Abstract
Background
Whether thyroid function would be affected by ablation remains controversial. This systematic review and meta-analysis aimed to investigate the effects of energy-based ablation on thyroid function in treating benign thyroid nodules.
Methods
EMBASE, PubMed, Cochrane Library, and Web of Science databases were searched. The mean difference (MD) or standard MD (SMD) was applied to assess changes in thyroid function, thyroglobulin (Tg), and antibodies after ablation. RevMan version 5.3 was used for data synthesis.
Results
Forty-two studies involving 6380 patients were eligible. The pooled results revealed significant decrease of 1-day thyroid-stimulating hormone (95% CI, −0.67 to −0.14), significant increase of 1-day, 1-week, and 1-month free thyroxine (95% CI, 1.57 to 5.28; 95% CI, 0.61 to 2.42; 95% CI, −0.76 to −0.15), 1-day and 1-week Tg level (95% CI, 0.40 to 0.81; 95% CI, 0.21 to 1.29), 6-month anti-thyroglobulin antibodies (95% CI, 0.02 to 0.26), 1- and 3-month thyroperoxidase antibody (95% CI, 0.02 to 0.22; 95% CI, 0.17 to 0.43), and 1-day, 1-, and 3-month thyrotrophin receptor antibody (95% CI, 0.10 to 0.43; 95% CI, 0.00 to 0.30; 95% CI, 0.13 to 0.36) after ablation. No statistically significant differences were found in these six indicators in the longer term. The results of subgroup analysis were similar to the pooled results. No significant publication bias was found.
Conclusions
Energy-based ablation was more likely to have negative effects on thyroid function and antibodies and led to transient increase in Tg level in the short term. However, most of the patients would not develop any thyroid dysfunction in the long-term follow-up.
Introduction
In recent years, the incidence of thyroid nodule has increased dramatically, and it becomes a common complication of endocrine surgery. Approximately 90% of the thyroid nodules are benign, of which only approximately 5% of the nodules could cause symptoms of compression [Citation1]. At present, the main management strategy of benign nodules is follow-up observation, and the follow-up period was determined according to risk stratification based on the ultrasound pattern [Citation2]. Based on 2015 ATA Management Guidelines, routine thyroid-stimulating hormone (TSH) suppression was not recommended to treat benign nodules. The surgery can only be considered when the nodule larger than 4 cm in diameter after repeated fine needle aspirations still presents as a benign nodule, compressive or structural symptoms appear, or patients have cosmetic requirement [Citation3]. Thus, there is an urgent need of novel treatments for patients who want to treat the nodule but are unwilling to undergo surgery.
The ‘image-guided ablation’ has been proposed for the last two decades as treatment of benign nodules that required treatment and plays an increasingly crucial role in the management of thyroid nodules [Citation4]. Compared with surgery, image-guided ablation has been proved to be effective and safe to relieve compressing symptoms and meet patients’ requirements for treatment without scars, especially for young or female patients [Citation5]. The types of ablation include chemical ablation and energy-based ablation. The latter consists of radiofrequency ablation (RFA), laser ablation (LA), microwave ablation (MWA), and high-intensity focused ultrasound (HIFU), and medical centers adopt different technologies. As the number of patients treated with energy-based ablation increased, the complications, side effects, and risks have been also observed. Pain is the most common complaint. Additionally, voice changes, perithyroidal hemorrhage, hematoma, and skin burn may also occur. Therefore, full informed consent was routinely required at pre-ablation [Citation6]. Recently, the effects of energy-based ablation on thyroid function have gained wide concern.
Previous studies have reported both the effects and complications of ablation therapy. Several studies by Kristina, Hong, and Korkusuz suggested that energy-based ablation could treat thyroid nodule and at the same time preserve thyroid function [Citation7–9]. However Valcavi et al., Cesareo et al., Zhi et al., and Lang et al. observed that some patients with normal thyroid function before therapy had developed thyroid dysfunction at follow-up for unknown reasons [Citation10–12]. In these studies, most thyroid dysfunctions occurred within 1 year after treatment.
