Abstract
Objectives
This clinical study was developed to primarily evaluate the Complete Cytopathological Response Rate of Cervical Intraepithelial Neoplasms to PDT using chitosan nanocapsules containing Chlorocyan-aluminum phthalocyanine as a photoactive agent. Analyses of the Free Recurrence Interval, toxicity profile (immediate and late), and complications (immediate and late), were secondarily analyzed.
Methods
This study was previously approved by the National Council of Ethics in Research of Brazil (CONEP), on May 28, 2014, under case number 19182113.4.0000.5009. On the surface of the cervix of each selected patient was applied one mL of the formulated gel, and after 30 min, the light was applied. Reports or the identification of adverse effects and/or complications were observed in follow-up visits, in addition to the collection of cervical oncotic cytology.
Results
Out of the total group, 11 (91.7%) primarily treated patients evolved with negative cervical oncotic cytology as soon as in the first evaluation following treatment, and one did not achieve any therapeutic benefit, even after reapplication. Two patients with initially positive response presented cytological recurrence determined by histopathology. A new round of PDT was developed, and both evolved with cytological remission three weeks later, remaining negative until the last follow-up. No important side effects were observed in all the patients.
Conclusions
Our trial demonstrates that treatment of CIN 1 and 2 lesions using our PDT formulation is feasible and safe. Large randomized clinical trials are required to establish efficacy.
Introduction
Drug delivery systems (DDS) represents one of the most promising fields in science, developed to contribute to the improvement of both human and animal health, offering several advantages to conventional pharmaceutical therapies and drug formulation. The investigation of controlled release systems, including the modulation of the dissolution process, reduction of toxicity, and increase of drug uptake, makes it an acceptable and convenient clinical protocol for the patient. Furthermore, these strategies can also make active drugs more available to the specific target tissues, inducing a better and faster therapeutic effect [Citation1]. The development of this new generation of pharmaceutical formulation, allowing the optimization of drug release speed, and the improvement of the dose regime through the controlled release of assets into specific target sites, has been the main goals of numerous studies in recent years [Citation2]. Among the biocompatible raw materials employed in such studies, liposomes, microparticles, and polymeric or lipid nanoparticles are highlighted [Citation3].
Considering the disease targeted in these studies (CIN), one of the DDS evaluated belongs to the class of the chitosan nanoparticles, which presents well-established mucoadhesive properties [Citation4], becoming an option to increase the time of contact with the active drug and to treat the target tissue with a fast release of the active compounds [Citation5]. Besides, the chitosan DDS is insoluble in pH above 6.5. and at low concentrations, it does not present cytotoxicity, as shown by in vitro studies [Citation6] and in more recent in vivo studies on cervical cancer [Citation7].
Cervical cancer is a common neoplasm in women [Citation8–10].
Most cervical neoplasms originate from the junction of the simple columnar epithelium (endocervix) with squamous epithelium (ectocervix). This cervical region is a site of intense alteration, especially in intrauterine periods, puberty, and during the first pregnancy, declining after the menopause. The term Cervical Intraepithelial Neoplasia (CIN), as proposed by Richard [Citation11], designates invasive carcinoma as the precursor of cervical cancer. Microscopic analysis findings corroborate the CIN diagnosis: cellular immaturity, cellular disorganization, nuclear abnormalities, and high mitotic activity. If mitoses and immature cells are present only in the lower third of the cervical epithelium, the lesion is usually designated as CIN1. When lesions compromise lower and middle thirds, they are classified as CIN2, and those that compromise up to the upper region are classified as CIN3 [Citation12].
