Abstract
Background
Stereotactic laser ablation(SLA) or laser interstitial thermal therapy (LITT) has been increasingly adopted as a treatment for primary and metastatic brain cancers. Here, we examined the published economic assessments of SLA, and review the current state of knowledge.
Methods
The PubMed database was queried for articles investigating the cost-effectiveness of LITT. 3068 articles were screened. Two studies that met the inclusion criteria were included in this review.
Results
Cost-effectiveness analysis(CEA) favored SLA(n = 8) relative to craniotomy (n = 92) for brain metastases (Mean difference [MD]=−US$6522; 95% confidence interval (CI) –$11,911 to –$1133; p = 0.02). SLA (n = 19) was found to be cost equivalent to craniotomy (n = 248) (MD=–US$1669; 95%(CI) –$8192 to $4854, p = 0.62) for primary brain tumors in general. CEA favored SLA for a subset of primary brain cancers. SLA was found to be cost-effective for difficult to access high-grade gliomas(HGG). When compared to ‘other’ existing treatments, the cost per life-years gained (LYG) through SLA was ∼$29,340, a threshold below that set for new technology adaptation in the U.S. Factors contributing to these cost-effectiveness were: (1) SLA of HGGs was associated with three-months prolongation in survival; (2) SLA of brain metastasis was associated with (i) shorter average length of stay (SLA: 2.3 days; craniotomy: 4.7 days), (ii) decreased discharge to inpatient rehabilitation facility (IRF), skilled nursing facility (SNF), or home healthcare (SLA: 14.8%; craniotomy: 52%), (iii) lowered 30-day readmission (SLA: 0%; craniotomy: 14.1%).
Conclusion
There is limited data on the cost-effectiveness of SLA. In the available literature, SLA compared favorably to craniotomy in terms of cost-effectiveness as a treatment for primary and metastatic brain cancers.
Introduction
Brain cancers can be classified into two major categories. Primary brain cancer refers to tumors that arise in the brain, while brain metastasis refers to cancer that spreads to the brain from cancers located in another part of the body [Citation1]. The most common form of primary brain cancer are tumors termed high-grade gliomas (HGGs) tha consists of anaplastic astrocytomas and glioblastomas [Citation2,Citation3]. These tumors are often grouped because of commonality in histology as well as the observation that anaplastic astrocytomas inevitably progress to glioblastomas [Citation4]. Relative to brain metastasis, primary brain cancers are rare, accounting for <2% of all cancers [Citation5]. In contrast, ∼25% of all cancer patients develop brain metastasis [Citation6].
Brain cancers are clinically managed based on guidelines issued by the National Comprehensive Cancer Network (NCCN) [Citation7]. Surgery plays a key role in this management [Citation8]. While maximal safe resection serves as the guiding principle, such resection may be associated with high morbidity for tumors located in regions that are difficult to access (DTA), such the deep gray matter [Citation9–13]. For these tumors, stereotactic laser ablation (SLA), also known as laser interstitial thermotherapy (LITT), has emerged as an attractive alternative. SLA is a minimally invasive procedure where a laser probe is stereotactically inserted into the tumor. Subsequent laser activation triggers thermocoagulation that leads to tumor destruction [Citation14]. A stereotactic needle-biopsy is often performed prior to SLA to secure tumor tissue for molecular analysis. Emerging data from single institutional experiences [Citation15] and multi-institutional registries [Citation16] support the efficacy and safety of SLA in the treatment of brain cancers.
Relative to the escalating number of studies documenting the efficacy and safety of SLA, there remains limited data on the cost-effectiveness of SLA [Citation17–19]. In this era of increasing focus on cost-containment and affordable healthcare, it is paramount to analyze the economic impact of treatment modalities as a function of survival or quality-of-life benefits. Here, we performed a systematic review of the existing literature to determine the cost-effectiveness of SLA relative to other therapies described in the NCCN guidelines for both primary and metastatic brain tumors, including craniotomy (with or without the use of carmustine wafers) and stereotactic needle biopsy.