With the above background, it remains unclear whether energy-based ablation therapy would have significant effects on thyroid function. Therefore, we conducted a systematic review and meta-analysis, combined with previous studies, to investigate the effects of energy-based ablation therapy on thyroid function in patients with benign thyroid nodules.
Materials and methods
Search strategy
Two independent investigators conducted a comprehensive literature search of EMBASE, PubMed, Cochrane Library, and Web of Science from their inception dates to 10 March 2020 with the restriction of English language. MeSH terms and free-text words were used to increase sensitivity. Conference abstracts, references of associated articles, and reviews were further hand-searched to identify potential eligible studies. The following key terms were used: ‘laser’ OR ‘radiofrequency’ OR ‘microwave’ OR ‘ablation’ and ‘thyroid nodule’. Endnote X8 was used for document management.
Eligibility criteria
The inclusion criteria of this meta-analysis were as follows: (i) original studies reported thyroid function or thyroid antibody before and after ablation of thyroid nodule; (ii) studies included patients with benign nodules, which were confirmed on fine needle aspiration biopsy; (iii) studies included patients with normal TSH levels (0.35–4.5 mIU/L) and normal free thyroxine (FT4) and free triiodothyronine (FT3) levels (12.0–22.0 and 3.60–7.50 pmol/L, respectively) before ablation therapy; and (iv) studies included patients without history of cervical radiation therapy and thyroid surgery.
The exclusion criteria were as follows: (i) reviews and meta-analyses, irrelevant literatures or editorials, comments, and case reports and (ii) nonhuman studies.
Study selection
All searched results were evaluated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [Citation13]. All studies were selected after duplication by two researchers independently according to titles, abstracts, and full texts. Two researchers evaluated potential studies strictly by inclusion/exclusion criteria. Any controversy was resolved by the third researcher and by team discussion.
Data extraction
Two researchers (Yuan Fei and Yuxuan Qiu) collected data from all eligible articles in an Excel sheet (Microsoft Corporation, Redmond, WA, USA) independently. The following data were extracted from all included studies: (i) study characteristics (first author, publication year, sample size, study country, age, sex, intervention, follow-up time, and outcome indicators); (ii) thyroid function and antibody parameters at pre-ablation and post-ablation, including TSH, FT3, FT4, thyroglobulin (Tg), anti-Tg antibody (TgAb), and thyroperoxidase antibody (TPOAb), and thyrotrophin receptor antibody (TRAb); (iii) number of participants with new onset of hypothyroidism (including subclinical and overt hypothyroidism) and hyperthyroidism at follow-up after ablation therapy.
Statistical analysis
Data synthesis was accomplished by Cochrane Systematic Review software Review Manager (RevMan; Version 5.3; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, 2014). The results were displayed as Forest plots, and a p < 0.05 was considered statistically significant. Continuous variables were shown as mean and standard deviation (SD). The mean difference (MD) or standardized MD (SMD) with corresponding 95% confidence interval (95% CI) was calculated, when necessary. Statistical heterogeneity among studies was determined by chi-squared statistic and reported by I2. If p < 0.10 or I2 > 50%, the random-effect model was used to calculate pooled effects. Otherwise, the fixed-effect model was applied. A subgroup analysis was also performed to explore whether country, study type, ablation technique, and sample size would affect the pooled results. Sensitivity analyses were conducted to verify the stability and reliability of outcomes by removing the data of one study at a time. Possible publication bias was evaluated via the funnel plot.
Quality assessment
The quality of all eligible studies was assessed with the Newcastle-Ottawa quality assessment scale (NOS) [Citation14]. The NOS consists of three parameters: selection, comparability, and outcome. Studies which earned at least score 6 were considered high-quality studies. Quality assessment was also completed by two independent researchers (Yuan Fei and Yuxuan Qiu).