Lesions classified as CIN1 have a low aggression condition and can either regress spontaneously over time or progress slowly toward CIN2 and CIN3. In clinical practice, patients with CIN1 only receive therapeutic intervention if there is a progression. CIN2 and CIN3 lesions present a risk of progressing more rapidly toward invasive lesions, but in the absence of local extension and lymphatic dissemination, they are treated using local therapeutic approaches such as High-Frequency Surgery (CAF), laser ablation, cryotherapy, or hysterectomy. Hysterectomy is considered a radical treatment for early cervical cancer, whereas the cervix and uterus are usually removed (simple hysterectomy). Laser ablation is a technique that uses a laser beam applied to the area to be treated to destroys the altered cells. Cryotherapy is a procedure that uses a cold chemical (liquid nitrogen) to destroy the wanted cells. High-Frequency Surgery uses electric energy (in high voltage) that is transformed into heat and can cut the desired tissue, avoiding at the same time bleeding. All these modalities have similar curative efficacy and must be chosen depending on the risk to the woman [Citation13]. It is worth noting that the structural damage of the cervix, caused by abrasive or invasive techniques, can compromise patients’ reproductive capacity. In this sense, Photodynamic Therapy (PDT), in cervical neoplasia, is known to preserve cervical competence, favoring the uterus' physiological preservation [Citation14].
Photodynamic Therapy emerges as an alternative noninvasive local treatment for CIN (14). PDT is a clinical protocol that involves the photoactivation of photosensitizer drugs (PS) with visible light in specific wavelengths of absorption. After absorbing the light, these molecules in the excited state induce a cascade of photochemical events that, in the presence of the molecular oxygen present in local tissues, induce a well-localized production of reactive oxygen species (ROS), especially singlet oxygen. These oxygen species react rapidly through type I or II photochemical reactions, leading to cellular death by an apoptotic or necrotic process. These reactive oxygen species can also destroy the tumor's vascularization and trigger a specific immune system response against it, increasing the final effect of cell death. As the main advantages compared to other classical anticancer therapies, such as chemotherapy or radiotherapy, it can be highlighted that PDT works as a rapid, harmless, and local treatment. Moreover, PDT does not induce systemic side effects and can be repeated several times without tumor resistance with a short delay between consecutive therapies [Citation15].
The class of molecules eligible to clinically work as PS should present a long life of the triplet reactive excited species generated by photoactive pathways (greater than 500 ns), enough time to react with molecular oxygen and induce ROS production. We also expected this molecule to present a high absorptivity coefficient in the electromagnetic spectral region, called phototherapeutic window, in the range of visible light, between 600 nm and 800 nm [Citation16]. Furthermore, this molecule should present good solubility and biocompatibility, with the absence of any phototoxicity in the dark, associated with a fast clearance of healthy tissues and body (generally from 24 to 48 h). With such characteristics, a good penetration of visible light, greater than 2 or 3 cm is possible even in slightly pigmented tissues, with minimal risk of undesired side effects such as the destruction of healthy tissues that do not contain the therapeutic agent, mainly when used topically or transmitted with target-specific delivery systems (DDS).
Considering the previous list of described properties for a good PS, three groups of photosensitive molecules can act as effective photosensitizer drugs: Porphyrins, Phthalocyanines, and Chlorins. The family of Phthalocyanines, as 2nd generation molecules, includes a variety of substances that have photosensitizing property, of which phthalocyanine stands out, with activation in the range from 670 to 690 nm and with a clear and well-defined tissue penetration in the target cells [Citation17]. However, most of these photosensitizing molecules are hydrophobic, requiring a designed formulation for their clinical use in drug delivery systems (DDS), such as liposomes, nanoemulsions, nanoparticles, or nanocapsules.
The use of Chloroaluminum phthalocyanine (AlClPc) formulated in nanostructured systems, mainly associated with chitosan nanocapsules, with mucoadhesive property, produces a photosensitizing drug ideal for topical application in mucous membranes. It could also be associated with Protoporphyrin IX as a second photoactive molecule for photodiagostic of the lesion. Its use on the vaginal and cervical epithelium, associated with light stimulation with visible light in the range of 650 nm, with good penetration into the tissue, demonstrates that the DDS has the desired properties for local therapy and theranostic behaviors in the treatment of cervical neoplasia, with preservation of the integrity of the surrounding tissues.