Methods
Search algorithm
A comprehensive PubMed database search was conducted on 12/30/2019 for articles investigating the cost effectiveness of laser interstitial thermal therapy for glioblastoma and brain metastases.
The initial search was performed using the following search terms and resulted in 3065 articles: ((cost effectiveness OR Cost Analysis OR life years gained OR LYG OR survival OR value OR QALY OR quality OR cost) AND (Thermal OR LITT OR laser OR interstitial OR thermo) AND (glioma OR glioblastoma OR multiforme OR GBM OR HGG OR high grade glioma OR metasta*)) (All Fields).
The search phrase was kept broad to capture all the possible relevant articles. References of relevant articles were screened to include any article that could have been missed in the initial PubMed search focusing on the cost-effectiveness of SLA in brain tumors. 8 additional articles were identified from such sources.
Article review
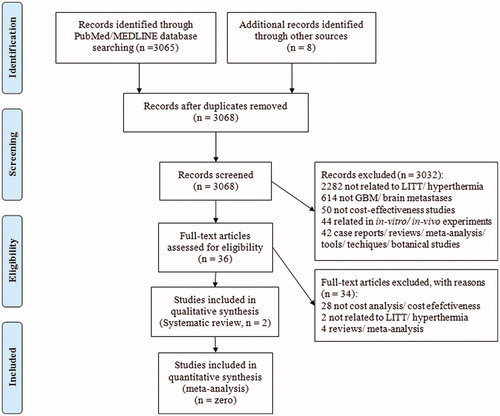
Articles were initially screened by title and abstract. Those articles that passed the initial screening were read in full text. Articles were excluded for the following reasons: 2282 articles were not related to SLA or hyperthermia in primary brain cancer or brain metastases, 614 articles were not related to either primary brain cancer or brain metastases, 50 articles did not focus on cost-effectiveness aspect of SLA, 44 articles focused on in vitro or in vivo studies, 42 articles were in the form of case series/reviews/meta-analysis/techniques/commentaries/proceedings. After these exclusions, 36 articles were evaluated in detail. 34 articles were excluded after full-text review and two articles were finally selected for inclusion in this review manuscript. The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (PRISMA) ().
Inclusion criteria
The inclusion criteria for the articles included in the review were: (1) written in English (or English language translation available), (2) abstract should be available, (3) articles evaluating cost-effectiveness of LITT as a treatment modality for GBM or brain metastases, (4) fully published peer-reviewed clinical research or cost-effectiveness modeling articles.
Results
Definitions
Cost-Effectiveness Analysis (CEA) to study interventions in healthcare involves the use of terms and acronyms which are specific to the field of health economics [Citation20]. lists terms and definitions pertinent to this manuscript.
Table 1. Important terms and definitions used in health economics studies.
Salient features of the included studies
We identified two studies that fulfilled our search criteria.
The study by Leuthardt et al. is a retrospective economic evaluation conducted in a university hospital setting that analyzed actual cost data [Citation17]. Consecutive patients with primary and metastatic brain cancer were categorized based on whether they underwent SLA (n = 27) or craniotomy (n = 340). Costs incurred during the peri and post-operative period were analyzed to determine cost-effectiveness.
Study by Voigt et al. is a computational economic modeling study that included patients with HGG located in regions that are difficult to access (DTA), including eloquent and deep-seated regions [Citation18]. This study utilized Medicare and reimbursement cost data to determine the cost-effectiveness of SLA over ‘other’ existing treatments including craniotomy-with or without the use of gliadel wafers, and biopsy. The study utilized cost per life-years gained (LYG) as the matrix for evaluating cost-effectiveness. compares the salient features of these two studies.
Table 2. Salient features and comparison of the two included studies.
