Abstract
Objective
To investigate the long-term reintervention of ultrasound-guided high intensity focused ultrasound (USgHIFU) treatment for uterine fibroids and analyze the factors affecting reintervention rate after USgHIFU.
Materials and methods
Three hundred and eight-one patients with uterine fibroids treated by USgHIFU at the third Xiangya Hospital of Central South University from April 2012 to December 2014 were retrospectively reviewed. The factors that affect the reintervention rate were analyzed.
Results
The mean follow-up time was 70.0 ± 9.0 months. During the follow-up period, 86.4% (329/381) of the patients reported symptomatic relief and the fibroids shrank after USgHIFU treatment. Seventy-nine patients received reintervention included myomectomy, a second session of HIFU, and hysterectomy. The overall reintervention rate was 20.7% (79/381). The reasons for reintervention included symptomatic recurrence in 50 (50/79, 63.3%) patients, psychological factors in 14 (14/79, 17.7%) patients, fertility requirement in three (3/79, 3.8%) patients, suspected uterine sarcoma in two (2/79, 2.5%) patients and others in 10 (10/79, 12.7%) patients. The reintervention rate has significant correlation with some factors including age, size, type and the signal intensity on T2 weighted image (T2WI) of the uterine fibroids.
Conclusion
USgHIFU for uterine fibroids is effective due to low reintervention rate in a long-term follow-up.
Introduction
Uterine fibroids are the most common benign gynecological tumors, with the prevalence of 20–40% in reproductive age women [Citation1]. Uterine fibroids are often accompanied with menorrhagia, lumbosacral pain, constipation and other symptoms, which can affect the quality of life and cause infertility [Citation2]. The treatments of uterine fibroids included medication, hysterectomy, myomectomy, uterine artery embolization (UAE) and high intensity focused ultrasound (HIFU) [Citation3]. Each treatment modality has its own advantages and disadvantages.
As a noninvasive treatment, HIFU has been widely used to treat uterine fibroids. Previous studies have shown that HIFU ablation for fibroids is safe and effective [Citation4,Citation5]. However, a 5-year follow-up study of 162 women treated with magnetic resonance-guided focused ultrasound (MRgFUS) by Quinn et al. showed that the overall reintervention rate was 58.64%. In patients with non-perfused volume (NPV) greater than 50%, the reintervention rate was 50% [Citation6]. Funaki et al. showed that the moderate volume reductions of hypointense or isointense uterine fibroids (signal intensity on T2 weighted images (T2WI) of MR) following MRgFUS were noted with relatively low reintervention rates [Citation7]. There is a possibility of reintervention when relapse or regeneration of the fibroid occurs. Therapeutic efficacy can be improved by studying the causes of local recurrence of fibroids and developing appropriate treatment modalities. Therefore, the aim of this current study was to evaluate the long-term outcomes and to investigate the factors that related to reintervention after ultrasound-guided high intensity focused ultrasound (USgHIFU) treatment.
Materials and methods
This study was approved by the Ethics Committee at the Third Xiangya Hospital of Central South University. All patients signed an informed consent before HIFU treatment.
Patients
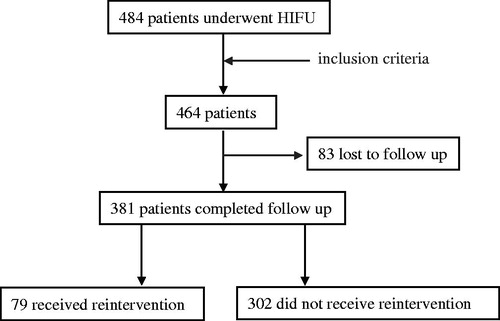
From April 2012 to December 2014, 484 patients with uterine fibroids were treated with HIFU in the Department of Gynecology at the Third Xiangya Hospital of Central South University. Among them, 20 patients who did not undergo MRI were excluded from this study ().
The inclusion criteria for USgHIFU treatment were as follows: (1) patients with symptomatic uterine fibroids and were premenopausal; (2) the diagnosis of uterine fibroids was confirmed by MRI; (3) patients were able to communicate with the nurse or the physician and received HIFU treatment voluntarily; (4) patients had a safe acoustic pathway.
