standard palliative care. However, less is known about the multidisciplinary and especially anesthesiological management of HIFU treatment although an interdisciplinary approach is crucial for treatment success.
Material and methods
Anesthesiological and radiological records of 71 HIFU-treated pancreatic cancer patients were analyzed with regard to the following items: intervention time, sonication time, total energy, anesthesia time, peri-interventional medication, body temperature maximum and minimum, pain scores before and 1 day, 6 weeks and 3 months after intervention, peri-interventional complications. Effects on pain scores were estimated with a mixed panel data model. Bivariate associations between interventional variables were examined with the Spearman’s correlation.
Results
HIFU treatment was performed without major adverse events. Peri-procedural hyperthermia >37.5 °C occurred in 2 patients, hypothermia <35 °C in 8 cases. Interventional variables did not correlate significantly with pain scores, opioid dose, nor body temperature. 85.5% of patients experienced significant early pain relief within the first week after intervention. Post-interventional pain relief is associated with morphine equivalent opioid dose (p = 0.025) and treatment time (p = 0.040).
Conclusion
While HIFU can be considered safe and effective treatment option, procedure-associated pain and temperature management represent challenges for the interdisciplinary HIFU intervention team. Especially short-term pain relief depends on the combined effort of the radiologist and anesthesiologist.
Introduction
Worldwide pancreatic carcinoma (PaC) is the 8th leading cause of death from cancer in men and the 9th leading cause of death from cancer in women [Citation1]. With a 5-year survival rate of 8% PaC has a very poor prognosis [Citation2]. At the time of initial diagnosis more than 80% of patients have an advanced disease and are considered inoperable either due to local infiltration of the mesentery root and mesenteric vessels, or due to the presence of metastases. Current standard therapy in advanced PaC using chemotherapy or radiochemotherapy has only limited efficacy in local tumor control and reduction of leading incapacitating symptoms, i.e., cancer pain [Citation2,Citation3].
Some minimally invasive thermal and non-thermal local treatment procedures in advanced PaC have been introduced for tumor mass reduction and symptom alleviation in recent years [Citation4]. One of these promising methods is ablation with high-intensity focused ultrasound (HIFU). HIFU is a noninvasive thermal procedure and uses ultrasound waves that can be focused on a tumor tissue inside the human body without damaging surrounding structures [Citation5–7]. At the focal point of the ultrasound beam the energy is sufficient to cause local cell death. In contrast to other local ablative methods, the main advantage of HIFU lies in its noninvasiveness: the treatment does not involve the use of needles, probes, or electrodes. With a very low complication rate, HIFU provides an effective treatment option in PaC patients in combination with standard palliative care to reduce pain and provide local tumor control [Citation8–11]. Despite of all its advantages and the clinical benefit for affected patients, the procedure itself has a few special features and requires a precise coordination between interventionalist, e.g., interventional radiologist or surgeon, and anesthesiologist during ablation to enhance accuracy of treatment and ensure patient safety. In a setting outside the operation room, the anesthesiologist is confronted with a specific patient positioning and has to adjust the equipment, ventilation strategy and drugs needed to reduce and prevent potential anesthetic and procedure-related complications.
Based on data of more than hundred patients with advanced PaC treated with HIFU during the last five years in our hospital, we analyzed relevant factors in multidisciplinary management particularly in relation to radiological as well as anesthesiological treatment in order to faciliate treatment, anticipate and avoid potential physiological derangements or injury and coping with the transient post-procedural pain.
