Abstract
Background
Based on patient and tumor characteristics, some authors favor laparoscopic microwave ablation (LMWA) over the percutaneous approach (PMWA) for treatment of hepatocellular carcinoma (HCC). We compared the two techniques in terms of technique efficacy, local tumor progression (LTP) and complication rates.
Study design
A retrospective comparative analysis was performed on 91 consecutive patients (102 HCC tumors) who underwent PMWA or LMWA between October 2014 and May 2019. Technique efficacy at one-month and LTP at follow-up were assessed by contrast-enhanced CT/MRI. Kaplan–Meier estimates and Cox regression were used to compare LTP-free survival (LTPFS).
Results
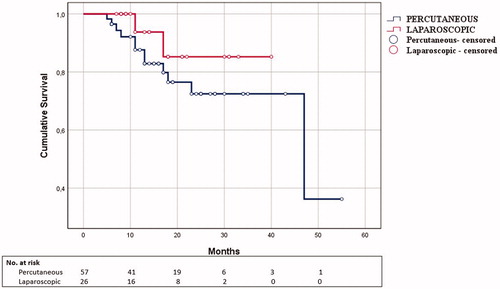
At baseline analysis, LMWA group showed higher frequency of multinodular disease (p < .001) and average higher energy delivered over tumor size (p = .033); PMWA group showed higher rates of non-treatment-naïve patients (p = .001), patients with Hepatitis-C (p = .03) and BCLC-A1 disease (p = .006). Technique efficacy was not significantly different between the two groups (p = .18). Among effectively treated patients, 75 (83 tumors) satisfied ≥6 months follow-up, 54 (57 tumors) undergoing PMWA and 21 (26 tumors) LMWA. LTP occurred in 14/83 cases (16.9%): 12 after PMWA (21.1%) and 2 after LMWA (7.7%). At univariate analysis, technique did not correlate to LTPFS (p = .28). Subgroup analysis showed a trend toward worse LTPFS after PMWA of subcapsular tumors (p = .16). Major complications were observed in six patients (6.6%), 2 after PMWA and 4 after LMWA (3.2% vs 14.3%, p = .049).
Conclusions
Technical approach did not affect LTPFS. Complications were reported more frequently after LMWA. Despite higher complication rates, LMWA seems a valid option for treatment of subcapsular tumors.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related death [Citation1] and therapeutic approach is often challenging. In a struggle toward less invasive therapies, thermal ablation has been recognized by EASL–EORTC guidelines as elective curative treatment and as a bridge to transplant of early HCC in patients who are not candidates for resection due to poor liver function, portal hypertension or elevated bilirubin [Citation2]. Thermal ablation techniques are also strongly recommended for treatment of very early HCCs (single lesion, up to 2 cm) even in patients amenable for surgery [Citation3].
In the past decade, microwave ablation (MWA) has emerged as a novel promising technique versus the conventionally used radiofrequency ablation (RFA) [Citation4]. The main advantages are more predictable and larger ablation zones over a shorter time with comparable complication rate [Citation5,Citation6]. A meta-analysis even suggested better outcomes after MWA of larger neoplasms [Citation7]. As for RFA, MWA has been used in both percutaneous and intraoperative approaches [Citation8,Citation9].
To date, few studies have compared laparoscopic and percutaneous ablation of HCCs, mostly using RFA [Citation10,Citation11] and no comparative data are available regarding the use of MWA. No clear indications are available regarding tumor or patient characteristics to guide the choice toward percutaneous or laparoscopic approach. The decision is therefore left to local multidisciplinary teams (MDT), resulting in great heterogeneity based on local expertise.
The aim of this retrospective study was to investigate differences between percutaneous and laparoscopic MWA in a cohort of patients with early stage HCC in terms of safety, technique efficacy and local tumor progression.
Methods
Study design
A retrospective analysis was performed on 91 consecutive patients with HCC who underwent MWA between October 2014 and May 2019. Following EC approval, data regarding patients and procedures were collected from an Institutional database (IRCCS Ospedale San Raffaele, Milan and Fondazione Poliambulanza Istituto Ospedaliero, Brescia).
