Abstract
Background
The impact of prolonged post-ablation fever (PAF) defined as persistent fever > 24 h after radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) had not been described before. This study aims to investigate the impact of prolonged PAF on early tumor recurrence in HCC patients after RFA.
Methods
From 2013 to 2015, a total of 135 patients with HCC meeting Milan criteria and all the tumors having confirmed complete ablation after RFA were enrolled. Study endpoint was any HCC recurrence within 1 year after ablation. Cox regression analysis was applied for multivariate analysis to determine the independent predictors of 1-year tumor recurrence.
Results
Post-ablation fever occurred in 42 (31.1%) patients after RFA, while prolonged PAF was found in 22 (16.3%) patients. Fifty-eight (42.8%) patients occurred any tumor recurrence within 1 year after complete ablation. Patients with prolonged PAF had a significantly higher rate of HCC recurrence within 1 year (72.7% vs. 37.1%, p = 0.002) and had a significantly shorter time-to-recurrence interval (19.6 vs. 40.5 months, Log rank test, p = 0.002) than those who had no prolonged PAF. Multivariate analysis by Cox regression showed the previous HCC recurrence history (aHR: 1.792, p = 0.0284), baseline AFP > 20 ng/ml (aHR: 1.868, p = 0.0211) and prolonged PAF (aHR: 2.092, p = 0.0138) were associated with early recurrence.
Conclusions
Prolonged PAF may associate with early HCC recurrence after complete ablation by RFA. Patients with prolonged PAF need to be more clinical attentions.
Keywords:
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related death worldwide [Citation1]. Radiofrequency ablation (RFA) is among the curative therapies for early-stage HCC [Citation2]. However, the 1-year tumor recurrence rate after RFA remains high [Citation3–5]. Self-limiting post-ablation syndrome, including low-grade fever, nausea, vomiting, and general malaise, has been reported in one-third of patients who recovered within 10 days post-RFA [Citation6,Citation7]. These clinical symptoms are similar to those of the post-embolization syndrome that presented in patients who underwent embolization of liver tumor. However, post-embolization fever (PEF) is related to heavier tumor burden and poorer liver reserve function, and the presence of PEF is associated with an unfavorable phenomenon correlating with poor treatment response in patients receiving trans-arterial chemoembolization (TACE) according to a previous study by Peng et al [Citation8]. The association between prolonged post-ablation fever (PAF) and prognosis, especially early tumor recurrence, remains uncertain because of limited data on the topic. Thus, the aim of this study was to investigate the incidence and predictors of prolonged PAF and the clinical impact of prolonged PAF on the prognosis of HCC patients receiving RFA.
Materials and methods
Patients
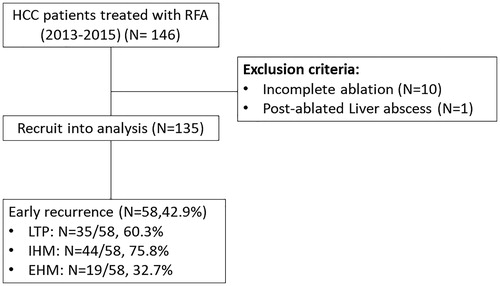
In total, 146 HCC patients meeting the Milan criteria and receiving RFA at our center between 2013 and 2015 were enrolled for analysis. HCC was diagnosed according to European Association for the Study of the Liver (EASL) guidelines by dynamic computer tomography (CT) or magnetic resonance imaging (MRI) and/or cytopathology [Citation9]. Patients with incomplete ablation and with a viable tumor (confirmed by dynamic CT/MRI 1-month post RFA) were excluded. Finally, a total of 135 patients were enrolled in the analysis (). Pretreatment variables including age, sex, etiology, tumor stage, hemogram findings, and liver biochemistry test results were retrieved from medical records. This was a retrospective study and the requirement for informed consent was therefore waived. The study was approved by our Institutional Review Board (201801499B0).