This clinical study was developed to primarily evaluate the Complete Cytopathological Response Rate of Cervical Intraepithelial Neoplasms to PDT using chitosan nanocapsules containing Chlorocyan-aluminum phthalocyanine as a photoactive agent. Analyses of the Free Recurrence Interval, toxicity profile (immediate and late), and complications (immediate and late), were secondarily analyzed.
Materials and methods
Materials
Protoporphyrin IX was purchased from Frontier Scientific®; aluminum phthalocyanine chloride (AlClPc) (Dye content ∼93%), Dimethyl Sulfoxide (DMSO), acetic acid, sodium hydroxide, acetone (HPLC grade) and, Pluronic-F127 were purchased from Sigma-Aldrich Co., USA. The water used in all experiments was ultrapure (Milli-Q).
Methods
Preparation of chitosan-based nanoparticles (CNP) for encapsulation of aluminum phthalocyanine chloride (AlClPc) and protoporphyrin IX (PPIX) photosensitizers
Initially, the apparatus used to prepare the formulations was sterilized using a horizontal benchtop autoclave at 120 °C (p = 2.3 Kg.Fcm−2) for 30 min. The preparation and manipulation of polymeric formulations were performed in aseptic conditions using a biological safety cabinet (ESCO Class II). First, a 10 mL chitosan (Sigma-Aldrich) solution of low molecular weight was prepared at a 0.25% concentration in an acid medium based on a 0.25 mol/L solution of acetic acid under controlled agitation. Afterward, a fraction of each ethanolic stock solution containing the active compounds protoporphyrin IX and aluminum phthalocyanine chloride was added to the reaction, respectively, for each photosensitizer. For the neutralization of the acid medium, 2.0 mL of a sodium hydroxide solution at a 0.25 mg/mL concentration (Sigma-Aldrich) was added during the homogenization process using an Ultra Turrax T25 disperser (IKA Labortechnik, Staufen, Germany) attached to a rod, processing volumes up to 10 mL. Chitosan nanoparticles (NQ) were recovered after centrifugation at 10,000 G for 60 min and then resuspended in ultrapure water and stored in a refrigerator at 4 °C for 12 h. Finally, the NQ were subjected to a gelling process using 23% (w/v) of Pluronic-F127 to obtain a hydrogel in the final pharmaceutical form of CNP (jellified chitosan nanoparticles), CNP-AlClPc, CNP-PPIX, or CNP-Mixed (chitosan nanoparticles loaded with AlClPc or PPIX).
Quantification of drug incorporation efficiency
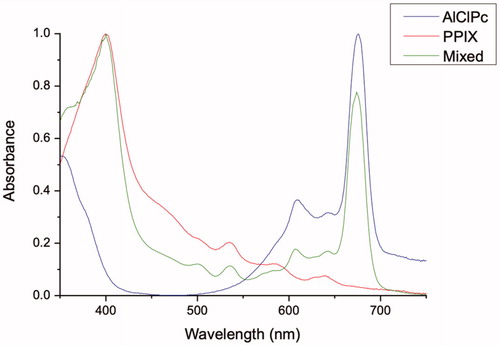
The absorption spectrum of CNP-AlClPc and CNP-PPIXs was determined by a UV-visible absorption spectroscopy technique in the stationary state using an Ultraspec 7000 Dual-beam Spectrophotometer (GE Healthcare Life Sciences, Sweden). The scan analysis to obtain the maximum wavelength (λmax) for the photosensitizers was performed through the dilution of the samples, executed ten times in a 1:1 ratio of saline phosphate buffer and acetone. All analyses were performed on quartz buckets of 1 cm optical path at room temperature, using the spectral interval of 350–750 nm.
To determine the amount of AlClPc or PPIX in the CNP, 50 μl of the formulation was dissolved into 50 μl of DMSO to ensure the full release of the drug from the formulation. Absorption and fluorescence measurements were performed in an acetonitrile medium. The reading was performed on the UV/VIS.