Results
Search process and study characteristics
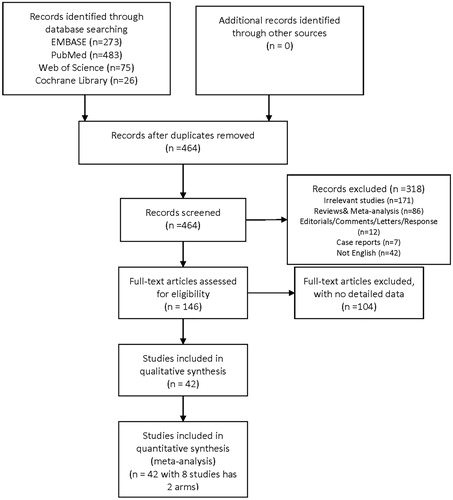
As shown in , a total of 857 related studies were identified from database searches. After removing duplicates, 464 papers were left to be screened. Full-text reading was performed, and 104 studies reporting incomplete data were then excluded. Finally, 42 remaining articles including 6380 patients were selected for qualitative synthesis [Citation7–9,Citation11,Citation12,Citation15–51]. Among these included studies, eight had two arms, and these arms were analyzed separately. Therefore, 49 reports were included for the final meta-analysis.
The study characteristics and quality assessment score are summarized in . Among them, eight reports described incidence of thyroid disorders (hypothyroidism and hyperthyroidism), while others presented detailed data regarding thyroid function and antibody parameters. The main parameters consist of TSH, FT3, FT4, Tg, TgAb, TPOAb, and TRAb.
Table 1 Characteristics of included studies
The types of ablation in the included studies were LA in 16 studies, RFA in 18, HIFU in eight, and MWA in seven.
Meta-analysis
Effects on TSH
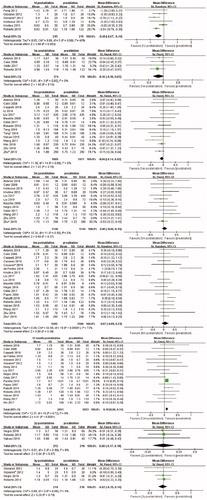
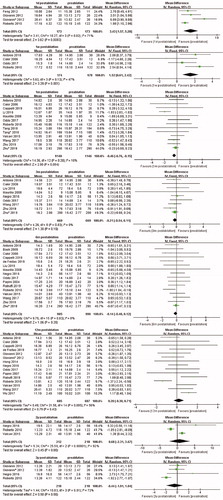
A total of six studies were analyzed. The random effects model was applied due to significant heterogeneity (I2 = 49%, p = 0.08), and the pooled results indicated significant decrease in TSH level at the first day after ablation (MD, −0.40; 95% CI, −0.67 to −0.14). No significant change in TSH level was found at 1 week (MD, −0.16; 95% CI,−0.36 to 0.03), 1 month (MD, −0.06; 95% CI, −0.14 to 0.02), 3 months (MD, 0.05; 95% CI, −0.06 to 0.15), and 6 months (MD, 0.07; 95% CI, −0.09 to 0.23) after ablation. Furthermore, no significant difference was noted at 2 years (MD, 0.00; 95% CI, −0.17 to 0.18) and 3 years (MD, −0.02; 95% CI, −0.19 to 0.14) after ablation ().
Effects on FT4
Four eligible articles reported FT4 levels at the first day after ablation. Random effects model was applied (I2 = 71%, p value for heterogeneity = 0.02), and significant increases were found (MD, 3.43; 95% CI, 1.57 to 5.28). Similarly, the FT4 levels at the first week was significantly higher than that before ablation (MD, 1.51; 95% CI, 0.61 to 2.42). However, results of analysis including 13 studies showed a significant decrease in FT4 level at 1 month after ablation compared with that before ablation (MD, −0.46; 95% CI, −0.76 to −0.15). Moreover, no significant difference in FT4 levels was observed at 3 months after ablation (MD, −0.21; 95% CI, −0.54 to 0.11), similar with that at 6 months (MD, −0.14; 95% CI, −0.40 to 0.12) after ablation. FT4 levels at 12 months, 2 years, and 3 years after ablation were available in 15, 3, and 3 studies, respectively. All results showed that FT4 levels did not present significant difference compared with that at pre-ablation ().
Effects on FT3
Only one study reported FT3 levels at the first day after ablation, so the analysis was abandoned. A total of three studies reported FT3 values at 1 week after ablation. The pooled MD was 0.04 (95% CI, −0.16 to 0.24), and the fixed-effects model was used (I2 = 0%, P value for heterogeneity = 0.58). However, no significant difference was found at 1 month, 3 months, 6 months, 12 months, and 2 years after ablation ().