Material and methods
Patient selection
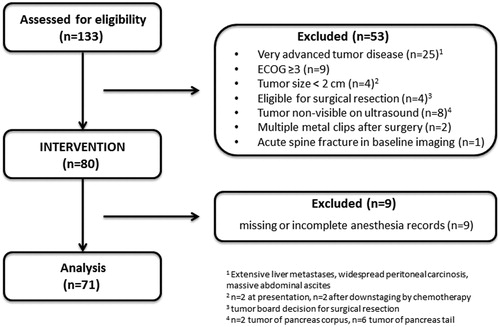
Charts of 80 consecutive patients with local advanced pancreatic cancer treated with US-guided HIFU between 2014 and 2017 were reviewed. Patients with missing or incomplete anesthesia or intervention records (n = 9) were excluded from analysis. Regarding eligibility for HIFU treatment, specific inclusion and exclusion criteria were considered according to the clinical routine. Eligibility for HIFU was confirmed previous to the intervention in a multidisciplinary tumor board. At time of presentation, all patients had exhibited cancer-related pain and/or local tumor growth leading to disease-associated findings as mesenteric or splenic vein occlusion, cholestasis, duodenal stenosis etc. The presence of metastasis or biliary drainages was not taken as an exclusion criterion. Following inclusion criteria should be fulfilled: [Citation1] 18 years or older; [Citation2] histological/cytological diagnosis of pancreatic adenocarcinoma; [Citation3] locally advanced tumor with a diameter ≥2cm verified either by computed tomography (CT) or magnetic resonance imaging (MRI) within 8 weeks prior to treatment; [Citation4] tumor sufficiently visible on ultrasound; [Citation5] estimated life expectancy of more than 3 months; [Citation6] Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Main exclusion criteria included: [Citation1] eligibility for surgical resection; [Citation2] non-eligibility for general anesthesia; [Citation3] tumor not sufficiently visible on ultrasound; [Citation4] extensive scarring along the acoustic path. shows a consort diagram of the study. For this retrospective analysis, informed consent was waived by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki.
Anesthesia
Preparation for anesthesia including informed consent was conducted according to current guidelines in our preoperative evaluation clinic at least 24 h prior to intervention. Patients were to remain fasted before the intervention according to current anesthesia guidelines [Citation12]. Once in the intervention room, standard anesthesia monitoring was established with ECG, SpO2 and noninvasive blood pressure measurement. Intravenous access was established with a single 1.1 to 1.3 mm peripheral cannula. Anesthesia induction was performed on the intervention table. For anesthesia induction, patients received propofol 2 mg/kg bodyweight and 0.1–0.2 mg of fentanyl or remifentanil 1 µg/kg bodyweight via continuous infusion. Neuromuscular blockade was achieved with cis-atracurium 0.1 mg/kg bodyweight or rocuronium 0.5 mg/kg bodyweight to facilitate orotracheal intubation. A nasogastric tube was inserted followed by the injection of 80 ml of simeticon to enhance ultrasound visibility of the pancreas and other organs in the upper abdomen. A urinary catheter was placed which was also used for temperature monitoring. Anesthesia was maintained with continuous propofol in decreased doses, usually starting with 10 mg/kg bodyweight/h, in combination with repeated doses of 0.05–0.1 mg fentanyl or continuous remifentanil 500–2000 µg/h. In most cases, fentanyl was used for induction and then switched to remifentanil. After induction, patients were placed in a prone position. The patients were suspended in a frame with the abdomen in a waterbath which contains the ultrasound diagnostic probe and the therapeutic transducer. A watertight foil was wrapped around arms and legs to avoid spilling of the water. Continuous warming of the back of the patient was performed with a convective air warming system once the patient was positioned correctly. Post-interventional analgesia was started during the intervention procedure with intravenous administration of metamizol and boluses of piritramide according to the clinical judgment of the anesthesiologist. Repeated piritramide boluses were administered after the intervention if the patient experienced pain. Some patients received up to 60 mg of Piritramide thus.
Pretreatment procedures and HIFU intervention
Pretreatment evaluation included medical history, physical examination and laboratory tests. Due to the lesion proximity to some parts of the gastrointestinal tract, e.g., stomach and duodenum, a specific bowel preparation similar to that prior to coloscopy is required to avoid possible complications. The bowel preparation starts one day before the procedure and consists of liquid food, no gas producing food, fasting for 12 h, and use of laxatives. On the treatment day, a stomach tube is placed to apply anti-foaming agents (e.g., simethicone) binding residual air bubbles in the stomach and bowel. The skin of the patient´s upper abdominal wall is shaved, degreased and degassed.