Determination of particle size and zeta potential
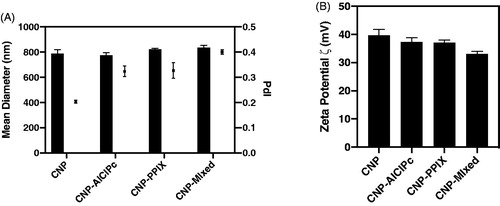
The mean particle diameter of the prepared hydrogels and their size distribution (polydispersion index – IPd) were determined by dynamic light scattering analysis (DLS) using a Zetasizer® (Nano ZS, Malvern Instruments, UK). The analyses were performed in a 173° scanning angle after the dilution of 20 μL of the formulations into 2 mL of ultrapure water. The zeta potential (ζ) was determined by the electrophoretic mobility of colloidal samples using the same previously mentioned apparatus (Zetasizer®). All determinations were performed in triplicate, at 25 °C, with dilution identical to that described above.
Shelf life determination
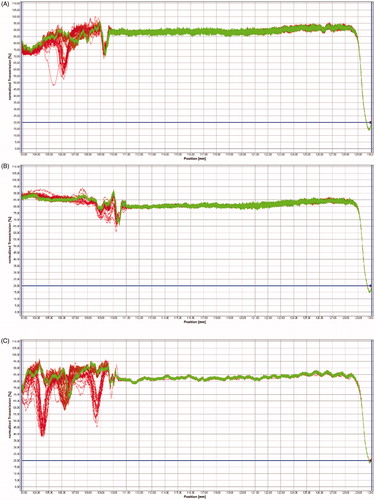
Shelf life studies were performed using the analytical centrifugation method with photometric detection characterized by STEP-Technology® (Space- and Time-resolved Extinction Profiles) in a LumiSizer 612v Dispersion Analyzer (LUM Ltd., Berlin, Germany), through which a 400 µL volume of hydrogels was placed in the LUM 2 mm, PC, Rect. Synthetic Cell (110–131xx), and subjected to a 3655 rpm rotation at 25 °C. A total of 255 profiles were recorded (in 880 nm) to evaluate the transmission profile and particle sedimentation (velocity), using the SEPView® 5.1 software.
Viscosity profile determination with temperature variation
The viscosity related to the temperature of the produced hydrogels was measured with a Compact Modular Rheometer – MCR 52 (Anton-Paar GmbH, Graz, Austria), using the RHEOPLUS software. The analysis was performed with a cone-and-plate measurement geometry CP50-1, (diameter: 50 mm; cone angle: 1°). Afterward, 0.5 mL of the samples were added at 4 °C ± 2, being measured at a constant rotation speed of 150 rpm, temperature variation of 4–40° C with a temperature increase of 2 °C.min−1. The data (viscosity vs. temperature) was then plotted into the RHEOPLUS software.
Clinical trial
As defined in the CONSENT Form of the National Council of Ethics in Research of Brazil (CONEP), approved by the Ministry of Health on 28 May 2014, under case number (Certificate of Presentation for Ethical Appreciation) 19182113.4.0000.5009, the patients included in this study should meet the following requirements: being female with 18 years of age or older, and having the diagnosis of CIN1 or CIN2 by histopathological analysis. The exclusion criteria, defined by the same instrument, were: the presence or suspicion of invasive neoplasia, photosensitivity or allergy previously reported to any photoactive substance (whether or not in the form of a biocompatible gel), patent hymen, vaginal atrophy, previous treatment for CIN, ongoing pregnancy, and patients with acquired immunodeficiency syndrome. The selected patients should expressly authorize that they approved their free participation in the trial by signing the Free and Informed Consent Form (TCLE).
Clinical procedures
In the first visit of the patient, anamnesis and analysis and recording of diagnostic and epidemiological data were performed to guide the treatment and deadlines of follow-up consultations, in addition to the reading and signing of the TCLE.