Effects on Tg
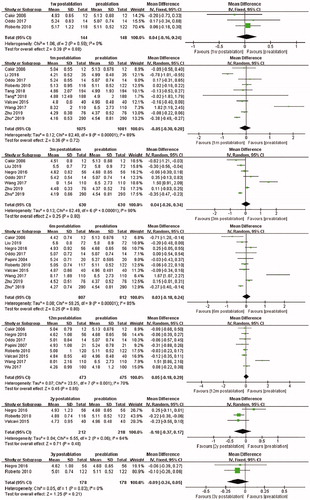
Significant increase in Tg level was found at 1 day (SMD, 0.16; 95% CI, 0.40 to 0.81) and 1 week (SMD, 0.75; 95% CI, 0.21 to 1.29) after ablation. However, the results were still not statistically different at 1, 3, 6, and 12 months of follow-up. Details are all shown in the Supplementary materials.
Effects on TgAb
No significant difference was observed at 1, 3, and 12 months after ablation. However, following analysis of six studies, a significant increase in TgAb level at 6 months after ablation was found (SMD, 0.14; 95% CI, 0.02 to 0.26). Details are shown in the supplementary materials.
Effects on TPOAb
Six studies recorded TPOAb levels at 1 month after ablation, and the pooled results present significant increase compared with that before ablation (SMD, 0.12; 95% CI, 0.02 to 0.22). At 3 months after ablation, the TPOAb level was also significantly higher than that before ablation (SMD, 0.30; 95% CI, 0.17 to 0.43). No significant difference was found at 6 months after ablation. Other details are shown in the Supplementary materials.
Effects on TRAb
Two studies reported TRAb levels at the first day after ablation, and pooled results present significant increase compared with that before ablation (MD, 0.27; 95% CI, 0.10 to 0.43). Three studies presented TRAb levels at 1 month after ablation, and the pooled MD was 0.15 (95% CI, 0.00 to 0.30). At 3 months after ablation, significant increase was also presented following analysis of three articles (MD, 0.25; 95% CI, 0.13 to 0.36). The fixed-effects model was applied in the data analysis of these three time points. Results of the analysis including the two studies showed no significant difference in TRAb levels at 6 months after ablation. Other details are shown in the Supplementary materials.
Cases of new onset of thyroid dysfunction in all included studies are shown in .
Table 2. New onset of thyroid abnormalities.
Sensitivity analysis
Sensitivity analysis was conducted in factors where heterogeneity existed. The TSH level at 6 months after ablation was significantly higher after omitting Liu et al.’s article [Citation34], and heterogeneity was decreased (MD, 0.13; 95% CI, 0.01 to 0.26, p = 0.04; I2 = 51%, P value for heterogeneity = 0.007). In Liu et al.’s study, the TSH level decreased significantly at 6 months after ablation and returned to pretreatment levels at 12 months. The results of the sensitivity analysis showed that the pooled results for the effects of ablation on FT3 were robust. The FT4 level at 12 months after ablation became lower significantly after omitting Valcavi et al.’s article [Citation46], and heterogeneity was reduced (MD, 0.42; 95% CI, 0.09 to 0.75, p = 0.01; I2 = 25%, P value for heterogeneity = 0.19). Four patients undertook therapy for hypothyroidism or hyperthyroidism in Valcavi et al.’s study [Citation46]; hence, the FT4 level was not very stable.
Similarly, after omitting Cakir et al.’s study [Citation50], the heterogeneity became zero, and the Tg level was significantly lower at 3 months than before ablation (SMD, −0.20; 95% CI, −0.39 to −0.01, p = 0.04; I2 = 0%, P value for heterogeneity = 0.86). The Tg level at other time points were very robust. After excluding Zhu et al.’s study [Citation15], the TgAb level at 3 months and TPOAb levels at 3 months and 6 months became significantly higher than those before ablation ().
Table 3. Sensitivity analysis.