The exclusion criteria were as follows: (1) patients with suspected or confirmed malignant diseases; (2) patients with bowel adhesions in the lower abdomen; (3) patients with prior surgical abdominal scar in acoustic pathway, causing obvious attenuation of B-model ultrasound in detecting tissue behind; (4) patients couldn’t lie prone for 1 h.
MRI examination
A contrast-enhanced MRI scan was obtained in all cases one day prior to the procedure. A series of standard T1 weighted images (T1WI), T2WI and contrast-enhanced T1WI sequences were performed with a 1.5 T MRI. Dynamic enhancement was performed 20 s after an intravenous injection of gadolinium.
Based on MRI, the position of the uterus, signal intensity of the fibroid on T2WI, location of the fibroids, and fibroid type were determined. Fibroids were classified according to their signal intensity as hypointense, isointense and hyperintense. The signal intensity of hypointense fibroids was comparable to that of skeletal muscle; isointense fibroids had a uniform intensity lower than that of the myometrium but higher than that of the skeletal muscle; the signal intensity of hyperintense fibroids had the signal intensity equal to or higher than that of the myometrium. Pre-USgHIFU enhanced MRI were used to determine the enhancement type of fibroids (mild, moderate, significant). Mildly enhanced fibroids had a weaker enhancement compared to that of the myometrium. Moderately enhanced fibroids had an enhancement similar to that of the myometrium. Significantly enhanced fibroids had a stronger enhancement compared to the myometrium in the enhanced area.
Post-USgHIFU and follow-up contrast enhanced MR images were used to measure the NPV and the residual volume of fibroids. NPV is the volume of coagulative necrosis. The volume of fibroids and NPV was obtained by measuring in three dimensions: longitudinal diameter (D1), transverse diameter (D2) and antero-posterior diameter (D3). The volume was calculated according to the following equation: V = 0.5233 × D1 × D2 × D3. NPV ratio is defined as NPV/Vfibroid × 100%.
USgHIFU protocol
To reduce the risk of adverse effects or complications, the patients were asked to have bowel preparation and skin preparation for three days before the USgHIFU treatment. The patients were asked to ingest a bland diet on day 1, followed by semi-liquid and liquid food without milk on the second and third day, respectively. In addition, fasting 12 h pretreatment and enema in the morning of treatment day were also required.
Skin preparation protocol was made on the treatment day included shaving hair from the umbilicus to the upper margin of the pubic symphysis, degreasing and degassing with 75% ethanol or degassed water.
Before USgHIFU treatment, a urinary catheter was inserted to help control the volume of bladder by filling the bladder with normal saline or releasing urine to improve the therapeutic acoustic window. Degassed water balloons of various sizes and tensions were applied to each patient to compress or push away the bowel from the acoustic pathway.
After USgHIFU ablation, patients were requested to lie prone for 2 h, to ingest liquid fluid 3 h after treatment. The patients were discharged from the hospital on first day after USgHIFU treatment.
USgHIFU ablation
USgHIFU ablation was performed under intravenous conscious sedation. The device used was a JC200 HIFU tumor therapeutic system (Chongqing Haifu Medical Technology, Co., Ltd., Chongqing, China). Therapeutic ultrasound energy was generated by a transducer with a frequency of 0.8 MHz, a focal length of 15 cm and a diameter of 20 cm. A Mylab 70 ultrasound imaging device (Esaote, Genova, Italy) was used to provide real-time imaging to localize and monitor the treatment. The patients were placed in a prone position on the HIFU table, with the anterior abdominal wall in contact with degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to help compress and push the bowel away from the acoustic pathway. Point scan was selected, and power set between 300 and 400 W. The distance from focal point to endometrium was at least 1.5 cm, and the distance from focal point to subserosal surface of the uterus was 1 cm. During the procedure, therapeutic energy was adjusted based on patient feedback and changes in grayscale on ultrasonographic imaging. This process was repeated until there was an absence of blood supply under contrast-enhanced ultrasound. The patients' vital signs such as heart rate, blood pressure, respiration and oxygen saturation were monitored during the procedure.
Statistical analysis
SPSS 23 (IBM Company, Chicago, IL) statistical analysis software was used for data analysis. Normally distributed data were depicted using mean ± standard deviation; skewed distributed data were depicted using median and interquartile range (IQR). The independent two sample t test, chi-square test and Mann–Whitney’s U test were utilized for univariate analysis. Binary logistic regression analysis was utilized for multivariate analysis. The test level was set at α = 0.05, p < .05 was considered statistically significant.
