Abstract
Objective
The purpose of this study was to evaluate the usefulness of CT for quantitative assessment of the neck structures after RFA in patients with benign thyroid nodules.
Materials and methods
This single-center, retrospective cohort study included 38 patients with benign thyroid nodules who had received RFA and had available pre- and post-treatment CT images. Changes in the tracheal anteroposterior (AP)/transverse diameter ratio, cross-sectional tracheal area, midline deviation of the trachea, and anterior neck angle after RFA were quantitatively measured using CT. Volume reduction rates (VRRs) for the thyroid gland and nodules were measured using CT and US, respectively, and the intraclass correlation coefficient (ICC) was calculated. The paired Wilcoxon signed-rank test was used to compare pre- and post-treatment CT-based measurements, and univariate linear regression analysis was performed to determine the association of VRR with the mean delivered radiofrequency energy, number of RFA sessions, and initial thyroid volume.
Results
After RFA, the tracheal AP/transverse diameter ratio and midline deviation were significantly decreased while the tracheal area and anterior neck angle were significantly increased (all, p < 0.001). The thyroid volume reduction was also significant (VRR, 42.1% ± 21.1%, p < 0.001), with moderate consistency between the CT-based thyroid VRR and US-based nodule VRR (ICC = 0.68, 95% confidence interval = 0.38–0.83, p < 0.001). The mean delivered radiofrequency energy (p = 0.565), number of RFA sessions (p = 0.209), and initial thyroid volume (p = 0.363) showed no significant association with VRR.
Conclusion
CT-based quantitative assessments may be useful for evaluating improvements in the neck structures after RFA for benign thyroid nodules.
Introduction
Ultrasound (US)-guided radiofrequency ablation (RFA) has been widely studied as a promising technique with good efficacy for the treatment of benign thyroid nodules [Citation1–5], recurrent thyroid cancers in surgical beds, and recurrent lymph nodes [Citation6–8]. For benign thyroid nodules, RFA is indicated for patients presenting with symptoms of neck pain/discomfort, dysphagia, foreign body sensation, cough, cosmetic problems, and those having autonomously functioning thyroid nodules. On average, RFA reduces the thyroid nodule volume by 84.8%, resulting in good clinical and cosmetic outcomes [Citation1,Citation9,Citation10] while avoiding the complications associated with open surgery.
Several recent studies have examined the volume reduction rate (VRR) for benign thyroid nodules as a measure of the treatment efficacy [Citation11,Citation12]. However, the current method of VRR measurement using US is confined only to VRR of the thyroid nodule and prone to variability because it is dependent on the operator and US device. Furthermore, evaluation of changes in the trachea relative to the surrounding cervical structures is often challenging with US. This is particularly important because patients with airway narrowing of >50% may develop symptoms and require invasive interventions [Citation13]. In this regard, CT deserves more attention as a potential tool for quantitative and objective structural evaluation of the treatment efficacy.
CT-based assessment of the entire thyroid gland may overcome the limitations of US-based measurements by clearly delineating the thyroid volume, tracheal area, and tracheal deviation, which can cause airway narrowing and breathing difficulties. Recently several international thermal ablation guidelines recommend RFA as an alternative to surgery for the treatment of benign thyroid nodules [Citation14–17]. The current perspective on the use of CT has been mentioned in one of the recent RFA guidelines [Citation14], which recommends CT as ‘selectively indicated’ for evaluation of the intrathoracic extent of benign thyroid nodules. While there have been studies evaluating the usefulness of CT for thermal ablations in various malignancies [Citation18,Citation19] and for assessing tracheal stenosis [Citation20,Citation21], no study has specifically evaluated the usefulness of CT as an assessment tool between RFA sessions for benign thyroid nodules.
Accordingly, the aim of this study was to evaluate the usefulness of CT for quantitative assessment of the neck structures after RFA in patients with benign thyroid nodules.