US-guided HIFU ablation was performed using the Focused Ultrasound Tumor Therapeutic System (JC HIFU, Chongqing HAIFU Technology, China) equipped with a 1–8 MHz ultrasound imaging device (MyLab 70, Esaote, Italy) for real-time guidance. The therapeutic ultrasound beam was transmitted by a 20 cm diameter ceramic transducer with a focal length of 15 cm, operated at a frequency of 0.8 MHz. The design of the HIFU system with the water basin and transducer below the table requires a treatment in prone position. Water is used as a coupling medium between the ultrasound device and patient´s skin. For planning and ablation a sagittal scanning mode is used; US energy is delivered to a circumscribed focal area using a dot mode. Repeated cycles of 1 s sonication followed by a 3 s break were delivered at each focal point. In case of visible grey-scale changes in the target area suggesting effective ablation or after a minimum of 50 s sonication time the transducer was moved to the next focal zone in the same slide, then in adjacent slides in order to achieve volume ablation. A safety margin of 1 cm to existing stents and structures at risk (bowel, stomach) was maintained to prevent potential damage by local temperature increase.
To avoid injury of gastrointestinal structures at risk in the acoustic pathway, a water-filled balloon is placed between patient´s upper abdominal wall and transducer. During HIFU ablation, the skin is cooled by water in the water basin and repeatedly checked by palpation after each 150 s sonication time.
Analysis and statistics
Data were retrospectively reviewed with regard to various anesthesiological and procedural parameters: intervention time, sonication time, therapeutic ultrasound energy used for ablation (total energy), anesthesia time, medication, body temperature maximum and minimum, pain scores before and 1 day, 6 weeks and 3 months after intervention (numeric rating scale, NRS, 0–10; 0 ‘no pain at all’, 10 ‘most intense pain imaginable’). Data were analyzed using Stata version 14 (Stata Corp, Lakeway Drive College Station, US). Primary statistical evaluation of NRS values was performed at baseline and follow-up (1 day, 6 weeks, 3 months) using a mixed panel data model. To estimate bivariate associations between interventional parameters, body temperature, peri-interventional medication and changes in pain score a Spearman´s correlation was used. Effect size and impact of different factors on pain score differences were estimated using a robust linear model (R2, partial Eta2, coefplot). Values of p < 0.05 were considered statistically significant.
Results
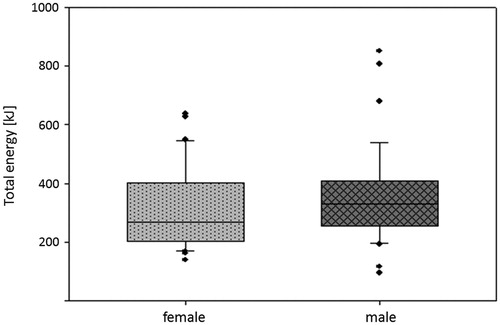
Charts with anesthesiological and radiological records of 71 HIFU-treated patients with advanced pancreatic cancer (UICC stage III, IV, local tumor recurrence after previous surgery) were reviewed with regard to different parameters as described above. Opioid doses were converted to morphine equivalents per kilogram of body weight. In , patient- and diagnosis-related characteristics are summarized. gives an overview of interventional and anesthesia data. Average sonication time was 936 ± 366 s, total applied energy 344 ± 152 kJ, power range 200–400 W, total treatment time 124 ± 45 min. There was no need for peri-interventional discontinuation of anticoagulant medication as it is the case in surgery.
Table 1. Patient demographics and anesthesia-related data.
Table 2. Intervention- and anesthesia-related data.