Subgroup analysis
We also performed subgroup analysis to explore the effects of country, study design, type of ablation, and sample size on the pooled outcomes. When the ablation type was limited to RFA, the TSH level was significantly lower at 6 months after ablation (MD, 0.18; 95% CI, 0.06 to 0.30, p = 0.002), and heterogeneity decreased (I2 = 17%, P value for heterogeneity = 0.30) (). At 1-month follow-up, the subgroup analysis of RFA studies showed that the FT3 level decreased significantly, and heterogeneity declined slightly (MD, 0.31; 95% CI, 0.08 to 0.53, p = 0.008; I2 = 83%, P value for heterogeneity <0.0001). At 6 months post-ablation, analysis of RFA studies presented significant decrease in FT3 level (MD, −0.11; 95% CI, −0.20 to −0.02, p = 0.02; I2 = 87%, p value for heterogeneity = 0.004) (). In the subgroup analysis at 12 months post-ablation, the FT4 level of studies from China (MD, 0.87; 95% CI, 0.25 to 1.49,p = 0.006; I2 = 0%, P value for heterogeneity = 0.41), studies with RFA therapy (MD, 1.05; 95% CI, 0.33 to 1.77, p = 0.004; I2 = 0%, P value for heterogeneity = 0.56), and studies with MWA therapy (MD, 0.95; 95% CI, 0.30 to 1.60, p = 0.004; I2 = 11%, P value for heterogeneity = 0.29) showed much higher level, and the heterogeneity decreased obviously ().
Table 4. Subgroup analysis of TSH level.
Table 5. Subgroup analysis of FT3 level.
Table 6. Subgroup analysis of FT4 level.
Publication bias
Analysis of publication bias was conducted for the results of TSH levels at 1, 3, 6, and 12 months, FT4 levels at 1, 6, and 12 months, and FT3 levels at 1 and 6 months after ablation. The funnel plot did not reveal significant publication bias ().
Discussion
Given that previous studies reported conflicting conclusions and some studies even paid more attention to ablation efficacy rather than thyroid function changes in the long term, it still uncertain whether energy-based ablation therapy would affect thyroid function.
To our knowledge, this is the first meta-analysis that exclusively investigated the effects of energy-based ablation of benign thyroid nodules on thyroid function and antibody levels. A total of 42 studies including 6380 patients, among which eight studies had two arms, were included in our analysis. We found a significant decrease in TSH level at 1 month and a significant increase at 12 months after ablation. Meanwhile, significant change in FT4 levels only occurred at 1 day, 1 week, and 1 month after ablation. The FT3 level was not significantly affected. The Tg level significantly increased at 1 day and 1 week after ablation, 6-month TgAb, 1- and 3-month TPOAb, and 1-day, 1-, and 3-month TRAb levels all presented significant increase after ablation.
A study acknowledged that energy-based ablation treats thyroid nodules directly through thermal injury and then causes denaturation and necrosis of the heated tissue to achieve the therapeutic purpose [Citation52]. We speculated there are two reasons responsible for our results. First, more FT4 in colloid is released into the blood throughout the therapy process. When the wound heals, the FT4 level returned to its normal level gradually. Moreover, the ablation therapy acts as a stressor to the patient; thus, normal thyroid tissues produce more FT4. However, in this study, the FT3 level did not present a similar change at the early time after ablation. One of the possible reasons was that the included studies were not sufficient to reveal the differences. On the contrary, approximately 75% of the FT3 molecule was transformed by FT4 in the peripheral tissue rather than being produced directly in the thyroid gland. Therefore, the direct effect of ablation is not obvious ultimately. The significant change in TSH levels at 1 day after ablation was due to the negative feedback mechanism of the hypothalamus-pituitary-thyroid (HPT) axis, in which the manifestations were up-regulation of the FT4 level leading to down-regulation of the TSH level. Further studies with larger sample size are needed to demonstrate the mechanisms behind the obvious increase in TSH level at 12 months post-ablation without significant change in FT3 and FT4 needs.