Photodynamic therapy procedures
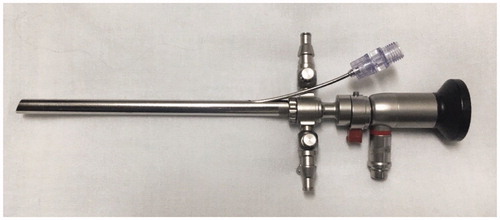
A continuous Laser Lighting system, developed in partnership with the DMC Equipamentos® Ltda (São Carlos-SP, Brazil), was used as a light source, operating from 300 to 400 mW of power in the range of 650 nm, with adjusted irradiation time. This system was adapted to promote diffuse irradiation, reaching every area involved in the treatment through an endovaginally introduced exchange. It is constituted by a lighting and image capture system, and an optic fiber passage channel that allows the targeting of the laser to the inside of the vagina. For PDT application, the patient was placed in a gynecological position, and the vaginal speculum was introduced to allow complete visualization of the cervix, which was cleaned with a physiological solution and gauze, followed by the application of a volume of 1 mL of the formulated gel with the aid of a plastic spatula on the surface of the cervix. The speculum was removed for light occlusion for 30 min. Then the Access vaginal trocater was introduced, along with an optical fiber for red laser lighting, observing a minimum dose of 200 J, power of 1.20 W, irradiance of 0.30 W.cm−2, and fluency of 50 J.cm−2 (). Reports or the identification of adverse effects and/or complications were observed in follow-up visits, in addition to the collection of cervical oncotic cytology in a liquid medium.
After undergoing treatment, patients were scheduled to be evaluated weekly in the first month (4 consultations). Each evaluation consisted of anamnesis, which analyzed adverse effects and complications, in addition to gynecological examination with video colposcope and collection of cervical oncotic cytology in a liquid medium. Those patients who did not meet cytological and/or videocolposcopic criteria for remission continued for weekly evaluations for another four weeks. After this limit, if the patient still showed signs of the disease, they were considered unresponsive and referred to conventional therapy (electrocauterization or conization), or even another section of TFD. Patients who presented complete cytopathological and/or videocolposcopic responses, within a maximum period of 8 weeks of immediate follow-up, were followed up on an outpatient basis with monthly medical consultations in the first trimester after remission, and bimonthly from the second to the fourth trimester.
Statistical analysis
Two statistical tests were carried out looking at the effect of the number of treatments on results (positive or negative) i) Logistic regression and ii) Chisq using Fisher's exact due to a low number of repetitions. Both tests showed no significant difference between the number of treatments. Odds ratio was 3.7 (CI 0.32–41.59).
Results
Drug loading
After loading the AlClPc or PPIX in CNP, the CNP was quantified by absorption spectroscopy () and presented a final concentration of 30 µmol/L and 20 µmol/L of PPIX and AlClPc respectively.
Diameter and zeta potential
The average size, PdI, and zeta potential () were used as indicators of stability.
Shelf life determination
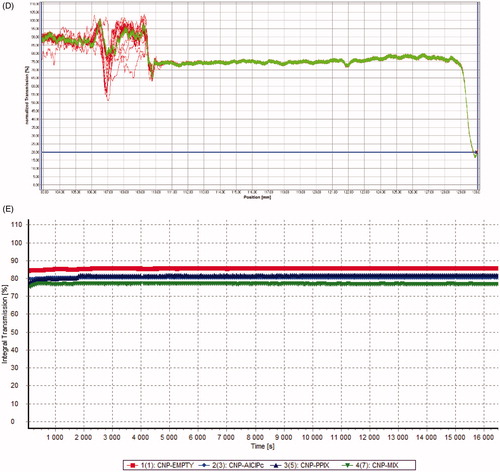
The determination evaluated the STEP Profiles and the transmission integral () after production at 25 °C, revealing that the formulation remains stable while stored at room temperature.