Cost-effectiveness of LITT for unselected primary and metastatic brain tumors
Of the two studies identified in our review, the study by Voigt et al. focused entirely on DTA HGGs [Citation18]. In contrast, the Leuthardt study [Citation17] provided cost data for consecutive surgically treated primary and metastatic brain tumor patients, irrespective of location. This latter dataset will be reviewed in this section.
The cost estimates reported in the Leuthardt study included the procedural and post-procedural costs associated with discharge to inpatient rehabilitation facility (IRF), or skilled nursing facility (SNF), or home healthcare [Citation17]. For patients with primary brain cancer, the average costs (US$) for craniotomy was $33,392 ± 13,773. For SLA, the corresponding cost was 35,061 ± 16,471 [Citation17]. No significant difference was noted in these cost estimates (Mean difference [MD] = –US$1669; 95% confidence interval (CI) –$8192 to $4854, p = 0.62) [Citation17].
For patients with metastatic brain cancer, the average costs (US$) for craniotomy was $35,941 ± 20,401. For SLA, the corresponding cost was 29,419 ± 4,965 [Citation17]. The cost-effectiveness assessment favored SLA as treatment for metastatic brain cancer (MD = –US$6522; 95% CI –$11,911 to –$1133; p = 0.02) () [Citation17]. The difference in cost-effectiveness was largely driven by fewer patients requiring inpatient rehabilitation facility (IRF), or skilled nursing facility (SNF), or home healthcare after SLA (0/8 for SLA versus 41/92 for craniotomy).
Figure 2. Median difference in acute care costs (in US $) including procedure costs and post-operative care by tumor type between Brain SLA vs craniotomy. SLA: stereotactic laser ablation; DTA: difficult to access lesions [Citation17].
![Figure 2. Median difference in acute care costs (in US $) including procedure costs and post-operative care by tumor type between Brain SLA vs craniotomy. SLA: stereotactic laser ablation; DTA: difficult to access lesions [Citation17].](/cms/asset/fccce4b5-3fee-49b1-aea3-e016db13e99f/ihyt_a_1774084_f0002_c.jpg)
Difficult to access (DTA) brain tumors
Because of the limited number of patients who underwent SLA (n = 19), there was insufficient data in the Leuthardt study [Citation17] to analyze DTA HGGs and metastatic brain tumors separately. When DTA HGG and brain metastasis results were aggregated, Leuthardt et al.. reported that the estimated cost for SLA was lower than that associated with craniotomy by $4,719 (SLA: 33,392 ± 13,773; craniotomy: 38,111 ± 17,139; MD = –US$4719; 95% CI –12,183 to 2745; p = 0.22) [Citation17]. However, this difference did not reach statistical significance.
The results reported by Voigt et al. in terms of cost-effectiveness of SLA as a treatment for DTA HGG are summarized in , which compared the overall survival (OS) and costs of SLA versus ‘other’ treatment modalities including craniotomy with or without gliadel wafers and biopsy [Citation18]. The cost of SLA was comparable to craniotomy + carmustine wafer ($89,839 for SLA and $87,654 for craniotomy + carmustine). The increased cost associated with SLA was associated with a prolongation in overall survival (19.0 months for SLA and 16.9 months for craniotomy + carmustine).
Figure 3. Comparison of base case between SLA and other treatments on outcomes of overall survival (in months) and cost (in US$). In parenthesis is mentioned the willingness to pay amount (in US$) [Citation18]. Other treatments include craniotomy (with or without carmustine wafer) and biopsy. LYG: life years gained; SLA: stereotactic laser ablation; OS: overall survival.