Results
Baseline characteristics
As shown in , among the 464 patients with uterine fibroids treated with USgHIFU, 83 patients lost and 381 completed the follow-up. The mean age of the 381 patients was 42.4 ± 5.0 (range: 28–52) years. The mean size of the uterine fibroids was 54.6 ± 15.2 (range: 15–110) mm. Of the 381 patients, 55 patients had submucosal uterine fibroids, 118 had subserosal uterine fibroids and 208 patients had intramural fibroids. Among the 381 patients, 258 had solitary uterine fibroid and 123 patients had multiple uterine fibroids. MRI showed that 241 uterine fibroids presented as hypointensity on T2WI, 78 fibroids presented as hyperintensity on T2WI and 62 fibroids presented as isointense on T2WI; the contrast enhanced T1WI showed slight enhancement in 76 fibroids, moderate enhancement in 67 fibroids and significant enhancement in 238 fibroids ().
Table 1. Comparison of baseline characteristics between patients with and without reintervention during follow-up.
Comparison of the baseline characteristics in patients with and without reintervention
Based on post-HIFU MRI, the median NPV ratio of the fibroids was 81.9% (IQR: 70.36%, 91.91%) in the 381 patients. The follow-up results showed that 297 patients reported a significant shrinkage of the fibroids and the symptomatic relief. During the average of 70.0 ± 9.0 (range: 58–88) months follow-up, 20.7% (79/381) of the patients had reintervention. We further compared the baseline characteristics between the patients with and without reintervention, and found a significant difference in age, size of uterine fibroids, type of the uterine fibroids, and the signal intensity on T2WI between the two groups (p < .05) ().
Reintervention analysis results
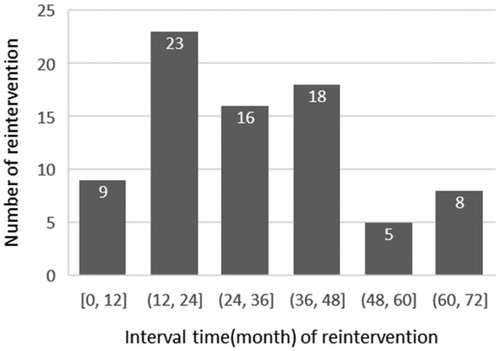
The mean interval of reintervention after USgHIFU was 33.7 ± 18.0 (range: 5–72) months. The reintervention mainly occurred within 2nd–4th years after USgHIFU, accounting for 72.2% (57/79) (). Among them, 46 patients had myomectomy, 30 had hysterectomy and three had a second session of USgHIFU treatment. The reasons for reintervention included symptomatic recurrence in 50 (63.3%) patients, psychological factors in 14 (17.7%) patients, fertility requirement in three (3.8%) patients, other surgical indications in 10 (12.7%) patients (five hysterectomy due to cervical, ovarian or endometrial lesions, and five myomectomy due to ovarian cyst, liver mass and cesarean section), and suspected uterine sarcoma in two (2.5%) patients after the 3-month follow-up MRI. The cumulative reintervention rate for symptomatic recurrence at the average of 70.0 ± 9.0 months after USgHIFU was 13.1% (50/381) ().
Table 2. The reasons for reintervention and interval time of reintervention.
Evaluation of the factors affecting reintervention
The binary logistic regression analysis was used to further analyze if any factor is independent risk factor for reintervention. The results showed that age, size of uterine fibroids, type of uterine fibroids (submucous) and hyperintensity signal intensity on T2WI were all independent risk factors for reintervention (p < .05). The younger patients (Exp(B) < 1), or patients with bigger size of uterine fibroids (Exp(B)>1) have greater risk of reintervention than older patients or patients with smaller size of uterine fibroids ().
Table 3. The binary logistic regression analysis of variance.