No major complications or death occurred in association with the HIFU procedure or anesthesia. Two patients presented with difficult airway and videolaryngoscopy had to be used. Hypothermia (<35 °C) during the interventional procedure occurred in 8 patients; only one patient had to be transferred to the post anesthesia care unit for rewarming while still ventilated. Hyperthermia (>37.5 °C) occurred in two patients. A second patient experienced hemodynamic instability after the intervention and was moved to the intensive care unit with vasopressor therapy.
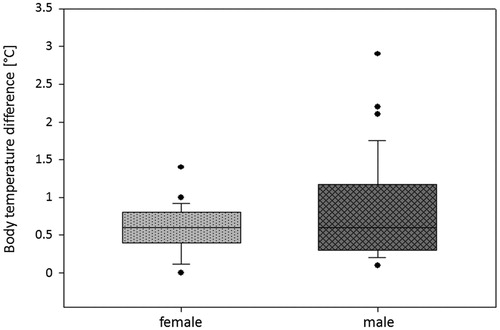
In 90% of patients a temperature difference of 1.3 °C between maximum and minimum body temperature was shown during the HIFU procedure. The observed temperature differences (median 0.6 °C, mean 0.7 °C, SD ±0.5) did not significantly correlate neither with the applied energy (rs = 0.086, p = 0.671) nor with the sonication or treatment time (rs=0.111, p = 0.582 and 0.079, p = 0.696, respectively). BMI (rs= −0.119, p = 0.554) also seems not to have an influence on temperature ranges. Peri-procedural differences in body temperature are presented in .
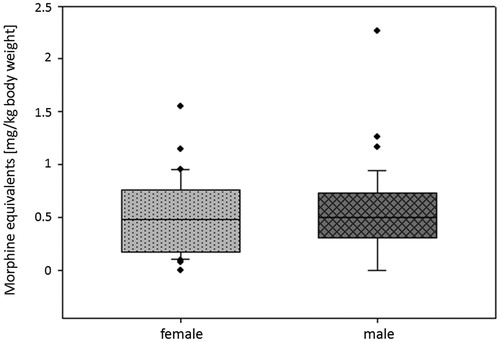
Similar to the findings regarding the temperature differences, the applied morphine equivalents (per kilogram of body weight, ) did not show any significant correlations to the interventional parameters (rs< −0.1, n.s., in all three parameters) and BMI (rs= −0.296, p = 0.134).
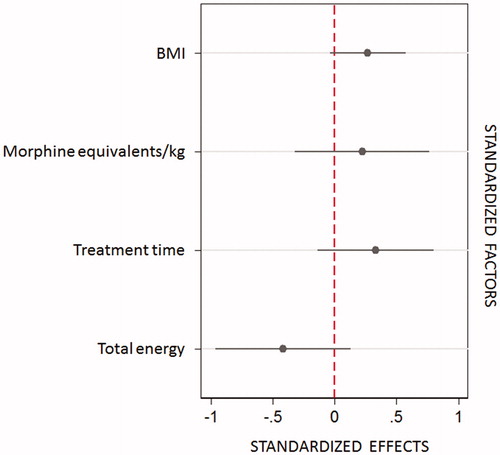
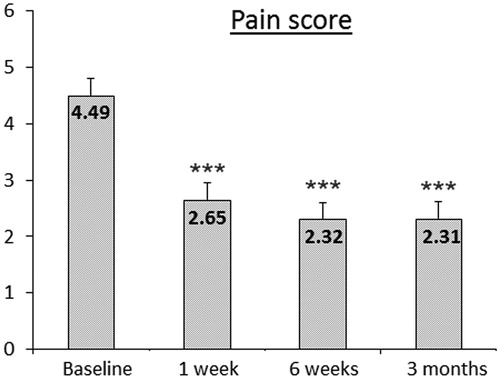
Before HIFU treatment, all patients suffered from cancer-related abdominal/back pain. After a single session of US-guided HIFU, cancer pain was considerably reduced in nearly 86% of patients, which lasted during follow-up. Pain scores during follow-up significantly decreased compared to baseline (mixed model, p < 0.001, ). The applied total energy in these patients during HIFU procedure () was associated with a trend of stronger and longer lasting effect on pain ().