The population under study had to fulfill the following inclusion criteria: clinical and imaging evidence of HCC (radiological diagnosis of tumors on pre-operative dynamic contrast-enhanced CT or MRI with a liver-specific acquisition protocol); disease stage 0, A, B deemed amenable of curative treatment (ablation alone or ablation combined with surgery); ablation within one month of last imaging. Patients were divided into those who underwent percutaneous ablation (PMWA group) and those treated with laparoscopic microwave ablation (LMWA group). Tumors undergoing effective treatment and imaging follow-up of at least 6 months were included in the survival analysis.
Preoperative planning
The indication for ablation was decided during weekly MDT, which included at least one radiologist, hepatobiliary surgeon, hepatologist, medical oncologist and radiation oncologist. Approach, percutaneous or laparoscopic, was agreed upon based on evaluation and consensus of both the interventional radiologist (IR) and surgeon on a case-by-case basis. The intraoperative approach was generally favored in presence of one or more of the following conditions: multifocal disease, sub-diaphragmatic location, subcapsular location, proximity to high-risk areas [Citation12] (adjacent to large vessels or extrahepatic organs).
Ablation technique
Percutaneous approach was free-hand ultrasound-guided, either under deep sedation or general anesthesia as per institutional protocol. Laparoscopic procedures were performed under general anesthesia using two 12-mm trocars for the laparoscope and ultrasound probe. The needle was inserted percutaneously through a different access to target the liver lesion under intraoperative US guidance.
All ablations were performed by experienced IRs (>100 ablation procedures) using a 2450 MHz/100 W Microwave generator (Emprint, Medtronic). Ablation protocol (power and time) was tailored to tumor size according to manufacturer instructions (Instructions for Use, Emprint™ percutaneous Antenna with Thermosphere™ technology, Ablation Zone Charts, R0065469).
Follow-up
Institutional follow-up defines CT/MRI using a liver-specific acquisition protocol 1 month after the procedure, then every 3 months for the first year and every 6 months thereafter. Technique efficacy was defined as absence of pathological enhancement at the ablation zone (residual tumor) on imaging at 1 month after ablation; Local Tumor Progression (LTP) was defined as appearance of foci of vital disease at any of the follow-up time points [Citation13].
Complications and side effects were defined according to the SIR classification system [Citation14] based on a combination of outcome, clinical severity, effect on hospitalization and presence of long-term sequelae based on clinical and radiological follow-up.
Data considered
For each patient, data regarding gender, age, history of previous HCC and liver interventions, child pugh class, etiology of cirrhosis, BCLC stage, number of lesions and all imaging (pre- and post-procedure) were collected. For each tumor, data regarding tumor size, location, amount of delivered energy over tumor size (W × mm/s), technique efficacy, LTP were collected. Actual tumor size was re-determined on the day of procedure by real-time US.
Statistical Analysis
Continuous variables were calculated as mean and standard deviation (SD), categorical variables as frequencies. Statistical analyses were performed using a commercially available software (SPSS v25, IBM). Distribution of categorical tumor- and patient-related variables between the two study groups was assessed through Chi-square analysis; continuous variables were compared through Mann–Whitney U-test. LTP free survival (LTPFS) was analyzed with Kaplan–Meier curves, comparing variables with log-rank analysis. Since occurrence of events did not reach 50% of the study sample, median LTPFS was not obtained. Univariate and multivariate Cox proportional hazard regression models were also performed. Hazard ratios (HR) and 95 percent confidence intervals (95% CI) were calculated. The significance level for all parameters was set at p ≤ .05 .
Results
Baseline analysis
One-hundred-two HCC tumors in 91 patients were included in the study. Sixty-seven tumors (PMWA group = 63 patients) were treated percutaneously, 35 tumors (LMWA group = 28 patients) were treated laparoscopically ().
Five patients underwent ablation of two tumors in a single session (one in the PMWA group, four in the LMWA group), three patients underwent ablation of three tumors in a single session (one in the PMWA group, two in the LMWA group). Eight patients in the LMWA group underwent concomitant hepatic resection.
Baseline analysis ( and ) showed higher frequency of multinodular disease (≥2 tumors) in the LMWA group (PMWA = 12.7% vs LMWA = 53.6%, p < .001). Ablation in the LMWA group was more frequently performed under general anesthesia (PMWA = 46% vs LMWA = 100%, p < .001). The PMWA group had more patients which were not-naïve to treatment for HCC (PMWA = 63.5% vs LMWA = 25%, p = .001), with Hepatitis C (PMWA = 49.2% vs LMWA = 25%, p = .03), and BCLC A1 stage disease (PMWA = 52.4% vs LMWA = 21.4%, p = .006). A higher amount of energy over tumor size was delivered in LMWA (PMWA = mean 1329.9 W × s/mm vs LMWA = mean 1616.4 W × s/mm, p = .033). All other patient, tumor and technical characteristics were homogeneous between the two groups .