![Figure 3. Comparison of base case between SLA and other treatments on outcomes of overall survival (in months) and cost (in US$). In parenthesis is mentioned the willingness to pay amount (in US$) [Citation18]. Other treatments include craniotomy (with or without carmustine wafer) and biopsy. LYG: life years gained; SLA: stereotactic laser ablation; OS: overall survival.](/cms/asset/be62f634-37ec-4dd9-be19-aa6e06853abd/ihyt_a_1774084_f0003_b.jpg)
When compared to craniotomy/biopsy without carmustine, the cost of SLA again exceeded that associated with craniotomy/biopsy by $7508 ($89,839 for SLA and $82,331). Similar to the above analysis, the overall survival for patients treated with SLA also exceeded that of craniotomy/biopsy (19.0 months for SLA and 15.9 months for craniotomy). When viewed through the matrix of Life Years Gained (LYG), these estimates translate into $29,340/LYG, or the cost of extending survival for one year. This estimate is considerably less than the value of $50,000/LYG that is frequently set as the threshold for new technology adaptation in the U.S [Citation18,Citation25].
Variables affecting the cost-effectiveness of SLA
Integrated analysis of the two studies identified in this review suggests that cost-effectiveness analysis favored SLA because it is associated with (1) shorter hospital stay (measured by length of stay (LOS)), (2) decreased discharge to rehabilitation/nursing facilities, and (3) lowered 30-day readmission.
In terms of hospital stay, Leuthardt et al. reported the average LOS as 2.33 ± 3.13 and 4.71 ± 3.16 days for SLA and craniotomy, respectively (p < 0.0001) () [Citation17]. Similar results were reported by Voigt et al. where LOS was 3 days for SLA and 7.5 days for craniotomy () [Citation18].
Figure 4. Average length of stay (LOS) in Brain SLA, craniotomy (with or without carmustine /gliadel wafers) and biopsy groups in the two included studies [Citation17,Citation18].
![Figure 4. Average length of stay (LOS) in Brain SLA, craniotomy (with or without carmustine /gliadel wafers) and biopsy groups in the two included studies [Citation17,Citation18].](/cms/asset/6a3a0f8c-11a1-461b-a997-e871204b464c/ihyt_a_1774084_f0004_b.jpg)
In terms of discharge outcome, Leuthardt et al. reported that 14.8% (4/27) of the LITT patients were discharged to service sites other than home, i.e., inpatient rehabilitation facility (IRF), or skilled nursing facility (SNF), or home healthcare as compared to 52% (177/340) of the patients who underwent craniotomy (p = 0.007) () [Citation17]. The cost associated with IRF, the most expensive of all discharge site facilities, was $24,367; reimbursement for care in SNF was $4284, while it was around $159/day for home health care [Citation17,Citation18].
Figure 5. Incidence of discharge to sites other than home like skilled nursing facility, inpatient rehabilitation facility and home healthcare. SLA: stereotactic laser ablation [Citation17].
![Figure 5. Incidence of discharge to sites other than home like skilled nursing facility, inpatient rehabilitation facility and home healthcare. SLA: stereotactic laser ablation [Citation17].](/cms/asset/05061dca-29a6-4743-9308-b8de6e6138b6/ihyt_a_1774084_f0005_b.jpg)
In terms of 30-day readmission, Leuthardt et al. reported that 14.1% (48/340) patients who underwent craniotomy were readmitted within 30 days of discharge from the hospital [Citation17]. In contrast, none of the SLA treated patients were re-admitted after discharge. The craniotomy readmissions in the metastatic tumor group further burdened the financial balance sheet by around $3400 [Citation17,Citation29].
Discussion
Our search of the available literature on the cost-effectiveness of SLA as a treatment for primary and metastatic brain cancer revealed only two publications. The analysis provided by these two studies generally painted a favorable cost-effectiveness profile for SLA. In both studies, the costs associated with SLA as a treatment for primary brain cancer is, at least, comparable to those associated with craniotomy. The two studies suggest that SLA may be more cost-effective as a treatment for (1) the subset of HGGs located in regions of the brain considered DTA, and (2) brain metastasis. The cost-effectiveness of SLA in the setting of DTA HGGs was built on the assumption that SLA treatment extended survival by three months. The cost-effectiveness of SLA as treatment for brain metastasis was attributed to (i) shorter average length of stay, (ii) decreased discharge to inpatient rehabilitation facility (IRF), skilled nursing facility (SNF), or home healthcare, and (iii) lowered 30-day readmission. Since the publication of the two studies reviewed, more mature information has emerged in the literature pertaining to these assumptions. This information will be reviewed below.