Radiofrequency ablation
RFA was performed percutaneously under ultrasound guidance and local anesthesia using an internal cool-tip or prong electrode (Radionics, Burlington, MA; Boston Scientific, CA). Straight electrodes with a 2 cm or 3 cm active tip were used. A multiple overlapping ablation technique was often used to create a sufficient ablation area. Artificial ascites or pleural effusion techniques were used when the target tumor was near critical organs or located in the hepatic dome area [Citation10,Citation11]. Radiofrequency energy was delivered for 9–12 min in each application. Adequate hyperechoic change of the ablation field with several pulses of impedance surge indicated complete application. The electrode tip cool-down temperature was >60 °C. An estimated safety margin of 0.5–1.0 cm was required. Needle tract ablation was performed by maintaining the temperature above 80 °C with slow withdrawal after each complete ablation. This was done to prevent hemorrhage or tumor seeding. Ablations were performed with a curative intent by three experienced hepatologists, each with 8–20 years of experience in intervention ultrasound.
Response evaluation and follow-up
CT/MRI scans were multiphasic and contrast-enhanced that performed 1-month post-RFA to determine tumor response. According to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), complete tumor response is defined as the absence of residual tumor or complete necrosis [Citation12]. Some patients underwent dynamic CT scan within 7 days after RFA to evaluate the sufficient ablative margin [Citation13]. We monitored HCC recurrence by dynamic CT/MRI every 3-4 months for the first 2 years and every 6 months thereafter, with the addition of serum alpha-fetoprotein (AFP) level measurements. HCC recurrence was diagnosed using the same criteria applied to HCC diagnosis. The radiologic images were assessed by experienced radiologists. Early recurrence is defined as local tumor progression (LTP), intrahepatic distant metastasis (IHM), or extrahepatic metastasis (EHM) detected less than 12 months post-RFA with complete ablation [Citation14].
Laboratory techniques
Hemogram and liver biochemistry tests were performed using automated techniques at the hospital’s clinical pathology laboratories. The upper limit of normal range (ULN) of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were set at 34 U/L and 36 U/L respectively, for both male and female participants. Commercial kits (Abbott Laboratories, North Chicago, IL, USA) were used for measuring serum Hepatitis B surface antigen and AFP levels (lower limit of detection: 2 ng/mL) and for the anti-hepatitis C virus assay.
Definitions
The body temperature of patients receiving RFA will be monitored regularly after the procedure. Post-ablation fever is defined when temperature is greater than 38 °C and antipyretics will be prescribed [Citation6]. Patient with persistent post-ablation fever more than 24 h even antipyretics use is defined as ‘prolonged post-ablation fever’. Sepsis work up will be arranged if SIRS or other clinical toxic sign like altered mental status were noted. The tumor-node-metastasis (TNM) stage is classified according to AJCC 7th Edition [Citation15]. AST to platelet ratio index (APRI) score is defined as (AST level/ULN AST)/Platelet counts (109/L)×100[Citation16]. Fibrosis-4 (FIB-4) score is defined as age (year)×AST (U/L)/Platelet count (109/L)×(ALT (U/L))1/2 [Citation17]. Albumin-bilirubin index (ALBI) score is defined as (log10 bilirubin [µmol/L] × 0.66) − (albumin [g/L] × 0.0852). ALBI grades 1, 2, and 3 were developed as follows: ALBI score ≤ −2.60 (ALBI grade 1), > −2.60 to ≤ −1.39 (ALBI grade 2), and > −1.39 (ALBI grade 3) [Citation18]. The fold increase in the neutrophil-to-lymphocyte ratio (NLR) is defined as post-RFA NLR/pretreatment NLR. A cutoff level >2 was set according to the literature [Citation18]. Edmondson–Steiner grade is defined according to that in the study by Edmondson et al. [Citation19]. Recurrent HCC history is defined as the patients having a new intrahepatic tumor(s) after previous curative treatments (e.g. surgical resection, ablation, TACE). Time-to-recurrence interval was defined as the interval between tumor complete response and HCC recurrence. Intrahepatic distant metastasis (IHM) was defined as tumor recurrence in different segment of liver. Extrahepatic metastasis (EHM) defined as tumor recurrence out of the liver to other organs, including the lungs, lymph nodes, bones, and adrenal glands [Citation20].