Materials and methods
Study cohort
The institutional review board approved this retrospective study with a waiver for consent forms. Data for patients with pathologically confirmed benign thyroid nodules treated with RFA between November 2007 and December 2018 were retrospectively reviewed. The indications for performing RFA were based on the consensus statement and recommendations of RFA of benign thyroid nodules [Citation22]. Patients who underwent CT both prior to and after RFA were initially enrolled (n = 46); those with images that did not include the entire thyroid gland (n = 8) were excluded. The interval between the initial CT scan and first RFA session was limited to <1.5 year. Eventually, 38 eligible patients were selected for analysis. Symptom and cosmetic scores were evaluated before RFA [Citation22].
Radiofrequency ablation
The benignancy of all thyroid lesions was pathologically confirmed prior to RFA. A single radiologist with 11 years of experience in RFA performed all RFA treatments on an outpatient basis. US was performed using the Aplio 500 Platinum (Toshiba Medical Systems, Tokyo, Japan) or HDI 5000 (Philips Medical Systems, Bothell, WA) device. A radiofrequency generator (Cool-Tip RF system, Covidien, Boulder; SSP-2000, Taewoong Medical, Gyeonggi, Korea) and a thyroid-dedicated, internally cooled radiofrequency electrode (Well-Point RF electrode, Taewoong Medical, Gyeonggi, Korea) were used. A 7-cm-long, 18-gauge electrode with a 0.5-, 0.7-, or 1.0-cm active tip was selected according to the tumor size [Citation23]. The patients’ blood pressure, venous oxygen saturation, pulse rate, and electrocardiograms were closely monitored throughout the procedures [Citation14].
To minimize complications, all procedures were performed with protective methods, including the trans-isthmic approach, moving shot technique, hydro-dissection technique, and pulled-away method as described previously [Citation14,Citation22,Citation24]. The hydro-dissection technique involves lidocaine (0.2%, up to 10 ml) injection into a space between the thyroid gland and surrounding critical structures such as the nerves, carotid artery, jugular vein, trachea, and esophagus; this provides local anesthesia and creates a protective barrier against thermal heat [Citation25,Citation26].
After treatment completion, all patients underwent regular follow-up clinical evaluations and US examinations of the neck at 2, 6, and 12 months and annually thereafter. Finally, all post-procedural reports have followed the criteria proposed by Mauri et al. [Citation27].
Image analysis
CT images were acquired using a multidetector row CT scanner (Siemens Definition AS Plus, Siemens Healthneers, Erlangen, Germany). Contrast-enhanced images were acquired following a 60-s delay after intravenous administration of 100 ml of an iodinated contrast medium (Ultravist 300; Bayer Schering Pharma, Berlin, Germany) at a rate of 2.5 ml/s. Contrast-enhanced CT images (slice thickness: 2.5 mm) were retrieved from the Picture Archiving and Communication System of our institution.
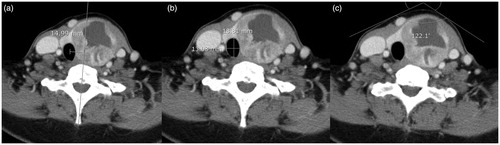
CT images with fully visible thyroid glands were reviewed. Before RFA, neck CT scans were acquired for the following reasons: 1) identification of other cervical lesions, 2) assessment of the extent of intrathoracic thyroid nodules, and 3) evaluation of the anatomical relationship between the thyroid nodules and the esophagotracheal groove. The volume of the entire thyroid gland was delineated while the cross-sectional tracheal area was measured at the narrowest level. Tracheal deviation from the midline was measured as the distance between the tracheal midline and a perpendicular line drawn along the midline of the cervical spinal process (). The ratio of the anteroposterior(AP)/transverse diameter of the trachea (tracheal AP/transverse diameter ratio) was also measured between RFA sessions (). Finally, the anterior neck angle was measured at the point where the surface neck contour showed maximum anterior protrusion ().
Figure 1. Contrast-enhanced CT images for a representative case involving a 49-year-old woman with a benign follicular nodule in the left thyroid gland prior to treatment. CT-based measurements of the (a) tracheal deviation distance, (b) ratio of anteroposterior/transverse diameter of the trachea, and (c) anterior neck angle.