Figure 4. Changes in pain score (NRS, numeric rating scale, range 0–10; 0 ‘no pain at all’, 10 ‘most intense pain imaginable’) after HIFU treatment of pancreatic cancer patients (n = 71). Average pain relief of 41% at 6 weeks and 48% at 3 months follow-up compared to baseline was observed after HIFU treatment.
No considerable correlation was found between the peri-interventional morphine administration and changes in NRS at follow-up time points 1 day, 6 weeks, and 3 months compared to baseline (rs=0.294, p = 0.137; rs=0.263, p = 0.186, and rs=0.1648, p = 0.412, respectively). Using a robust linear model across pain score differences in all patients, the highest impact for the observed pain relief between baseline and 1 day post-HIFU is referred to the morphine equivalent dose (p = 0.025) and the treatment time (p = 0.040). In contrast, no significant impact of these parameters on pain reduction at 6 week- and 3-month follow-up were found. The model explains about 21.7% of the short-term pain score change (R2), 12.2% (partial Eta2) of this change are attributable to the effect of the morphine dose. One day after the HIFU procedure, 85.5% of treated patients experienced a significant pain relief (p < 0.001). In contrast, 15% of very severely affected patients showed initially an increase in pain score 1 day post-HIFU, this decreased to baseline values during the follow-up period in most cases. Interestingly, a significantly different application of morphine equivalents was found between these two groups: patients with reduction in pain score on the first post-interventional day received 0.432 mg morphine equivalent/kg, and patients with initial increase in pain score 0.627 mg (p = 0.021).
Discussion
Even though there is an increasing interest in minimally and noninvasive local ablative procedures in pancreatic carcinomas, there is still a lack of knowledge on the influence of intra- and peri-procedural treatment measures on the outcome. To date, there is only one review article partially covering not only interventional, but also anesthesiological aspects of HIFU procedures [Citation13].
As far as inoperable pancreatic cancer is concerned, the locally invasive tumor growth causes a plethora of symptoms ranging from bile congestion to tumor-associated pain and substantial reduction of health-related quality of life [Citation14,Citation15]. Cancer pain is reported in around 80% of all cases of pancreatic cancer, making it one of the most prevalent symptoms [Citation1]. It is caused not only by the compression by growing tumor masses, but also by the local infiltration of nerve fibers around the celiac trunk. Despite systemic therapies (chemotherapy, analgesic medication with high potency) with a wide range of side effects, in most cases it is not possible to manage these symptoms adequately which results in potentially detrimental effects on patient’s life. To combat this problem, more and more local ablative procedures are being utilized in the symptomatic treatment of locally invasive pancreatic carcinoma [Citation4,Citation16–18]. These local ablative techniques can be divided into two groups: thermal and non-thermal methods. The thermal methods summarize microwave ablation, radiofrequency ablation, and cryoablation, the non-thermal include the irreversible electroporation (IRE, NanoKnife®) as well as the stereotactic body radiation therapy (SBRT, CyberKnife®). Most of these methods, except for the radiotherapy, need insertion of probes, electrodes or needles to achieve their functions. All these procedures are invasive or minimally invasive which directly increases the risk of complications. In majority of cases, an open surgical approach is used; in rare cases percutaneous approach has been tried but with a different rate of success. In all methods, insertion of needles, probes or electrodes is necessary which needs well-trained staff and makes them not available in many hospitals. All this taken together increases the risk of complications like bleeding, organ injury, or needle track seeding. In some methods such as RFA the treatment efficiency is reduced due to the heat-sink effect near the large vessels.