Statistical analyses
Descriptive data with normal distribution were reported as mean ± standard deviation or as median (range). To determine the difference in pretreatment factors between patients with and those without prolonged PAF, independent Student’s t-test and the Mann–Whitney U-test were applied for variables with normal and non-normal distributions, respectively, whereas the Chi-square test was applied for comparison of categorical variables. Logistic and cox proportional hazard regression analysis was carried out to determine predictors of prolonged PAF and 1-year tumor recurrence. The Kaplan–Meier method, with the log rank test, was used to compare 1-year recurrence rate between patients with and those without prolonged PAF. A two-tailed p value less than 0.05 was considered statistically significant. Statistical analyses were carried out using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Prolonged post-ablation fever
PAF was observed in 42 (31.1%) of the 135 patients. Of these 42 patients, 22 (16.3%) had prolonged PAF. After complete work-up of the patients with prolonged PAF, none of the cases of fever were determined to be sepsis-related. The baseline clinical variables (age, sex, HCC treatment history, Child–Pugh score, BCLC stage, APRI, FIB-4, ALBI grade, and AFP level) of the patients with and those without prolonged PAF were statistically comparable. Prolonged PAF tends to occur in patients at TNM stage II [N = 10/22 (44.5%) vs. 22/113 (19.5%), p = 0.0087], those having a large tumor size (>3 cm) [N = 9/22 (40.9%) vs. 22/113 (19.5%), p = 0.0287], and those with a higher proportion of NLR fold increase (>2) post-RFA [N = 13/22 (59.1%) vs. 35/113 (30.9%), p = 0.0117] (). Logistic regression analysis showed that TNM stage II (adjusted odds ratio (OR): 3.185, 95% CI: 1.17-8.64, p = 0.0231) and increased NLR fold (> 2) (adjusted OR: 2.68, 95% CI: 1.008-7.17, p = 0.0483) were independent predictors for prolonged PAF ().
Table 1. Comparisons of baseline features of the patients with prolonged post-ablation fever (PAF) and others.
Table 2. Logistic regression for predictors of prolonged post-ablation fever.
One-year tumor recurrence
After a median follow-up period of 52 months post-ablation, 58 (42.9%) patients were found to show tumor recurrence within 1 year after complete ablation. Of the 58 patients with recurrent HCC, IHM, LTP, and EHM occurred in 44 (75.8%), 35 (60.3%), and 19 (32.7%), respectively. The occurrence rates of IHM and LTP were significant among patients with prolonged PAF compared to those in patients without prolonged PAF [IHM: 12/22 (59.1%) vs. 32/113 (28.3%), p = 0.005; LTP: 10/22 (45.5%) vs. 25/113 (22.1%), p = 0.0223], whereas the occurrence rate of EHM was comparable between patients with and those without prolonged PAF [5/22 (22.7%) vs. 14/113(12.3%), p = 0.2021]. The ablation parameters including ablation volume, time and power were was all not correlated with prolonged PAF and early recurrence. One-year tumor recurrence tended to occur in patients with a history of recurrent HCC [N = 30/58 (51.8%) vs. 22/77 (28.5%), p = 0.0062], a higher baseline AFP level (> 20 ng/mL) [N = 32/58 (55.1%) vs. 22/77 (28.5%), p = 0.0018], and a higher prolonged PAF rate [N = 16/58 (27.5%) vs. 6/77 (7.8%), p = 0.0021] (). Cox proportional hazard regression analysis showed that a history of recurrent HCC (adjusted HR: 1.792, 95% CI: 1.064–3.019, p = 0.0284), AFP > 20 ng/mL (adjusted HR: 1.868, 95% CI: 1.098–3.176, p = 0.0211), and prolonged PAF (adjusted HR: 2.092, 95% CI: 1.162–3.764, p = 0.0138) were independent predictive factors of 1-year tumor recurrence (). Similarly, Kaplan–Meier analysis showed that patients with prolonged PAF had a higher 1-year tumor recurrence rate than patients without prolonged PAF (median time-to-recurrence interval: 19.6 vs. 40.5 months, 1-year cumulative tumor recurrence rate: 72.7% vs. 37.1%, Log rank test, p = 0.002) ().