On US scans, three orthogonal diameters—the longest and two perpendicular diameters—of target nodules were measured and the volume was calculated using the following equation: V = abc × 0.524 (V: volume, a: longest diameter, b and c: two perpendicular diameters) [Citation28]. The thyroid VRR and nodule VRR on CT and US images, respectively, was calculated using the following equation: [(initial volume – final volume) ×100/initial volume].
All image analyses were performed by one radiologist and subsequently reviewed by another radiologist (6 and 20 years of experience in thyroid radiology, respectively), and a consensus was established. Both radiologists were blinded to the patients’ clinical information. All segmentations were performed using a 3 D slicer [Citation29] (www.slicer.org). Distances and angles were measured using the built-in functions of the Picture Archiving and Communication System of our institution.
Statistical analysis
Continuous variables were compared using the paired Wilcoxon signed-rank test, while categorical variables were compared using Fisher’s exact test. The consistency between CT-based and US-based VRR measurements was evaluated by calculation of the intraclass correlation coefficient (ICC). A subgroup analysis comparing VRR of US and CT was performed for patients with intrathoracic masses. Univariate linear regression analysis was performed to determine the association of the CT-based thyroid VRR with the mean delivered radiofrequency energy, number of RFA sessions, and initial thyroid volume. The Kruskal–Wallis test was used to determine if there were significant differences in VRR according to the number of RFA sessions. All statistical analyses were performed using R software (version 3.4.4., Vienna, Austria), and a p-value of <0.05 was considered statistically significant.
Results
Study population
The baseline characteristics of the study cohort are summarized in . The mean age of the patients was 51.3 ± 16.1 (range, 26–78) years. The most common pathological diagnosis was benign follicular nodule (29/38, 76.3%) as confirmed by either fine-needle aspiration or core-needle biopsy. Fourteen patients (36.8%) had intrathoracic extension of mass. Twenty-four patients (63.2%) received two RFA sessions. Neck CT was the most frequently used modality in both initial (37/38, 97.4%) and follow-up evaluations (31/38, 81.6). The mean interscan intervals were 38.6 ± 25.5 (range, 2–99) and 39 ± 24.9 (range, 2–89) months for CT and US, respectively. All patients reported cosmetic and symptomatic improvements.
Table 1. Baseline characteristics of patients who underwent radiofrequency ablation for benign thyroid nodules.
CT-based measures before and after RFA
shows CT images with segmented thyroid volumes and tracheal areas for a representative case, and CT-based measurements obtained before and after RFA are summarized in . The thyroid volume was significantly decreased after RFA (p < 0.001), with a mean CT-based thyroid VRR of 42.3% ± 20.3%. The mean US-based nodule VRR was 66.3% ± 50.7%, which was significantly higher than the CT-based thyroid VRR (p < 0.001). The US- and CT-based VRRs showed moderate consistency (ICC = 0.68, 95% confidence interval = 0.38–0.83, p < 0.001). The tracheal area was significantly increased from 0.46 cm2 before RFA to 0.59 cm2 after RFA (p < 0.001); the mean increase of tracheal area was 0.197 ± 0.3 cm2. shows the mean (with standard errors) VRRs according to the number of RFA sessions; no significant differences were noted for both US-based nodule VRR (p = 0.924) and CT-based thyroid VRR (p = 0.384). Images showing the changes between RFA sessions for a representative case are presented in .
Figure 2. Contrast-enhanced axial (a, b) and coronal (c, d) CT images for a representative case involving a 32-year-old woman with benign nodular hyperplasia in the left thyroid gland. Volumetric segmentations of the thyroid glands are shown in red while cross-sectional segmentation of the trachea is shown in blue.