Tg level was usually used to evaluate thyroid mass or thyroid injury. Energy-based ablation leads to massive release of Tg and increase in TgAb level, which is consistent that reported by Valcavi et al. [Citation46]. Cakir et al. [Citation50] reported that Tg increased until the first month after ablation and tended to decrease until 6 months, and the TgAb level had an increasing trend until 3 months and returned to baseline at 12 months. However, in our meta-analysis, the increase in TgAb levels occurred much later than the change in Tg level. It may be related to the number of included studies and sample size. Carracio et al. [Citation53] have put forward a hypothesis that patients would develop autoimmune thyroiditis after ablation because of Tg release. However, in that study, the ablation method was ethanol ablation rather than energy-based ablation. Among those included, some patients developed hyperthyroidism and some need anti-thyroid drugs. In addition, one patient developed Graves’ disease after MWA therapy, but TRAb level was normalized without any treatment; however, the TRAb level of this patient was above the normal limit at pre-ablation; thus, this patient is not representative. In another study, three patients were positive for TRAb after treatment, but all turned negative after 1 year. With regard to the elevation of TRAb following treatment of thyroid nodules, Yu et al. [Citation54] have put forward the following hypotheses: 1) TSH receptor, Tg antigen, and microsomal antigen were secreted from the thyroid, and helper T cells were stimulated to generate autoantibodies, and 2) the abnormality of antigen-presenting cells controlled the activation of inhibitory or regulatory cells and then attacked the immune system. However, Yu et al. had observed that patients underwent thyroid surgery, so the mechanism of ablation deserved further research. We hold the idea that maybe these patients were genetically susceptible, so antibodies increased after ablation. However, none of these concepts has been tested so far. In brief, the onset of autoimmune thyroiditis needs further research.
In Zhu et al.’s study, the ablation procedure in one group was performed using ethanol ablation combined with RFA, and the antibody levels were significantly higher after ablation, which was persistent with Bennedbaek et al.’s [Citation55,Citation56] opinion that the appearance of thyroid autoimmune was one of the major complications of ethanol ablation. After omitting this arm, 3-month TgAb and 3- and 6-month TPOAb levels were still significantly higher level with lower heterogeneity. Therefore, we should attach more importance to the effect of energy-based ablation on antibodies.
The effects of energy-based ablation vary with different types. RFA usually uses less than 900 KHZ of alternating current. It is believed that RFA works by inducing excitation of electrons with a subsequent increase in temperature at the active site of the electrode, which led to tissue necrosis and production of microbubbles at the same time [Citation57]. The clinical phenomenon that RFA is likely to affect thyroid function has been reported in some studies [Citation58,Citation59], which was in line with the subgroup analysis of the TSH level at 6 months, FT3 level at 1 and 6 months, and FT4 level at 12 months after RFA therapy in the present study.
Microwave is applied into thyroid nodules directly through the antennas under ultrasound guidance, which brings about an increase in local kinetic energy, rapid rise of tissue temperature in targeted region, and thermal coagulation of nodules. Microwave wavelengths of 950–2450 MHz produced the desired effects [Citation45]. Up to now, research concerning the effects of MWA therapy to benign nodules on thyroid function is insufficient. Therefore, the subgroup analysis did not clearly indicate the statistical significance of thyroid function between before and after ablation.
LA was first conducted in animal experiments by Brown et al. Seventeen years later, Pacella et al. [Citation60] applied it into thyroid tissue and concluded that LA could be an effective therapeutic tool on selected thyroid tumors. Laser diodes or neodymium:yttrium aluminum garnet was the source of energy. The scattering of laser photons causes an increase in temperature up to 60 °C in the target lesion, which leads to tissue necrosis and subsequent fibrosis. Thyrotoxicosis has been reported in a small number of patients after LA treatment [Citation16,Citation61]. However, in our study, the subgroup analysis showed that FT3 and FT4 levels were not significantly different, except that the TSH level markedly increased at 12 months after ablation. Thus, whether this change has specific clinical significance needs further discussion.
HIFU takes advantage of heat induced by focused ultrasound beams applied by probe without any skin penetration. The focused ultrasound beam reaches an energy concentration and results in local hyperthermia and tissue coagulation. The whole process usually takes one to a few hours to achieve goals of treatment, and concomitant analgesic therapy is required. In 2004, Esnault et al. [Citation62] once chose ewe as an animal model to test the feasibility of HIFU to ablate thyroid tissue. The application of HIFU in human treatment gradually developed thereafter. However, its application is still not as much as the former three methods. Hence, whether thyroid function was influenced by HIFU still needs detailed explorations and large sample-size studies.