Figure 2. Kaplan–Meier curve showing cumulative recurrence rate for all patients. Of all the 135 patients, prolonged PAF had higher 1-year tumor recurrence rate comparing to those without prolonged PAF (median time-to-recurrence interval: 19.6 vs. 40.5 months, 1-year cumulative tumor recurrence rate: 72.7% vs. 37.1%, Log rank test, p = 0.002).
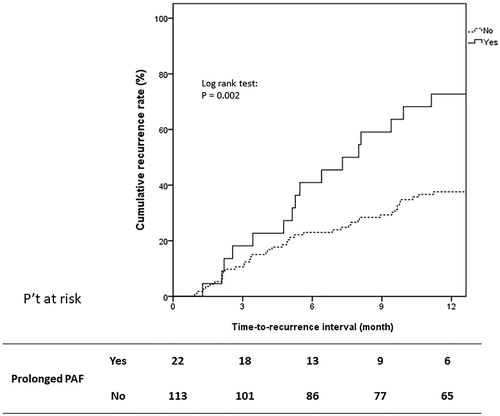
Table 3. Comparisons of baseline features of the patients with early recurrence and nonrecurrence.
Table 4. Cox’s proportional hazards model for predictors of progression free survival (PFS).
Of the patients with tumor recurrence, more than half received subsequent RFA (N = 33, 56.8%), whereas the others received TACE (N = 17, 29.3%), sorafenib (N = 4, 6.8%), systemic chemotherapy (N = 1, 1.7%), and radiotherapy (N = 3, 5.1%).
TNM stage I and II subgroups
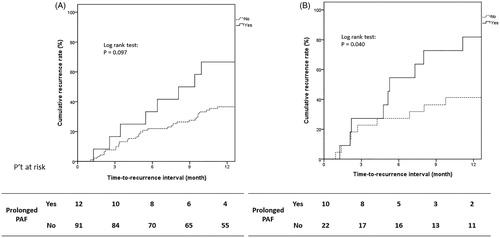
The impact of prolonged PAF on 1-year tumor recurrence in patients with either TNM stage I or II was investigated separately. As shown in , the impact in both subgroups (TNM stage I, N = 103; TNM stage II, N = 32) is that patients with prolonged PAF tend to have a higher early recurrence rate than those without prolonged PAF. This impact is not significant for patients within the TNM stage I subgroup (1-year cumulative tumor recurrence rate: 63.6% vs. 36.2%, Log rank p = 0.097) (), whereas for the TNM stage-II subgroup, the impact of prolonged PAF is statistically significant (1-year cumulative tumor recurrence rate: 81.8% vs. 40.9%, Log rank p = 0.040) ().
Figure 3. Kaplan–Meier curve showing cumulative recurrence rate for different TNM stages. (A) In TNM stage I (N = 103), the patients with prolonged PAF had a trend to have a higher early recurrence rate (prolonged PAF vs. without: 63.6% vs. 36.2%, Log rank p = 0.097). (B) As for TNM stage II (N = 32), patients with prolonged PAF had significantly higher early recurrence rate (prolonged PAF vs. without: 81.8% vs. 40.9%, Log rank p = 0.040).