The difference in favorable YLG (year of life gained) associated with SLA by Voigt et al. suggests survival benefit for brain tumor patients undergoing SLA relative to those undergoing conventional craniotomy or biopsy [Citation18]. This survival benefit is extracted from the published literature, which consisted of Level III evidence [Citation30,Citation31]. There is currently no level I or II evidence in support of SLA associated survival benefit in HGG patients. A consistent trend observed in published retrospective series was that improved survival was associated with maximal tumor coverage by SLA, constituting level III evidence supporting potential survival benefit [Citation32–34]. However, the benefits observed in these studies are modest and confounded by patient selection [Citation32–34]. As such, the contribution of selection bias as well as other forms of biases to the observed benefit cannot be excluded.
The best available class III data compared the survival of 24 SLA-treated patients with newly diagnosed glioblastoma in DTA locations to 24 matched patients who underwent biopsy only [Citation35]. The patients were matched in terms of age, gender, tumor location, and tumor volume. All patients subsequently underwent standard-of-care radiation/chemotherapy. The overall survival for the SLA treated patients and the biopsied patients were 15.8 and 14.4 months, respectively, and did not reach statistical significance. In this context, the assumption by Voigt et al.. that SLA of DTA HGG was associated with an average of 3-months survival prolongation is subject to question [Citation18] and may only be pertinent to patients in whom SLA achieved favorable tumor coverage [Citation32–34].
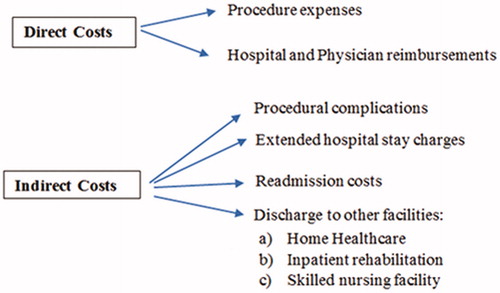
Different variables determine the direct and indirect costs associated with a procedure (). Direct costs include the procedural expenses, and Medicare reimbursements to the hospital and physicians based on the diagnostic related group (DRG) and current procedural terminology (CPT) codes. Indirect costs comprise of costs incurred due to procedural complications, extended LOS, readmission costs and discharge to facilities other than home like IRF, SNF, and home healthcare. The cost-effectiveness of SLA relative to craniotomy was based on assumptions of lowered indirect costs secondary to procedural safety, LOS, discharge needs, and re-admission. These assumptions have largely been born out in subsequent studies. In the first 100 patients enrolled in a multi-institutional registry that tracked the clinical outcome of brain tumor patients after SLA, the median LOS was 27 h, with 84.8% of the patients discharged home and one mortality within 30 days [Citation36]. These reported statistics are largely consistent with those reported in independent, published case series [Citation37]. It is important to note, however, that the LOS associated with craniotomy utilized in the two studies are likely to be historical rather than reflecting of current reality, where uncomplicated craniotomy patients are discharged on post-operative day one or two [Citation38]. Moreover, selection criteria for craniotomy likely differ from those for SLA. Cost-effectiveness analysis should be analyzed factoring these considerations.