This study has some limitations. First, a high heterogeneity was found in some pooled results which may be due to the (i) retrospective study design, (ii) source and number of populations, and (iii) varied ablation types. We attempted to explore it between studies by pooling the data by sensitive analysis and subgroup analysis. Second, the change in thyroid hormone level was often overlooked or not recorded in detail in energy-based ablation studies. Thus, more attention is needed to pay for this complication. Third, not all thyroid antibodies of patients were normal among included studies. As a result, thyroid antibody could be considered a confounding factor, which was not addressed by performing more subgroup analyses or adjusting results due to lack of original data. Finally, all included studies were published in English, which means that some eligible studies might have been excluded because of language restrictions.
In conclusion, energy-based ablation was more likely to have negative effects on thyroid function and antibodies in the short term and led to transient increase in Tg level. However, the majority of patients would not develop any thyroid dysfunction during long-term follow-up. These conclusions need to be verified in the future.
Consent
The authors obtained consent from participants to participate in the study and to publish their data. All authors have read and approved the manuscript.
Supplemental Material
Download TIFF Image (16.4 MB)Supplemental Material
Download TIFF Image (18.6 MB)Supplemental Material
Download TIFF Image (16.5 MB)Supplemental Material
Download TIFF Image (10.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data collected to support the findings of this study are included within the article.
References
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926–935.
- Durante C, Grani G, Lamartina L, et al. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319:914–924.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133.
- Døssing H, Bennedbaek FN, Karstrup S, et al. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation–initial experience. Radiology. 2002;225:53–57.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376–382.
- Shi YF, Zhou P, Zhao YF, et al. Microwave ablation compared with laser ablation for treating benign thyroid nodules in a propensity-score matching study. Front Endocrinol. 2019;10:874.
- Korkusuz H, Sennert M, Fehre N, et al. Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Fortschr Röntgenstr. 2015;187:1011–1015.
- Ji Hong M, Baek JH, Choi YJ, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. JVIR. 2015;26:55–61.
- Heck K, Happel C, Grunwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. 2015;31:560–567.
- Zhi X, Zhao N, Liu Y, et al. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34:644–652.
- Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab. 2017;61:173–179.
- Valcavi R, Tsamatropoulos P. Health-related quality of life after percutaneous radiofrequency ablation of cold, solid, benign thyroid nodules: a 2-year follow-up study in 40 patients. Endocr Pract. 2015;21:887–896.
- Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
- Zhu Y, Zhang M, Jin Z, et al. Solid benign thyroid nodules (>10 ml): a retrospective study on the efficacy and safety of sonographically guided ethanol ablation combined with radiofrequency ablation. Int J Hyperthermia. 2020;37:157–167.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomised, parallel, open-label, trial (LARA trial). Thyroid. 2020;30:847–856.
- Rabuffi P, Spada A, Bosco D, et al. Treatment of thyroid nodules with radiofrequency: a 1-year follow-up experience. J Ultrasound. 2019;22:193–199.
- Negro R, Greco G. Unfavorable outcomes in solid and spongiform thyroid nodules treated with laser ablation. EMIDDT. 2019;19:1041–1045.
- Liu SY, Guo WH, Yang B, et al. Comparison of stress response following microwave ablation and surgical resection of benign thyroid nodules. Endocrine. 2019;65:138–143.
- Lang BHH, Woo YC, Chiu KW. Two sequential applications of high-intensity focused ultrasound (HIFU) ablation for large benign thyroid nodules. Eur Radiol. 2019;29:3626–3634.
- Lang BHH, Wong CKH, Ma EPM, et al. A propensity-matched analysis of clinical outcomes between open thyroid lobectomy and high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Surgery. 2019;165:85–91.
- de Freitas RMC, Miazaki AP, Tsunemi MH, et al. Laser ablation of benign thyroid nodules: a prospective pilot study with a preliminary analysis of the employed energy. Lasers Surg Med. 2020;52:323–332.
- Cappelli C, Franco F, Pirola I, et al. Radiofrequency ablation of functioning and non-functioning thyroid nodules: a single institution 12-month survey. J Endocrinol Invest. 2020;43:477–482.