The high-intensity focused ultrasound (HIFU) belongs to the thermal local ablative methods. In addition to heat generation causing coagulation necrosis [Citation6,Citation19,Citation20], the mechanisms of action also include cavitation and potential immunological effects [Citation21–23]. The noninvasive approach of HIFU makes it an innovative and potent treatment modality for local tumor control and symptomatic improvement in inoperable pancreatic cancer. The HIFU effectiveness on pancreatic cancer pain has been repeatedly shown [Citation7,Citation8,Citation24–27]. While the method is per se noninvasive, the procedure itself is often associated with peri-procedural pain for the patient. Therefore, general anesthesia for ablation of tumor lesions in the upper abdomen is crucial toward improvement of treatment accuracy. In principle, controlled ventilation is recommended [Citation13], as through breathing movements displacement of ventrally located structures such as the stomach, duodenum or colon can occur, even though respiration usually results in less movement of the retroperitoneal located pancreas. Air in the stomach or displaced bowel loops requires a prompt reaction by the interventionalist in order to interrupt the therapy if necessary and select a different acoustic window to avoid injuries.
The effect of anesthetic practice on post-interventional outcome currently remains unknown. It seems from our results that some patients experience a great, treatment-associated pain for about 6–24 h after the intervention while others do not. This peri-procedural pain is due to a high extent on the procedure itself and in majority of cases short-lasting. It can be explained by the treatment-associated edema of the upper abdominal wall, in some cases also of abdominal fat tissue and parts of the stomach and duodenum, which lie along the acoustic pathway. To counteract the post-interventional pain, different doses of opioids are used according to expected patient requirements as judged by the anesthesiologist during the procedure and according to pain scores after anesthesia. Tumor size or energy applied do not seem to play a significant role in this respect. Due to the US-guided technology used, it is currently not technically possible to state which energy was ultimately released in the focus or in the tumor or which temperature was actually reached. For the anesthesiologist this means that post-interventional pain in HIFU-treated patients is hard to predict. Novel approaches may be applied, such as the use of nociception monitors that can even be used during general anesthesia so that early and objective measurements could be provided to ensure an adequate opioid therapy [Citation28,Citation29]. However, this still remains to be proven.
With the advent of noninvasive therapeutic approaches, today’s anesthesiologist faces an increasing number of workplaces outside the operating room. HIFU treatment is one of these. As in the operative setting, an interdisciplinary team approach is necessary to provide patient safety and therapeutic success. Hardly any information is available so far about the implications of HIFU therapy for anesthesiologists or considerations for the interdisciplinary team radiology/anesthesiology. From previous reports the HIFU ablation is considered as a safe, low-risk procedure with only very low rate of severe complications [Citation30]. Our data on 71 patients covering a time-span of five years confirm this finding, since only two patients had to be admitted to the ICU, one for vasopressor treatment and the other due to hypothermia.
Airway management during HIFU may represent a problem, since radiology workplaces are located at a distance from central operating room facilities and support with equipment or personnel may be short. Although the HIFU patient by himself is not associated with a higher risk of a difficult airway, sufficient emergency equipment such as supraglottic devices and videolaryngoscopy are needed at the HIFU anesthesia workplace, as it should be the case in every anesthesia workplace [Citation31]. In two of our patients we experienced unexpected difficult intubation which was managed with videolaryngoscopy.
It seems that in the HIFU setting, temperature management represents a challenge for both the anesthesiologist as well as the interventional radiologist. In our patient cohort, 8 patients experienced hypothermia and 2 patients hyperthermia. It is the underlying principle of HIFU therapy to cause local hyperthermia inside the tumor in order to destroy this tissue with the focused energy of the ultrasound beam. On the other side, the abdominal wall has to be cooled during the procedure in order to prevent skin burn and injury. This is especially important in case of previously damaged abdominal wall, for example in patients after previous surgery causing extensive scarring in the acoustic pathway. We could show that the applied energy has some, although limited effect on body temperature. The importance of temperature monitoring is also suggested by Yao et al. [Citation13] as well as the risk of local thermal injury. Hypothermia is even more prevalent in HIFU-treated patients. During the procedure, 27 (14%) of the patients experienced hypothermia as defined by a core body temperature <36 °C. In 8 patients, body temperature even dropped to <35 °C. Since the patient is suspended in a water bath, water spilling is possible and may lead to additional temperature loss by evaporation despite constant warming, as we have experienced in some patients. All this underlines the great importance of an interdisciplinary team approach and adequate communication in order to provide patient safety.