Bundled episodes of payment care is an alternative reimbursement model introduced by Center for Medicare and Medicaid Services (CMS), to keep a check on the increasing health care costs [Citation27]. In contrast to the ‘quantity’ of care focused fee-for-service reimbursement model, in which the doctors, hospitals, post-acute care providers and external discharge facilities file for individual claims, the ‘quality’ of care focused Bundled Payments for Care Improvement (BPCI) model focuses on one combined average payment for a single episode of care (see ) [Citation39–41]. Under this model, the global period associated with each procedure varies from 0 to 92 days covering the expenses associated with hospital care, discharge facilities and readmissions for up-to 90 days in the post-operative period [Citation42]. Currently, cranial neurosurgical procedures are not included under the Bundled Payments for Care Improvement model [Citation40]. Considering the lower indirect costs associated with SLA as mentioned above, SLA could be highly cost-effective compared to craniotomy in the BPCI reimbursement model.
Finally, the measure by which cost-effectiveness is determined warrants discussion. The Voigt study leaned heavily on LYG, a mortality measure, as a means for determining cost-effectiveness. The measure suffers from a failure to consider the quality of life associated with extended survival. Moreover, LYG data from a younger age patient cannot be compared with the data from an older patient. In this context, measures such as Quality Adjusted Life Years (QALY) warrant consideration in future cost-effectiveness studies for SLA (See for definitions), since QALY considers both the survival duration and health-related quality of life (HRQoL) weight [Citation43].
Conclusion
While imperfect, the available data support SLA as a cost-effective alternative to craniotomy, especially in light of data supporting procedural safety, shortened LOS, decreased discharge to facilities, and reduction of 30-day readmission. Given the limited cost-effectiveness literature for SLA, further investigation is warranted.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Nathoo N, Chahlavi A, Barnett GH, et al. Pathobiology of brain metastases. J Clin Pathol. 2005;58(3):237–242.
- Prisco FE, Weltman E, de Hanriot RM, et al. Radiosurgical boost for primary high-grade gliomas. J Neurooncol. 2002;57(2):151–160.
- Fetcko K, Lukas RV, Watson GA, et al. Survival and complications of stereotactic radiosurgery: a systematic review of stereotactic radiosurgery for newly diagnosed and recurrent high-grade gliomas. Medicine (Baltimore) 2017;96(43):e8293.
- Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100(12):2235–2241.
- Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423–1430.
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54.
- NCCN Clinical practice guidelines in oncology. Center nervous system cancers. Version 2. 2014. [cited 2015 July 2]. Available from: NCCN.org.
- Young RM, Jamshidi A, Davis G, et al. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3(9):121
- Li YM, Suki D, Hess K, et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? JNS. 2016;124(4):977–988.
- Bakhsheshian J, Strickland BA, Jackson C, et al. Multicenter investigation of channel-based subcortical trans-sulcal exoscopic resection of metastatic brain tumors: a retrospective case series. Oper Neurosurg (Hagerstown). 2019;16(2):159–166.
- Gassie K, Wijesekera O, Chaichana KL. Minimally invasive tubular retractor-assisted biopsy and resection of subcortical intra-axial gliomas and other neoplasms. J Neurosurg Sci. 2018;62(6):682–689.
- Iyer R, Chaichana KL. Minimally invasive resection of deep-seated high-grade gliomas using tubular retractors and exoscopic visualization. J Neurol Surg A Cent Eur Neurosurg. 2018;79(4):330–336.
- Jackson C, Gallia GL, Chaichana KL. Minimally invasive biopsies of deep-seated brain lesions using tubular retractors under exoscopic visualization. J Neurol Surg A Cent Eur Neurosurg. 2017;78(6):588–594.
- Salem U, Kumar VA, Madewell JE, et al. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19(1):65.
- Hawasli AH, Bagade S, Shimony JS, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017.
- Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–811.
- Leuthardt EC, Voigt J, Kim AH, et al. A single-center cost analysis of treating primary and metastatic brain cancers with either brain laser interstitial thermal therapy (LITT) or craniotomy. Pharmacoecon Open. 2017;1(1):53–63.
- Voigt JD, Barnett G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff Resour Alloc. 2016;14:6.
- Williams D, Loshak H. Laser interstitial thermal therapy for epilepsy and/or brain tumours: a review of clinical effectiveness and cost-effectiveness [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health 2019.