Figure 3. Kaplan–Meier curve for local tumor progression-free survival according to tumor location in the PMWA subgroup.
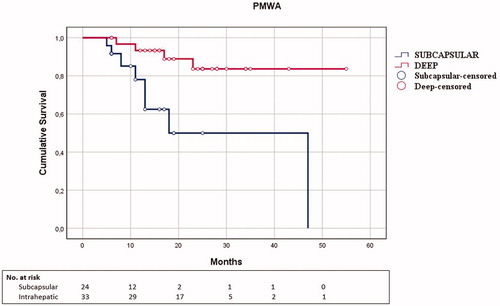
Table 1. Patient characteristics.
Table 2. Tumor characteristics.
Technique efficacy
At one-month follow-up, 94/102 tumors were effectively treated (technique efficacy rate = 92.2%); of these, 34/35 were effectively treated laparoscopically (97.1%) and 60/67 percutaneously (89.6%), with a statistically not significant trend toward better results in the LMWA group (p = .18). Tumors which did not achieve effective treatment were re-treated: four cases were re-ablated (three in the PMWA group, one in the LMWA group) and 4 cases were converted to TACE (two in the PMWA group, two in the LMWA group).
Local tumor progression free survival
Among patients with imaging-proven technique efficacy at 1 month, 75 patients with 83 tumors satisfied imaging follow-up of at least 6 months. Mean follow-up time, albeit groups, was 18.2 months (SD 10.7; range: 6–55 months). Of these, 57 tumors (54 patients) were in the PMWA group (follow-up 6–55 months, mean 18.9, SD 11.3) and 26 tumors (21 patients) in the LMWA group (follow-up 6–40 months, mean 16.8 SD 9.5). Overall LTP rate was 14/83 (16.9%) with 1- and 2-year LTPFS of 95.3% and 74.9%, respectively. Twelve LTPs occurred in PMWA group (21.1%) and 2 in the LMWA (7.7%). LTPs were re-ablated in five cases (four in the PMWA group, one in the LMWA group). The remaining cases showed disease progression outside the ablation zone and not deemed fit for re-ablation and thus required different therapeutic approaches (in the PMWA group 6 cases underwent TACE, 2 cases refused further treatment; one case in the LMWA group was scheduled to receive sorafenib). Univariate Cox regression analysis ( and ; ) showed that the operative approach was not correlated to LTPFS (PMWA: mean time to LTP 14.9 months; LMWA: mean time to LTP 14 months, p=.26). In a multivariate model comprising technique, subcapsular location of tumors, energy delivered and tumor size (), subcapsular location of tumors was the only independent predictor of worse LTPFS (HR = 4.727, p=.009).
Table 3. LTPFS univariate cox regression analysis according to subgroups based on technique.
Table 4. LTPFS multivariate cox regression analysis (n = 83).
Since no deaths occurred during the follow-up period, overall survival was not analyzed.
Subgroup analysis
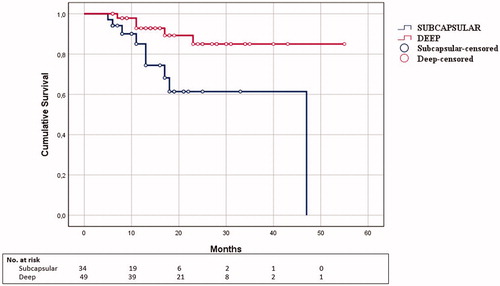
Considering PMWA and LMWA separately (), subcapsular tumors had worse LTPFS compared to deep tumors in the PMWA group (subcapsular: mean time to LTP 15.1 months; deep: mean time to LTP 14.5 months; p = .005) (), whereas no statistically significant differences were seen in the LMWA group (subcapsular: mean time to LTP 17 months; deep: mean time to LTP 11 months p = .9).