Figure 4. STEP Profiles of CNP (A), CNP-AlClPc (B), CNP-PPIX (C), CNP-Mixed (D), and transmission integral (E) measured after production at 25 °C.
In the 12-month study of long-term stability (shelf life), we did not detect any substantial changes for any hydrogels when comparing the final transmission profiles with the initial profiles, as the slope reaches almost zero and the initial and final profiles stay in the same position. This result indicated that the particles remained stable throughout the experiment. These parameters predicted that all produced hydrogels remain stable for at least one year.
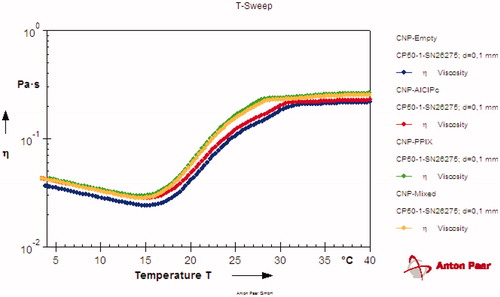
Viscosity profile determination with temperature variation
The gelation temperature of the formulations is an essential parameter for this work, as the goal of these hydrogels are intra-vaginal delivery. The parameters observed by rheometry are available in .
This result shows that the hydrogel stays in sol form between 4 and 20 °C, and turn into gel form around 30 °C. Considering this, at 36–37 °C (human body temperature), the formulations remain a gel for application, and since the pH of the region is acidic (between 2 and 4), the acid present in the region will protonate the chitosan and then release the encapsulated photosensitizers for the treatment.
Clinical trial
A total of 14 patients were treated, although only 12 were considered for analysis. One patient resigned treatment without justification, nor provided any information on her decision, along with another patient diagnosed with pregnancy one week after PDT. It is important to report that, although the case was removed from the analysis, it was observed that PDT does not bring any harm to pregnancy or the formation of the conceptus.
Patient's ages ranged from 18 years to 52 years, with an average of 24.5 years. Epidemiologically, we did not identify any factors that could have relevance in this analysis other than those reported in the usual literature. According to the histological classification, 91.7% (11) of the patients presented Low-Grade Intraepithelial Lesion, CIN 1, and 8.3% (1) presented High-Grade Intraepithelial Lesion, CIN 2 ().
Table 1. Patients’ characteristics and treatments.
In the group of patients subjected to the analysis, 15 PDT sessions were performed since three patients were treated for a second time due to the recurrence of the pathology (). As this was a pilot study, for the first section, there were variations in the amount of luminosity (200–300 J), power (1.20–2.00 W), irradiance (0.40–0.70 W/cm2), fluence (50–80 J/cm2), and the laser modality was continuous light (12). All reapplications, in relapses, used doses of 300 J of pulsed light and fluence of 80 J/cm2. The applications occurred in an average time of 92 s (80–106).
Table 2. Baseline patient: clinical: trial characteristics; and patient outcome.
Regarding immediate toxicity, there were only mild complaints of heat in the lower abdomen during laser application. However, no patient presented any situation that limited the continuity or required treatment modification. Only one patient brought clinical complaints of pelvic pain and frequent urination in her first follow-up visit, which was diagnosed as a low urinary infection, solved with a urine culture-guided antibiotic therapy.
Out of the total group, 11 (91.7%) primarily treated patients evolved with negative cervical oncotic cytology as soon as in the first evaluation following treatment, which ranged from 2 to 4 weeks (average of 2.5 weeks), and one only did not achieve any therapeutic benefit, even after reapplication. The first two patients with initially positive response presented cytological recurrence determined by histopathology. The first patient was diagnosed with CIN2 and relapsed after three months of negativity, and her histological classification of relapse was CIN1 (). The second patient maintained the CIN1 diagnosis after four months of the initial remission. These patients received initial applications with continuous light at the power of 1.20 W in a 200 J dose, the same parameters used for the refractory patient. New TFD was performed with pulsed light at the power of 2.00 W, in a 300 J dose, and both evolved with cytological remission three weeks later, remaining negative until the last follow-up. The mean follow-up of the patients was 6.16 months (3–10 months).