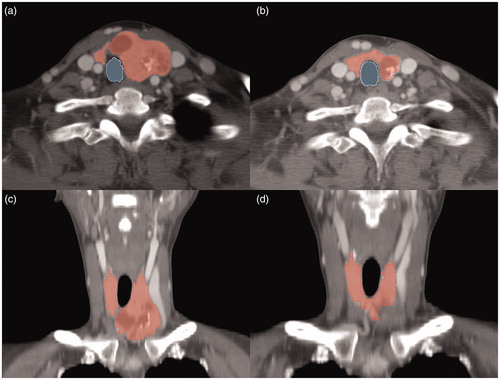
Figure 3. Mean volume reduction rates (with standard errors of means) measured by CT and US according to the number of radiofrequency ablation (RFA) sessions; no significant differences in volume reduction rates was observed according to the number of RFA sessions.
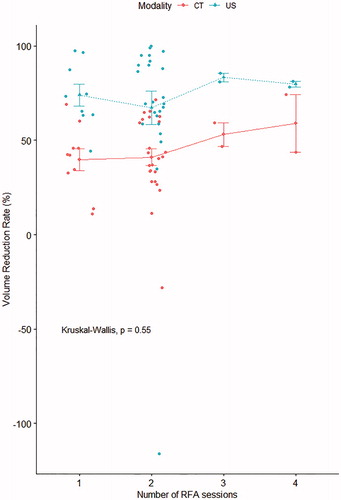
Figure 4. Contrast-enhanced CT images obtained (a) before and (b) after two radiofrequency ablation sessions (interscan interval: 4.5 years) for a 50-year-old woman with a benign nodule in the right thyroid gland. The volume reduction rate was high (65.5%). Normalization of adjacent structures, including an increase in the tracheal area (4 mm2) and anterior neck angle (20°), a decrease in the ratio of anteroposterior/transverse diameter of the trachea (0.31), and a reduction in midline deviation of the trachea (2.3 mm), could be observed.
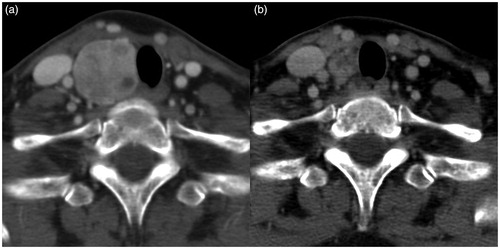
Table 2. Comparison of CT-based measurements obtained before and after radiofrequency ablations for benign thyroid nodules.
Subgroup analysis of patients with intrathoracic masses
In patients with intrathoracically extending masses (n = 14), the mean US-based nodule VRR was 62.4% ± 54.2%, which was significantly higher than the CT-based thyroid VRR (42.3 ± 26.0, p < 0.004) (). The US-based nodule VRR and CT-based thyroid VRR showed good consistency (ICC = 0.75, 95% confidence interval = 0.23–0.92, p < 0.008). Other measurements are summarized in .
Complications
Complications were those previously defined by the Society of Interventional Radiology [Citation30,Citation31]. There were two major complications (5.8%; one permanent voice change and one nodule rupture with abscess formation) and one minor complication (2.9%; hematoma). The patient with the ruptured nodule was hospitalized for 2 days, during which the necrotic tissue was surgically removed by a plastic surgeon. On a follow-up US scan after 4 months, the ruptured abscess showed complete resolution. The patient with hematoma showed spontaneous resolution of the lesion on a follow-up US scan.
Linear regression analysis
Univariate linear regression analysis showed no significant relationship of the CT-based thyroid VRR with the number of RFA sessions (coefficient = 5.81, standard error = 4.54, p = 0.209), mean delivered radiofrequency energy (coefficient = 1.58 × 10−3, standard error = 2.79 × 10−3, p = 0.574), and initial thyroid volume (coefficient = 0.122, standard error = 0.132, p = 0.363).
Discussion
The present study involved CT-based evaluation of the thyroid glands and adjacent neck structures after RFA in patients with benign thyroid nodules. CT was found to be valuable for estimating the structural improvements in the thyroid glands and adjacent neck structures after RFA. Relative to conventional US-based measurement of VRR for the thyroid nodules, CT provided a more objective assessment of VRR for the entire thyroid gland. Furthermore, CT enabled objective assessment of improvements in the neck structures after RFA, including reduced anterior neck protrusion and tracheal deviation and narrowing.