- Ben Hamou A, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules?. Int J Hyperthermia. 2019;36:666–676.
- Xiaoyin T, Ping L, Dan C, et al. Risk assessment and hydrodissection technique for radiofrequency ablation of thyroid benign nodules. J Cancer. 2018;9:3058–3066.
- Wei Y, Qian L, Liu JB, et al. Sonographic measurement of thyroid nodule changes after microwave ablation: relationship between multiple parameters. Int J Hyperthermia. 2018;34:660–668.
- Rahal Junior A, Falsarella PM, Mendes GF, et al. Percutaneous laser ablation of benign thyroid nodules: a one year follow-up study. Einstein (Sao Paulo, Brazil). 2018;16:eAO4279.
- Oddo S, Felix E, Mussap M, et al. Quality of life in patients treated with percutaneous laser ablation for non-functioning benign thyroid nodules: a prospective single-center study. Korean J Radiol. 2018;19:175–184.
- Lang BHH, Woo YC, Chiu KW. Changes in serum thyroglobulin and antithyroglobulin shortly following high-intensity focused ablation of benign thyroid nodules in patients with positive antithyroglobulin status. Int J Hyperthermia. 2018;35:637–643.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19:167–174.
- Wu W, Gong X, Zhou Q, et al. US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr J. 2017;64:1079–1085.
- Wang B, Han ZY, Yu J, et al. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia. 2017;33:459–464.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:1–9.
- Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med (Maywood). 2017;242:1515–1523.
- Lang BHH, Woo YC, Chiu KWH. The percentage of serum thyroglobulin rise in the first-week did not predict the eventual success of high-intensity focussed ablation (HIFU) for benign thyroid nodules. Int J Hyperthermia. 2017;33:882–887.
- Lang BHH, Wong CKH, Ma EPM. Single-session high intensity focussed ablation (HIFU) versus open cervical hemithyroidectomy for benign thyroid nodule: analysis on early efficacy, safety and voice quality. Int J Hyperthermia. 2017;33:1–74.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7:9554.
- Negro R, Salem TM, Greco G. Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia. 2016;32:822–828.
- Li XL, Xu HX, Lu F, et al. Treatment efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation for benign thyroid nodules. BJR. 2016;89:20150858.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100:460–466.
- Gambelunghe G, Fede R, Bini V, et al. Ultrasound-guided interstitial laser ablation for thyroid nodules is effective only at high total amounts of energy: results from a three-year pilot study. Surg Innov. 2013;20:345–350.
- Døssing H, Bennedbaek FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules – a prospective randomized trial. J Clin Endocrinol Metab. 2013;98:E1213–E1217.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166:1031–1037.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20:1253–1261.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142.
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34:784–791.
- Papini E, Guglielmi R, Bizzarri G, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid. 2007;17:229–235.
- Cakir B, Topaloglu O, Gul K, et al. Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest. 2006;29:876–884.
- Papini E, Guglielmi R, Bizzarri G, et al. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract. 2004;10:276–283.
- Hegedus L. Therapy: a new nonsurgical therapy option for benign thyroid nodules? Nat Rev Endocrinol. 2009;5:476–478.
- Caraccio N, Goletti O, Lippolis PV, et al. Is percutaneous ethanol injection a useful alternative for the treatment of the cold benign thyroid nodule? Five years’ experience. Thyroid. 1997;7:699–704.
- Yu HM, Park SH, Lee JM, et al. Graves’ disease that developed shortly after surgery for thyroid cancer. Endocrinol Metab (Seoul). 2013;28:226–230.
- Bennedbaek FN, Karstrup S, Hegedus L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol. 1997;136:240–250.
- Bennedbaek FN, Hegedus L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid. 1999;9:225–233.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361–367.
- Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009;33:1971–1977.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation – a feasibility study. Radiology. 2000;217:673–677.
- Gambelunghe G, Fatone C, Ranchelli A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest. 2006;29:Rc23–Rc26.
- Esnault O, Franc B, Monteil JP, et al. High-intensity focused ultrasound for localized thyroid-tissue ablation: preliminary experimental animal study. Thyroid. 2004;14:1072–1076.