HCC treatment-naive and single tumor patient subgroups
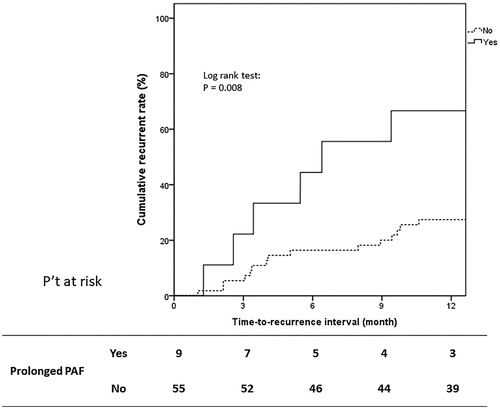
There was a total of 64 HCC treatment-naive and single tumor patients. Among these 64 patients, nine (14.1%) encountered prolonged PAF. There was no significant difference between patients with and without prolonged PAF including age, gender, liver preserved function and tumor burden except increased NLR fold >2 after RFA (crude OR: 7.194, 95% CI: 1.355–38.19, p = 0.0205). Throughout the follow-up period [median 57.9 (3.8–76.7) months], one-year tumor recurrence occurred in 21 (32.8%) patients. Cox proportional hazard regression analysis showed that prolonged PAF (adjusted HR: 2.880, 95% CI: 1.091–7.602, p = 0.0372) was the only independent predictive factor of 1-year tumor recurrence (Supplement Table 1). Kaplan–Meier analysis showed that patients with prolonged PAF had a higher 1-year tumor recurrence rate (median time-to-recurrence interval: 6.4 vs. 20.8 months, 1-year cumulative tumor recurrence rate: 66.6% vs. 27.2%, Log rank test, p = 0.008) ().
Figure 4. Kaplan–Meier curve showing cumulative recurrence rate for HCC treatment-naïve and single tumor patients. Of the subgroup of 64 patients with HCC treatment-naïve and single tumor, prolonged PAF had higher 1-year tumor recurrence rate comparing to those without prolonged PAF (median time-to-recurrence interval: 6.4 vs. 20.8 months, 1-year cumulative tumor recurrence rate: 66.6% vs. 27.2%, Log rank test, p = 0.008).
Discussion
This study provides data on the incidence, predictors, and clinical impact of prolonged PAF in an RFA-treated cohort of substantial sample size. RFA is an evolving and growing treatment option for HCC patients with a relatively low risk of major complications. However, 1-year tumor recurrence after RFA remains high among patients with reported risk factors for recurrence, including a (large tumor size, higher AFP level, and increased NLR) [Citation21,Citation22]. In our study, the incidence of 1-year tumor recurrence was 42.9% (58/135). A history of recurrent HCC, a higher AFP level (>20 ng/mL), and prolonged PAF was independent predictive factors for tumor recurrence. Furthermore, similar findings were also documented in treatment-naive and single-tumor patients.
Although RFA is a minimally invasive treatment modality, some complications are still associated with this procedure [Citation6,Citation23]. Major complications post-RFA, including liver abscess, intra-abdominal hemorrhage, and liver failure, are rare (incidence rate of 2.2–3.1%) [Citation23–27]. However, post-ablation syndrome featuring idiopathic fever after RFA was quite common (incidence rate of 19–71.2%) according to previous reports [Citation23,Citation28]. This is similar to our current findings (incidence rate of PAF is 31.1%, prolonged PAF is 16.3%). Previous studies mostly focused on major complications and post-ablation syndrome was considered as a transient, self-limiting minor complication without a significant clinical impact [Citation7,Citation29]. Nevertheless, this study elucidated that prolonged PAF may be an independent factor in predicting tumor recurrence post-RFA.