Discussion
Currently, HIFU is the only noninvasive procedure that can be used to treat uterine fibroids [Citation8]. With the increasing demand for minimally invasive treatment, more and more patients with uterine fibroids choose USgHIFU treatment in China [Citation9]. Over the last decades, many studies have shown that USgHIFU is safe and effective in the treatment of uterine fibroids [Citation10,Citation11]. However, no study reported the 5 years reintervention rate of patients with uterine fibroids after USgHIFU treatment. Recently, several studies have shown that the 3-year reintervention rate in patients with uterine fibroids after MRgFUS was 19–31% [Citation12–15], and Quinn et al. [Citation6] reported that the 5-year reintervention rate reached 58.64% after MRgFUS because of the low NPV ratio. In this study, the median NPV ratio of the fibroids was 81.9%, which was higher than those in the previous studies. After the average of 70.0 ± 9.0 (range: 58–88) months follow-up, a significant shrinkage of the fibroids was observed in 297 patients. Among the 381 patients, 79 (20.7%) patients had reintervention; however, only 50 (13.1%) patients were due to symptomatic recurrence. The cumulative reintervention rate at 5 years was lower than that in the previous studies using MRgFUS [Citation6]. In comparison with the previous studies, this lower cumulative reintervention rate can be explained by the high NPV ratio [Citation6,Citation12–15]. We also reviewed the papers regarding the reintervention after UAE or myomectomy, the cumulative reintervention rate after USgHIFU in this study is comparable to the 5-year reintervention rate after UAE or myomectomy [Citation16,Citation17].
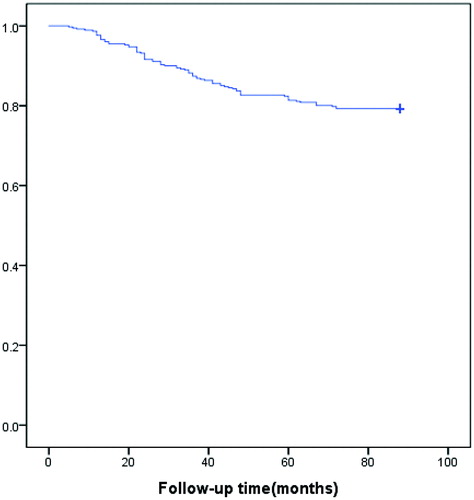
In this study, we only found the symptomatic recurrence in 50 (account for 63.3%) patients. However, 14 (17.7%) patients had reintervention due to psychological factors because subjective mental stress from the follow-up image of ablated residual fibroid which was even asymptomatic and did not grow, which indicated that it is crucially important to perform a fully communication with patients and emphasize that no further intervention is required as long as fibroids no longer grow and asymptomatic. Interestingly, we also found the average time to reintervention was 33.7 ± 18.0 (range: 5–72) months, and 72.2% (57/79) of the patients received reintervention in 2nd–4th years after USgHIFU. Our results suggested that the 2nd–4th years after USgHIFU is an important period of follow-up, and the follow-up period could be appropriately extended. In the analysis of subgroup who had reintervention for persistent or recurrent symptoms, the mean time to reintervention was 35.2 ± 18.2 (range: 5–71), 68% (34/50) of them received reintervention in 2nd–4th years, and 12.0% (6/50) of them received reintervention within one year. Although the life table plot showed a flattening of the curve at 5 years (), long-term follow-up beyond 5 years is needed.
The previous studies have demonstrated that the long-term symptomatic relief of patients with uterine fibroids is related to NPV ratio of the treated fibroids. Many factors included the size of uterine fibroids, distance from fibroid ventral side to the skin, location of the uterus, location of uterine fibroids, signal intensity on T2WI, and enhancement type may affect the treatment results [Citation18–22]. Our results showed that the reintervention rate was negatively correlated with the NPV ratio, number of uterine fibroids, and enhancement degree on T1WI. The further analyzed results showed that the age, size of the uterine fibroids, type of the uterine fibroids (submucous), and signal intensity on T2WI (hyperintense) were independent risk factors for reintervention. The younger patients or the patients with bigger size of the uterine fibroids have a greater risk than the older patients or the patients with smaller size of uterine fibroids for reintervention. The possible reasons are as follows: (1) with the increase of age, the level of hormone decreased, and the risk of recurrence is decreased; (2) the large uterine fibroids may have large feeding blood vessels, thus more therapeutic ultrasound energy is required to ablate large fibroids completely. If the therapeutic energy deposited in the fibroids is insufficient, vascularization of fibroids may occur; (3) blood flow in vascular fibroids could dissipate some heat and therefore the acoustic energy deposition in these fibroids is less than that of avascular uterine fibroids. Some studies have shown that multiple uterine fibroids is a risk factor for recurrence after myomectomy [Citation23,Citation24]. However, we did not find a significant difference in reintervention rate between the patients with solitary and multiple uterine fibroids. This may be due to the small uterine fibroids that could not be removed by surgical myomectomy were ablated by USgHIFU under the guidance of ultrasound.