Comparing the two techniques according to a ‘per single variable stratification’, no significant differences in LTPFS were observed. Subcapsular tumors () showed a non-significant trend toward worse LTPFS after percutaneous procedures compared to laparoscopic (PMWA: mean time to LTP 15.1 months; LMWA mean time to LTP 17 months, p = .16).
Table 5. LTPFS univariate cox regression analysis of technique on specific variables’ subgroups.
Subcapsular tumors received a significantly higher amount of energy in LMWA (PMWA = mean 1281 W × s/mm; LMWA = mean 1821.7 W × s/mm, p = .023) ().
Table 6. Results of Mann–Whitney U-test for amount of delivered energy per tumor size according to technique in subcapsular tumors.
Complications and side effects
No fatal events occurred. All complications were major, with significantly different distribution between the two groups: 2 in the PMWA group (bilio-bronchial fistula, hematoma) and 4 in the LMWA group (pneumothorax, respiratory failure, hematoma, thrombosis of the right main portal branch) (3.2% vs 14.3%, p = .049). Pneumothorax and bilio-bronchial fistula required percutaneous drainage, respiratory failure was managed conservatively with noninvasive ventilation. Both cases of hematoma were self-limited but required prolonged hospitalization with conservative treatment. Portal thrombosis required anticoagulant treatment (LMW Heparin 100 IU/kg bid); although no recanalization was seen on follow-up imaging, a cavernoma developed with preserved parenchymal portal vascularization. Regarding side effects, post-ablation syndrome, i.e., unexplained fever after ablation [Citation13], was reported after a single laparoscopic procedure.
Discussion
Based on the data collected in our cohort, despite differences in patient and tumor characteristics in the two study groups, the two approaches did not significantly differ in terms of technique efficacy and local tumor control. Complications were more frequently reported following LMWA. On the other hand, LMWA seems to offer greater reliability in treatment of subcapsular tumors.
Already at baseline analysis, some substantial differences between the two groups were observed which are most likely linked to decisions strategies within the MDTs. Firstly, the higher frequency of multinodular disease in the LMWA group. The reason lies mostly because MDTs tend to agree on a combined ablative/surgical treatment of multinodular HCC with curative intent. The second finding at baseline was the higher number of non-treatment – naïve-patients in the PMWA group; most patients with previous treatment history for HCC, in particular hepatic resection, were deemed not amenable for surgery during multidisciplinary discussions and scheduled to receive the less invasive approach. Tumor size did not significantly differ between the two groups. Tumor size does not affect the choice of which approach to use and therefore, rightly so, did not show any significance. Despite general preference of LMWA over the PMWA when dealing with tumors in difficult areas of the liver, no significant differences were observed in variables regarding tumor location.
In our study, technique efficacy of the two different approaches was comparable and consistent with what reported in literature [Citation15]. The choice of the technical approach did not impact significantly on LTPFS. However, better local tumor control at follow-up was observed following LMWA of subcapsular tumors, consistent with previously published literature [Citation10].
Traditionally, thermal ablation is performed using a percutaneous approach with results, in terms of local disease control, comparable with resection [Citation16,Citation17]. However, tumors located closer to the liver surface are known to exhibit a higher tendency to recurrence [Citation18], as confirmed by our data. In these cases, literature suggests an intraoperative approach, either during laparotomy or laparoscopy, may be applied to improve outcome. An interesting finding was the average higher energy delivered over tumor size observed in the LMWA group, particularly in subcapsular tumors. A better visualization and monitoring of the ablation zone through laparoscopic guidance enables the IR to radically ablate tumors which would go untreated with the percutaneous approach due to their position.
Even though the number of tumors treated in the laparoscopic group was nearly half (36 vs 78), procedure-related complications reached statistical significance for the LMWA approach over PMWA. Less technical invasiveness and lower amounts of energy delivered per tumor in PMWA may explain these differences in the number of complications recorded.