Discussion
Cervical neoplasms are a serious public health problem in several countries, usually developing from superficial epithelial lesions. The treatment of these superficial lesions is very variable in medical practice; however, the structural damage of the cervix, caused by abrasive or invasive techniques, can compromise the reproductive capacity of patients. It can be a differential of PDT in cervical neoplasia, as it is known to preserve cervical competence, favoring the physiology of the uterus [Citation14]. This data should be highlighted when we verify that our patients presented a mean age of 24.5 years, during the fertile age.
Our study evaluated PDT in the treatment of initial lesions of the cervix, with good efficacy in 91.7% of cases. Moreover, no toxicity or complications were observed, especially in the anatomical and tissue structure of the cervix.
Patients that presented recurrence or refractoriness were the first included in the study (patients 1, 2 and 3), when the parameters were still being adjusted, receiving applications with lower irradiance values (0.30 W/cm2) and fluence (50 J/cm2) compared to those applied in subsequent patients. These factors may explain early recurrence and are also reinforced by the fact that there was no second remission, after the retreatment, at the dose of 300 J, irradiance of 0.50 w/cm2, and fluence of 80 J/cm2. Besides, all the other patients that received higher fluence had a positive response to the treatment. The non-responder patient presented an extensive lesion in the uterine cervix, affecting more than 50% of the mucous surface, which may have made it difficult to approach her entire cervix, perhaps requiring sequential applications. However, for safety reasons, this patient received conventional treatment as required by the trial protocol.
Even though this pilot study used, in most cases, patients with low-grade of intraepithelial injury, CIN 1, a negative cytopathological examination collected in patients in a short time (average of 2.5 weeks) is observed after a single application of therapy, an unusual fact in the natural history of the disease [Citation18].
Success rates in studies published with PDT and cervical neoplasia vary from 68% to 90% [Citation14,Citation19–21]. This difference can be explained by the heterogeneity of techniques, ranging from the systemic or topical use of the photosensitizer, the light source employed, and the group of neoplasms included, some with CIN3. What draws the most attention is the difference in toxicity, since the drug's application route is intravenous, with reports of skin photosensitivity, sometimes requiring hospitalization for the use of a protocol of luminous desensitization, despite high rates of cytological response [Citation22]. In studies using topical aminolaevulinic acid in the cervix, the tolerability data on toxicity are very close to what we found in our sample [Citation20–23]. A significant differential of the method employed in this research is the absence of toxicity when compared to the use of systemic photosensitizers, besides the shorter time in the application of the therapy, since the time between the application of dye and lighting does not require a long absorption interval, decreasing the discomfort of the patient [Citation22].
As a perspective, the CNP can also serve as a platform for combined therapies and for improving the release of the photosensitizers. The co-encapsulation of photosensitizers and superparamagnetic nanoparticles or photothermal agents, for instance, can enable us to combine two different modalities of cytotoxic strategies. The combination of PDT and magnetohyperthermia has shown to be more effective than the isolated approaches against different malignant cells in vitro [Citation24,Citation25]. Magnetically or photogenerated heat can also be used to increase the release of the photosensitizers to the target cells, once the formulation is applied to the affected area [Citation26,Citation27].
Conclusion
Based on the above, we believe that the treatment with AlClPc-chitosan nanoparticles in gel form, associated with red laser stimulation, is a promising and safe therapeutic modality of CINs and, if considering the absence of toxicity, perhaps a preventive therapy that can be employed in high-risk populations even in the absence of routine screening tests.
Author contributions
All authors read and approved the final manuscript.