Our findings can be compared with the results of previous studies that evaluated VRR after RFA for benign thyroid nodules. The US-based nodule VRR in the present study was slightly lower than that reported in a previous meta-analysis (66.3% vs. 77.8%) [Citation32]; this could be attributed to the smaller sample size in the present study. Furthermore, other factors affecting VRR, such as the number and size of nodules and the delivered radiofrequency energy, were not accounted for in the meta-analysis.
In the present study, VRR for the entire thyroid gland was calculated using CT-based measurements and compared with the nodule VRR measured by conventional US. Further, subgroup analysis was performed for a smaller number of patients who were partially treated for intrathoracic portion of thyroid masses. The overall thyroid VRR was found to be lower, which is plausible considering the measurement was based on the total thyroid volume rather than the volume of individual nodules. However, ICC for the association between the CT-based thyroid VRR and US-based nodule VRR indicated moderate consistency. The results were also reproduced in subgroup analysis, suggesting the potential application of CT-based thyroid VRR for patients with intrathoracically extending masses.
Univariate linear regression analysis showed no significant influencing factor for VRR in the present study. Jung et al. [Citation33] reported that the mean radiofrequency energy delivered per milliliter of the pretreatment nodule volume was an independent predictor of VRR. However, the previous study failed to detect a significant association between the number of RFA sessions and VRR [Citation2]. Hence, further study is warranted to evaluate factors influencing VRR of the benign thyroid masses after RFA.
Of importance, the advantage of CT over US is the delineation of intrathoracic portion of mass. Assessment of intrathoracic portion of the masses would allow better clinical decision-making prior to RFA treatment. The current study demonstrated significant improvements in various CT-based measurements in patients with intrathoracically extending masses. Despite the small number of patients, the results would provide evidence for future consensus statement on RFA treatment.
The major complication rate in the present study (2 of 38, 5.3%) was higher than the previously reported rate (2.11%) [Citation6]. A possible reason for this discrepancy could be the higher number of RFA sessions performed in the present study. Both patients with major complications had received two RFA sessions, which increased their susceptibility to complications. Another possible reason is selection bias, because we only included patients with availability of both pre- and post-RFA CT scans.
The strength of this study is that the neck structures were quantitatively evaluated, with the generation of reproducible data. CT allows objective nodule measurements and trachea evaluation, which is not feasible with other noninvasive diagnostic investigations. This is particularly important in patients with multinodular goiters, large masses with intrathoracic extension, and airway compression [Citation34]. A recent case report by Shin et al. [Citation35] documented the use of CT for assessment of the tracheal area between RFA sessions and tracheal stent placement. Consistent with their finding, our results showed that the tracheal area was significantly increased after RFA. While the patients in this study were evaluated in terms of subjective cosmetic scores as per physician inspection, we also assessed the anterior neck angle because it reflects the degree of visible protrusion (an acute angle is possibly associated with greater cosmetic concerns). As expected, the angle was significantly increased (more obtuse) after RFA. These findings are in line with those of previous studies reporting cosmetic improvements after RFA in patients with benign thyroid nodules [Citation36,Citation37], thus indicating the feasibility of quantifying cosmetic improvements on the basis of an increase in the anterior neck angle.
This study has several limitations. First, only a small sample size was included because neck CT is not routinely performed for patients with benign thyroid nodules. The main concern is the ionizing radiation exposure to patients with benign diseases, and the cost-benefit of CT needs to be considered. Second, thyroid function tests were not performed between RFA sessions because serum samples were sporadically collected from most patients; however, all patients showed normal thyroid function at the last follow-up visit. In fact, a previous meta-analysis on complications of RFA has reported that post-procedural hyperthyroidism is usually asymptomatic and transient while hypothyroidism is very rare [Citation6]; indeed, one guideline does not recommend routine post-procedural blood test [Citation14]. Third, a single radiologist performed all RFA treatments, and the results may not be generalizable to other settings. Fourth, the relationship between tracheal parameter changes and symptoms was not evaluated. However, intrathoracic thyroid masses are difficult to treat completely, and a longer follow-up could show regrowth of treated intrathoracic thyroid masses, which was documented in one study [Citation38]. Finally, the follow-up interval was relatively short which might have been responsible for relatively less VRR than previous reports with longer-term follow-up [Citation11].