- Cost Effectiveness Analysis. Available from: https://www.cdc.gov/policy/polaris/economics/cost-effectiveness.html (accessed 20 December 2019).
- “Glossary”. National Institute for Health and Care Excellence. Available from: https://www.nice.org.uk/glossary.
- Pliskin JS, Shepard DS, Weinstein MC. Utility functions for life years and health status. Oper Res. 1980;28(1):206–224.
- Pieto L, Sacristan JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80.
- Incremental cost-effectiveness ratio (ICER) [online]. 2016. York: York Health Economics Consortium; 2016. Available from: https://yhec.co.uk/glossary/incremental-cost-effectiveness-ratio-icer/.
- Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;52(25):2119–2126.
- Cost to Charge ratio. Available from: https://www.hcup-us.ahrq.gov (accessed 20 December 2019).
- CMS.gov. Bundled payments for care improvement (BPCI) initiative: general information. [cited 2019 Dec 20]. Available from: https://innovation.cms.gov/initiatives/bundled-payments/
- Willingness-to-Pay [online]. York: York Health Economics Consortium; 2016. Available form: https://yhec.co.uk/glossary/willingness-to-pay/.
- Medicare Payment Advisory Committee (MEDPAC). Report to the Congress. Medicare payment policy. March 2015. p. 253. [cited 2019 Dec 20]. Available from: http://www.medpac.gov/., http://www.medpac.gov/-documents-/reports.
- Oxford Centre for Evidence-Based Medicine. [cited 2020 Apr 27]. http://www.cebm.net/index.aspx?o=5653
- Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310.
- Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979.
- Shah AH, Semonche A, Eichberg DG, et al. The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery. 2019 (published online ahead of print). doi:10.1093/neuros/nyz424.
- Rennert RC, Carroll KT, Ali MA, et al. Safety of stereotactic laser ablations performed as treatment for glioblastomas in a conventional magnetic resonance imaging suite. Neurosurg Focus. 2016;41(4):E7.
- Mohammadi AM, Sharma M, Beaumont TL, et al. Upfront magnetic resonance imaging-guided stereotactic laser-ablation in newly diagnosed glioblastoma: a multicenter review of survival outcomes compared to a matched cohort of biopsy-only patients. Neurosurgery. 2019;85(6):762–772.
- Rennert RC, Khan U, Bartek J, et al. Laser ablation of abnormal neurological tissue using robotic neuroblate system (LAANTERN): procedural safety and hospitalization. Neurosurgery. 2020;86(4):538–547.
- Alattar AA, Bartek J, Jr, Chiang VL, et al. Stereotactic laser ablation as treatment of brain metastases recurring after stereotactic radiosurgery: a systematic literature review. World Neurosurg. 2019;128:134–142.
- Richardson AM, McCarthy DJ, Sandhu J, et al. Predictors of successful discharge of patients on postoperative day 1 after craniotomy for brain tumor. World Neurosurg. 2019;126:e869–e877.
- Shih T, Chen LM, Nallamothu BK. Will bundled payments change health care? examining the evidence thus far in cardiovascular care. Circulation. 2015;131(24):2151–2158.
- Medress Z, Ugiliweneza B, Parker J, et al. Simulating episode-based bundled payments for cranial neurosurgical procedures. Neurosurgery. 2019 (published online ahead of print) doi:10.1093/neuros/nyz353.
- Greenwald AS, Bassano A, Wiggins S, et al. Alternative reimbursement models: bundled payment and beyond: AOA critical issues. J Bone Joint Surg Am. 2016;98(11):e45.
- CMS.gov. Bundled Payments for Care Improvement (BPCI) initiative: general information. [cited 2020 Apr 20]. Available from: https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf.
- Robberstad B. QALYs vs DALYs vs LYs gained: what are the differences, and what difference do they make for health care priority setting?. Nor J Epidemiol. 2009;15(2):183–191.