In summary, HIFU treatment in pancreatic cancer can be considered safe from an interdisciplinary standpoint. Peri-procedural pain and temperature management are critical points where an adequate communication between the anesthesiologist and the interventional radiologist is mandatory. More innovative approaches may be necessary to counteract the short-lasting peri-procedural pain in these special patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049.
- Carrato A, Falcone A, Ducreux M, et al. A systematic review of the burden of pancreatic cancer in Europe: real-world impact on survival, quality of life and costs. J Gastrointest Canc. 2015;46:201–211.
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–2615.
- Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced pancreatic cancer: high-intensity focused ultrasound (HIFU) and other local ablative therapies. Rofo. 2019;191:216–227.
- Gao HF, Wang K, Meng ZQ, et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60:1906–1910.
- Sofuni A, Moriyasu F, Sano T, et al. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:295–303.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4:294–302.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound in patients with advanced pancreatic cancer. Ultraschall Med. ahead of print. 2019;40:625–637.
- Marinova M, Rauch M, Mucke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26:4047–4056.
- Marinova M, Strunk HM, Rauch M, et al. High-intensity focused ultrasound (HIFU) for tumor pain relief in inoperable pancreatic cancer: evaluation with the pain sensation scale (SES). Schmerz. 2017;31:31–39.
- Strunk HM, Henseler J, Rauch M, et al. Clinical use of high-intensity focused ultrasound (HIFU) for tumor and pain reduction in advanced pancreatic cancer. Fortschr Röntgenstr. 2016;188:662–670.
- De Hert S, Staender S, Fritsch G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2018;35:407–465.
- Yao CL, Trinh T, Wong GT, et al. Anaesthesia for high intensity focused ultrasound (HIFU) therapy. Anaesthesia. 2008;63:865–872.
- Vedie AL, Neuzillet C. Pancreatic cancer: best supportive care. Presse Med. 2019;48:e175–e185.
- Vincent A, Herman J, Schulick R, Hruban RH, et al. Pancreatic cancer. Pancreatic Cancer. Lancet. 2011;378:607–620.
- Ierardi AM, Lucchina N, Bacuzzi A, Marco de C, et al. Percutaneous ablation therapies of inoperable pancreatic cancer: a systematic review. Ann Gastroenterol. 2015;28:431–439.
- Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119:483–498.
- Keane MG, Bramis K, Pereira SP, et al. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:2267–2278.
- Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967–975.
- Hynynen K, Lulu BA. Hyperthermia in cancer treatment. Invest Radiol. 1990;25:824–834.
- Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34.
- Liu F, Hu Z, Qiu L, et al. Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation. J Transl Med. 2010;8:7.
- Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 2007;23:165–171.
- Dimitrov D, Andreev T, Feradova H, et al. Multimodality treatment by FOLFOX plus HIFU in a case of advanced pancreatic carcinoma. A case report. JOP: Journal of the Pancreas. 2015;16:66–69.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27:101–107.
- Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol. 2006;15:26–35.
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10:123–129.
- Boselli E, Daniela-Ionescu M, Begou G, et al. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br J Anaesth. 2013;111:453–459.
- Jeanne M, Clement C, De Jonckheere J, et al. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. 2012;26:289–294.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40:1080–1086.
- Law JA, Broemling N, Cooper RM, et al.; for the Canadian Airway Focus Group. The difficult airway with recommendations for management–part 1–difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anesth/J Can Anesth. 2013;60:1089–1118.