Randomized studies evaluating clinical factors that could predict tumor recurrence and survival post RFA are still lacking. A retrospective study reported by Lee et al. [Citation30] showed that tumor location, size, and number were crucial parameters for predicting recurrence. Several biomarkers, such as AFP, des-γ-carboxy prothrombin (DCP), Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), histones, myeloperoxidase (MPO), inflammatory cytokines (IL-1β, IL-6, IL-10, TNF-α), and microRNAs could also predict HCC recurrence post-RFA [Citation31]. In our study, we showed that a history of recurrent HCC, a higher AFP level (> 20 ng/mL), and prolonged PAF (a previously unstudied factor) were independent risk factors for 1-year tumor recurrence. On the other hand, according to previous study, death of cancer cells by hyperthermia induced tumor antigen releasing, expression of danger signals that activate a specific T-cell response and this effect is ineffective to avoid recurrence [Citation32]. Therefore, the change of tumor microenvironment maybe associated with LTP and IHM instead of EHM. Besides, the median interval from HCC complete ablation to occurrence of EHM is longer than 1-year (median: 17.1 (2.6–44.8) months) that prolonged PAF could not predict early EHM recurrence.
Prolonged PAF as an independent predictor for tumor recurrence is intriguing, especially considering its simplicity. However, the mechanism of PAF is still uncertain. It may reflect the degree of hepatic cell damage after loco-regional therapy [Citation33,Citation34] and also reflect a strong inflammatory process that may have roles in anti-tumor immune activity [Citation35]. NLR has also been widely studied in patients with HCC; it is regarded as a predictive factor for survival and it is correlated with some immune dysregulations associated with tumor immune responses [Citation35]. In our study, we failed to demonstrate the association between NLR and early tumor recurrence. However, we showed that increased NLR post-ablation was highly correlated with prolonged PAF, again implying that prolonged PAF may represent some immune activity. Because the prolonged PAF is considered to be associated with tumor burden that the more advanced tumor burden including larger tumor size or more tumor numbers, the easier of prolonged PAF occurred. We therefore further to investigate if prolonged PAF still have the impact of tumor recurrence on different tumor stage especially in those patients with treatment-naive and only single tumor. Results still revealed that prolonged PAF was associated with a poor outcome in different tumor stage. This finding supports the clinical practice of earlier or more frequent HCC surveillance for patients who exhibit prolonged PAF post-RFA. A timely shift to a different therapy or a combination with other therapies, if feasible, may also be considered.
This study has several limitations. First, this was a single-center and retrospective study. Second, pathologic assessment of tumor extent and non-tumor necrosis between patients with and without prolonged PAF could not be carried out in this study. Third, some patients (7 of 22, 31.8%) with prolonged PAF did not have their blood drawn for culture because of the absence of signs of clinical toxicity (defined as systematic inflammatory response syndrome, SIRS [Citation36]). Some patients also requested to be discharged despite persistent fever, hence prolonged PAF may be underestimated.
In conclusion, the presence of prolonged PAF may have some role in predicting early tumor recurrence post-RFA (in both treatment-naïve and recurrent HCC patients). However, further studies are required to explore the underlying mechanism of prolonged PAF and its impact on tumor progression.
Author contributions
Ping-Hung Ho: data acquisition, statistical analysis, draft writing; Wei Teng: study concept, design and supervision, manuscript revision; Chen-Chun Lin: study concept, design and supervision; Wen-Juei Jeng: study concept and design; Wei-Ting Chen: data acquisition; Chun-Yen Lin: study concept, design and supervision, manuscript revision; Shi-Ming Lin: study concept, design and supervision, manuscript revision; I-Shyan Sheen: study concept, design, statistical analysis.