Acknowledgements
The author also thanks DMC – São Carlos, Sao Paulo, Brazil, and Research and Education Center for Phototherapy in Health Science (NUPEN), São Carlos, SP, Brazil, for the adjustment and development of the light activation device used in all this research and clinical studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Evangelista R. Sistemas de liberação controlada de fármacos. Araraquara: Tese. 2006.
- Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1–2):3–9.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:117739280700200. 117739280700200002.
- Liang Y, Zhao X, Ma PX, et al. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci. 2019;536:224–234.
- Hanafy NA, Leporatti S, El-Kemary MA. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl Sci. 2019;9(5):825.
- Lu B, Lv X, Le Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers. 2019;11(2):304.
- Yang J, Li S, Guo F, et al. Induction of apoptosis by chitosan/HPV16 E7 siRNA complexes in cervical cancer cancer cells. Mol Med Rep. 2013;7(3):998–1002.
- INCa. 2020. Incidência de Câncer no Brasil. Estimativa 2016. Instituto Nacional de Câncer José Alencar Gomes da Silva 2016; [cited 2020 July 31]. Available from: http://www.inca.gov.br/estimativa/2016. 2020.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136.
- Lando M, Holden M, Bergersen LC, et al. Gene dosage, expression, and ontology analysis identifies driver genes in the carcinogenesis and chemoradioresistance of cervical cancer. PLoS Genet. 2009;5(11):e1000719.
- Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–328.
- DeVita VT, Lawrence TS, Rosenberg SA. Cancer: principles and practice of oncology-advances in oncology. Philadelphia: Lippincott Williams & Wilkins; 2010.
- Martin-Hirsch PP, Paraskevaidis E, Bryant A, Dickinson HO, et al. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001318.
- Muroya T, Suehiro Y, Umayahara K, et al. Photodynamic therapy (PDT) for early cervical cancer. Gan to Kagaku Ryoho. 1996;23(1):47–56.
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545.
- Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol, B. 1997;39(1):1–18.
- Reddi E, Jori G. Steady-state and time-resolved spectroscopic studies of photodynamic sensitizers: porphyrins and phthalocyanines. Rev Chem Intermed. 1988;10(3):241–268.
- Östor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192.
- Monk BJ, Brewer C, VanNostrand K, et al. Photodynamic therapy using topically applied dihematoporphyrin ether in the treatment of cervical intraepithelial neoplasia. Gynecol Oncol. 1997;64(1):70–75.
- Hillemanns P, Korell M, Schmitt-Sody M, et al. Photodynamic therapy in women with cervical intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Int J Cancer. 1999;81(1):34–38.
- Wierrani F, Kubin A, Jindra R, et al. 5-aminolevulinic acid-mediated photodynamic therapy of intraepithelial neoplasia and human papillomavirus of the uterine cervix-a new experimental approach. Cancer Detect Prev. 1999;23(4):351–355.
- Yamaguchi S, Tsuda H, Takemori M, et al. Photodynamic therapy for cervical intraepithelial neoplasia. Oncology. 2005;69(2):110–116.
- Bodner K, Bodner-Adler B, Wierrani F, et al. Cold-knife conization versus photodynamic therapy with topical 5-aminolevulinic acid (5-ALA) in cervical intraepithelial neoplasia (CIN) II with associated human papillomavirus infection: a comparison of preliminary results. Anticancer Res. 2003;23(2C):1785–1788.
- Bolfarini GC, Siqueira-Moura MP, Demets GJF, et al. In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbit. J Photochem Photobiol B Biol. 2012;115:1–4.
- Primo FL, Rodrigues MMA, Simioni AR, et al. Photosensitizer-loaded magnetic nanoemulsion for use in synergic photodynamic and magnetohyperthermia therapies of neoplastic cells. J Nanosci Nanotechnol. 2008;8(11):5873–5877.
- Lin J, Wang S, Huang P, et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7(6):5320–5329.
- Hong EJ, Choi DG, Shim MS. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm Sin B. 2016;6(4):297–307.