After HIFU treatment, the treated fibroids do not disappear immediately and thus need to explain to patients clearly. It is different from surgery, no pathological results obtained after HIFU. Therefore, pre-HIFU MRI is mandatory for patients with uterine fibroids who want to have HIFU treatment. Although MRI has the best soft tissue resolution and plays a key role in the differential diagnosis of uterine fibroids and uterine sarcomas, it is difficult to confirm the diagnosis based only on MRI in some atypical cases. Hence, the follow-up protocol is another important measure to prevent missed diagnosis of sarcoma. In this study, a 34-year-old patient presented with increased menstrual volume, MRI showed the tumor with hyperintensity on T2WI and moderate enhancement on contrast enhanced MRI. The initial diagnosis of uterine fibroid was made and the patient underwent USgHIFU treatment. She returned to our department for routine follow-up at 3 months after HIFU, she complained of irregular vaginal bleeding and the ultrasound examination did not show shrinkage of the treated fibroid. We reviewed the pre-HIFU MRI again and had a multiple discipline team discussion, thus uterine sarcoma was suspected and we ordered a follow up pelvic MRI for her. The MRI showed regrowth of the treated fibroid tumor with the features of endometrial stromal sarcoma. The patient then had hysterectomy and the pathological diagnosis of endometrial stromal sarcoma was confirmed. This patient has been followed up for 5 years without recurrence of endometrial stromal sarcoma.
This study is limited because it is a retrospective study and some bias may occur. This study is also limited because many patients did not complete the symptomatic evaluation, so we could not report the score of symptomatic relief. Future studies are needed in evaluating the quality of life to assess the symptomatic relief. Future studies with a prospectively compared with the contemporaneous selection of laparoscopic hysteromyomectomy are needed.
In summary, USgHIFU is safe and effective in the treatment of uterine fibroids with a low reintervention rate. Fully communication with patients is very important, and the key period of follow-up is 2nd–4th years after USgHIFU. In addition, indication optimization of USgHIFU should be performed based on age of patients, the size and type of uterine fibroids, the signal intensity on T2WI to further reduce the reintervention rate.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
- Lethaby A, Vollenhoven B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin Evid. 2015;2015:0814.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73(2):396–403.
- Tempany CM, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589.
- Rueff LE, Raman SS. Clinical and technical aspects of MR-guided high intensity focused ultrasound for treatment of symptomatic uterine fibroids. Semin Intervent Radiol. 2013;30(4):347–353.
- Sirkeci RF, Belli AM, Manyonda IT. Treating symptomatic uterine fibroids with myomectomy: current practice and views of UK consultants. Gynecol Surg. 2017;14(1):11.
- Fan HJ, Zhang C, Lei HT, et al. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine (Baltimore). 2019;98:e14566.
- Chen JY, Chen WZ, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Thiburce AC, Frulio N, Hocquelet A, et al. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015;31(7):764–770.
- Gorny K, Borah B, Brown D, et al. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol. 2014;25(10):1506–1514.
- Himabindu Y, Sriharibabu M, Nyapathy V, et al. Early evaluation of magnetic resonance imaging guided focused ultrasound sonication in the treatment of uterine fibroids. Indian J Med Res. 2014;139:267–272.
- Kim HS, Baik JH, Pham LD, et al. MR-guided high intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol. 2011;18(8):970–976.
- Moss JG, Cooper KG, Khaund A, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118(8):936–944.
- Reed S, Newton KM, Thompson LB, et al. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Fan HJ, Cun JP, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35(1):534–540.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine (Baltimore). 2015;94(13):e650.
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184e1–184e6.
- Zhao WP, Zhang J, Han ZY, et al. A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep. 2017;7(1):10812.
- Shiota M, Kotani Y, Umemoto M, et al. Recurrence of uterine myoma after laparoscopic myomectomy: what are the risk factors? Gynecol Minim Invasive Ther. 2012;1(1):34–36.
- Radosa MP, Owsianowski Z, Mothes A, et al. Long-term risk of fibroid recurrence after laparoscopic myomectomy. Eur J Obstet Gynecol Reprod Biol. 2014;180:35–39.