Disclosure of interest
The authors have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
| Abbreviation | ||
| AFP | = | alpha-fetoprotein |
| ALBI | = | albumin-bilirubin index |
| ALT | = | alanine aminotransferase |
| APRI | = | AST to platelet ratio index |
| AST | = | aspartate aminotransferase |
| BCLC | = | Barcelona Clinic Liver Cancer |
| CTP | = | Child–Turcotte–Pugh |
| HBV | = | hepatitis B virus |
| HCV | = | hepatitis C virus |
| EASL | = | European Association for the Study of the Liver |
| FIB-4 | = | fibrosis-4 |
| HCC | = | hepatocellular carcinoma |
| NLR | = | Neutrophil-to-lymphocyte ratio |
| PAF | = | post-ablation fever |
| LTP | = | local tumor progression |
| IHM | = | intrahepatic distant metastasis |
| EHM | = | extrahepatic metastasis |
| RFA | = | radiofrequency ablation |
| SIRS | = | Systemic inflammatory response syndrome |
| TACE | = | trans-arterial chemoembolization |
| TNM | = | tumor-node-metastasis |
Supplemental Material
Download PDF (230.7 KB)Additional information
Funding
References
- M.D. Augusto Villanueva. Hepatocellular carcinoma. N Engl J Med. 2019;381(1):e2.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9(1):79–86.
- Wang J-H, Wang C-C, Hung C-H, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56(2):412–418.
- Lin S-M, Lin C-J, Lin C-C, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma≤ 4 cm. Gastroenterology. 2004;127(6):1714–1723.
- Wah TM, Arellano RS, Gervais DA, et al. Image-guided percutaneous radiofrequency ablation and incidence of post–radiofrequency ablation syndrome: prospective survey. Radiology. 2005;237(3):1097–1102.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Peng C-W, Teng W, Hsieh Y-C, et al. Postembolization fever after transarterial chemoembolization is a sign of unfavorable therapeutic response in hepatocellular carcinoma patients. Adv Dig Med. 7:83–92.
- Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Kondo Y, Yoshida H, Shiina S, et al. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006;93(10):1277–1282.
- Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Onc. 2017;15(1):126.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–060. © Thieme Medical Publishers,
- Teng W, Liu K-W, Lin C-C, et al. Insufficient ablative margin determined by early computed tomography may predict the recurrence of hepatocellular carcinoma after radiofrequency ablation. Liver Cancer. 2015;4(1):26–38.
- Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98(1):119–127.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474.
- Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526.
- Sterling RK, Lissen E, Clumeck N, APRICOT Clinical Investigators, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558.
- Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503.
- Han T-S, Ban HS, Hur K, et al. The epigenetic regulation of HCC metastasis. IJMS. 2018;19(12):3978.
- Lee SH, Kim SU, Jang JW, et al. Use of transient elastography to predict de novo recurrence after radiofrequency ablation for hepatocellular carcinoma. OncoTargets Ther. 2015;8:347.
- Doyle A, Gorgen A, Muaddi H, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. 2019;70(5):866–873.
- Park JG, Park SY, Tak WY, et al. Early complications after percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1,843 ablations in 1,211 patients in a single centre: experience over 10 years. Clin Radiol. 2017;72(8):692.e9-692–e15.
- Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30(4):409–418.
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–451.
- Rhim H, Yoon KH, Lee JM, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23(1):123–134.
- Song SY, Chung JW, Han JK, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001;12(3):313–320.
- Choi JB, Rhim HC, Kim YS, et al. Radiofrequency thermal ablation of malignant hepatic tumors: post-ablation syndrome. J Korean Radiol Soc. 2000;43(1):63–68.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Lee MW, Kang D, Lim HK, et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma< 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391–2400.
- Canale M, Ulivi P, Foschi FG, et al. Clinical and circulating biomarkers of survival and recurrence after radiofrequency ablation in patients with hepatocellular carcinoma. Crit RevOncol/Hematol. 2018;129:44–53.
- Hansler J, Wissniowski TT, Schuppan D, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12(23):3716–3721.
- Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–1942.
- Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551.
- Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39(11):2008–2023.
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655.