Massive thrombosis of the right main portal branch was diagnosed one-month after LMWA effectively treated a cranially located tumor in proximity of the middle hepatic vein. The thrombosis did not respond to medical treatment with heparin, and at the three-month follow-up, collateral revascularization of the liver parenchyma was observed. This complication is particular and has been previously described in literature [Citation19] associated to tumors adjacent to portal vessels. This type of late complication has also been described in an experimental setting [Citation20] as potential consequence of reduced portal flow following intra-procedural CO2 insufflation of the peritoneal cavity [Citation21] which leads to an increased degree of thermal injury even in vessels not immediately surrounding the treated lesion [Citation20]. This particular case may have been also influenced by the high energy delivered due to tumor size (>3 cm) which was far above the mean. Respiratory failure with hypercapnic acidosis is also a potential complication related to laparoscopic surgery due to the synergistic effect of anesthesia-induced respiratory depression and laparoscopic CO2 insufflation of the peritoneum [Citation22]. Post-ablation syndrome (PAS) [Citation23], i.e., hyperthermia due to release of inflammatory mediators in response to tissue necrosis, occurred in one patient after treatment of a multifocal disease (3 tumors). This finding is consistent with what suggested by Andreano et al. [Citation24] that total volume of ablation correlates with occurrence of this side effect.
Both complications observed in the PMWA group occurred after treatment of subcapsular tumors located in segment VII. This is consistent with literature which suggests that percutaneous ablation of tumors located closer to vulnerable structures, such as the diaphragm in this case, are associated to a higher rate of complications [Citation12]. Interestingly, no complications in the LMWA group occurred after treatment of subcapsular tumors, as laparoscopy-induced pneumoperitoneum allows isolation of the tumor from surrounding tissues and permits direct surgical hemostasis and repair.
Limitations of the present study are linked to the limited sample size of LMWA compared to PMWA, non-consistent follow-up time for LTPS which led to exclusion of a number of patients and the retrospective nature of the study.
In conclusion, LMWA and PMWA are both safe and effective options for treatment of HCC. Accurate case-to-case discussion during MDT meetings is necessary in order to evaluate the best treatment option and achieve comparable results between approaches. LMWA showed a tendency toward better outcome in the treatment of subcapsular tumors. However, given the greater risk of complications, it should be performed by expert interventionalists.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1–8.
- Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236.
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203.
- Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(8):1054–1063.
- Kamal A, Elmoety AAA, Rostom YAM, et al. Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial. J Gastrointest Oncol. 2019;10(3):562–571.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperth. 2016;32(3):339–344.
- De Cobelli F, Marra P, Ratti F, et al. Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions: new advances in interventional oncology: state of the art. Med Oncol. 2017;34:49.
- Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104(7):822–829.
- Eun HS, Lee BS, Kwon IS, et al. Advantages of laparoscopic radiofrequency ablation over percutaneous radiofrequency ablation in hepatocellular carcinoma. Dig Dis Sci. 2017;62(9):2586–2600.
- Wong J, Lee KF, Yu SCH, et al. Percutaneous radiofrequency ablation versus surgical radiofrequency ablation for malignant liver tumours: the long-term results. HPB (Oxford). 2013;15(8):595–601.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43(5):1101–1108.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting Criteria-A 10-year update. Radiology. 2014;273(1):241–260.,
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Vogl TJ, Nour-Eldin NEA, Hammerstingl RM, et al. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms - review article. Fortschr Röntgenstr. 2017;189(11):1055–1066.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012;57(4):794–802.
- Di Sandro S, Benuzzi L, Lauterio A, et al. Single hepatocellular carcinoma approached by curative-intent treatment: a propensity score analysis comparing radiofrequency ablation and liver resection. Eur J Surg Oncol. 2019;45:1691–1699.
- Shiozawa K, Watanabe M, Wakui N, et al. Risk factors for the local recurrence of hepatocellular carcinoma after single-session percutaneous radiofrequency ablation with a single electrode insertion. Mol Med Rep. 2009;2(1):89–95.
- Kim KR, Thomas S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin Intervent Radiol. 2014;31(2):138–148.
- Ng KKC, Lam CM, Poon RTP, et al. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg. 2004;91(5):632–639.
- Jakimowicz J, Stultiëns G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12(2):129–132.
- Gutt CN, Oniu T, Mehrabi A, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21(2):95–105.
- Dodd GD, Napier D, Schoolfield JD, et al. Percutaneous radiofrequency ablation of hepatic tumors: postablation syndrome. Am J Roentgenol. 2005;185(1):51–57.
- Andreano A, Galimberti S, Franza E, et al. Percutaneous microwave ablation of hepatic tumors: prospective evaluation of postablation syndrome and postprocedural pain. J Vasc Interv Radiol. 2014;25:97–105.e1-2.