In conclusion, the results of this study provide preliminary evidence of the usefulness of CT as a modality for quantitative evaluations of the neck after RFA for benign thyroid nodules. These findings further support the established role of RFA as an effective treatment modality for benign thyroid nodules.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session–prospective randomized study. Radiology. 2012;263:909–916.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361–367.
- Shin JH, Baek JH, Chung J, et al.; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–395.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19:219–225.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:920–930.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25:163–170.
- Choi Y, Jung SL, Bae J-S, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36:358–367.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25:890–896.
- Aldea Martinez J, Aldea Viana L, Lopez Martinez JL, et al. Radiofrequency ablation of thyroid nodules: a long-term prospective study of 24 patients. J Vasc Interv Radiol. 2019;30:1567–1573.
- Vuong NL, Dinh LQ, Bang HT, et al. Radiofrequency ablation for benign thyroid nodules: 1-year follow-up in 184 patients. World J Surg. 2019;43:2447–2453.
- Walters DM, Wood DE. Operative endoscopy of the airway. J Thorac Dis. 2016;8:S130–S139.
- Kim JH, Baek JH, Lim HK, et al.; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632–655.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376–382.
- Dobnig H, Zechmann W, Hermann M, et al. Radiofrequency ablation of thyroid nodules: “Good Clinical Practice Recommendations” for Austria: an interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr. 2020;170:6–14.
- Kim J-h, Baek JH, Lim HK, et al. Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography. 2019;38:125–134.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–879.
- Kim S, Kim D-Y, An C, et al. Evaluation of early response to treatment of hepatocellular carcinoma with yttrium-90 radioembolization using quantitative computed tomography analysis. Korean J Radiol. 2019;20:449–458.
- Koletsis EN, Kalogeropoulou C, Prodromaki E, et al. Tumoral and non-tumoral trachea stenoses: evaluation with three-dimensional CT and virtual bronchoscopy. J Cardiothorac Surg. 2007;2:18.
- Binar M, Serindere M, Bozlar U, et al. Determining the thyroid gland volume causing tracheal compression: a semiautomated 3D CT volumetry study. Medicina. 2019;55:143.
- Na DG, Lee JH, Jung SL, et al.; Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125.
- Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33:1971–1977.
- Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15:836–843.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W331–W336.
- Baek JH, Lee JH, Sung JY, et al.; Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29:611–618.
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34:784–791.
- Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341.
- Burke DR, Lewis CA, Cardella JF, et al.; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243–S246.
- Lewis CA, Allen TE, Burke DR, et al. Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1997;8:475–479.
- Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100:1903–1911.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Rios A, Rodriguez JM, Canteras M, et al. Surgical management of multinodular goiter with compression symptoms. Arch Surg. 2005;140:49–53.
- Shin JH, Baek JH, Oh Y-M, et al. Combination therapy of temporary tracheal stenting and radiofrequency ablation for multinodular thyroid goiter with airway compression. Korean J Radiol. 2013;14:805–809.
- Cervelli R, Mazzeo S, De Napoli L, et al. Radiofrequency ablation in the treatment of benign thyroid nodules: an efficient and safe alternative to surgery. J Vasc Interv Radiol. 2017;28:1400–1408.
- Ji Hong M, Baek JH, Choi YJ, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol. 2015;26:55–61.
- Sim JS, Baek JH. Long-term outcomes following thermal ablation of benign thyroid nodules as an alternative to surgery: the importance of controlling regrowth. Endocrinol Metab. 2019;34:117–123.
