Abstract
Objectives
To establish a modified strategy of the feeding artery ablation (FAA) procedure as an add-on to percutaneous radiofrequency ablation (RFA) for patients with hypervascular hepatocellular carcinoma (HCC), and to evaluate the outcomes.
Materials and methods
For this prospective, single-arm study, from June 2014 to August 2016, twenty-five patients with hypervascular HCC, 2–5 cm in diameter were treated by contrast-enhanced ultrasound (CEUS)-guided FAA before conventional RFA. Technical success of FAA and subsequent perfusion change of the tumor were evaluated by CEUS. Technical efficacy and ablation sizes were evaluated by CT/MRI at 1 month. Therapeutic outcomes, including local tumor progression (LTP), overall survival (OS), and recurrence-free survival (RFS) were evaluated using the Kaplan–Meier method.
Results
One or two target feeding arteries were visible on CEUS for 52.6% (61/116) of the hypervascular HCCs 2–5 cm in diameter. The technical success rate of the FAA was 100%; 13/25 (52.0%) target tumors were evaluated as complete perfusion response, while 12/25 (48.0%) were evaluated as partial perfusion response. The ablation volume was 41.9 ± 17.5 cm3 (14.9–78.2 cm3) and the ablative safety margin was 8.2 ± 1.9 mm (4–12 mm) at the 1-month evaluation. These parameters did not differ significantly between the complete and partial subgroups. The cumulative rates of LTP at 1-, 2-, and 3-year follow-ups were 0.0%, 4.2% and 4.2%, respectively. The 3-year OS and RFS were 70.3% vs. 59.8%, respectively. There were no treatment-related deaths. Major complications occurred in one patient (4.0%).
Conclusion
As an add-on to conventional percutaneous RFA, tailored CEUS-guided FAA can reduce tumor perfusion and provide good local control of HCC.
Introduction
Currently, radiofrequency ablation (RFA) is the most widely used method to treat small malignant hepatic tumors [Citation1]. However, RFA has several intrinsic limitations compared to surgical resection, the most important one being the higher rate of local tumor progression (LTP) due to an inadequate ablative margin [Citation2]. An ablative margin greater than 5 mm, ideally 10 mm, could reduce LTP rates after RFA [Citation1]. Therefore, the creation of a larger ablation volume without technical and device complexity is being continually explored. Since hepatocellular carcinoma (HCC) is rich in arterial blood supply, a common and effective strategy to enlarge ablation volume is to reduce the heat loss caused by substantial effects of capillary level tumor microperfusion [Citation1,Citation3]. Several techniques reduce perfusion-mediated cooling, ranging from pharmacologically decreasing blood flow [Citation4,Citation5], through temporary vascular balloon occlusion of a specific vessel during ablation [Citation6,Citation7], to intraarterial embolization or chemoembolization [Citation8,Citation9], or performing a Pringle maneuver while performing RFA at laparotomy (i.e. temporary hepatic pedicle occlusion by direct compression of the vessels) [Citation10].
Another way to reduce or block tumor perfusion is the direct ablation of the target blood vessel before conventional RFA, as a one-step strategy. Previous studies have demonstrated that ultrasound-guided feeding artery ablation (FAA) before conventional RFA, either through percutaneous or laparoscopic access, could safely decrease LTP after HCC ablation [Citation11–15]. However, most of the researchers only use color Doppler imaging to evaluate feeding artery structure and tumor perfusion change [Citation11–14], which are obviously not precise. Another major drawback of these previous studies is that operators do not make a clear distinction between feeding artery ablation and tumor ablation [Citation11–15]. They usually insert one or more electrodes within the tumor and coagulate a relatively large amount of tissue including the feeding vessel, sometimes non-feeding vessels, and part of the tumor, which makes the accurate evaluation of perfusion change not possible and the independent effect of FAA confusing, or even worse, causes injury to non-feeding vessels and ischemia of normal liver parenchyma.
In the present study, with the assistance of contrast-enhanced ultrasound, we were able to precisely evaluate the feeding artery structure and tumor perfusion change [Citation16]; with the unique application of the Cauterization Mode, it was possible to create a relatively small ablation zone to occlude the feeding artery. With the improved and detailed FAA technique, we tried to select the feeding artery and to spare non-feeding branches, avoid the tumor, create a minimal ablation area and evaluate the independent impact of FAA on tumor perfusion. Therefore, the purpose of this study was to investigate a modified FAA procedure and evaluate the therapeutic outcomes.
Materials and methods
Patients
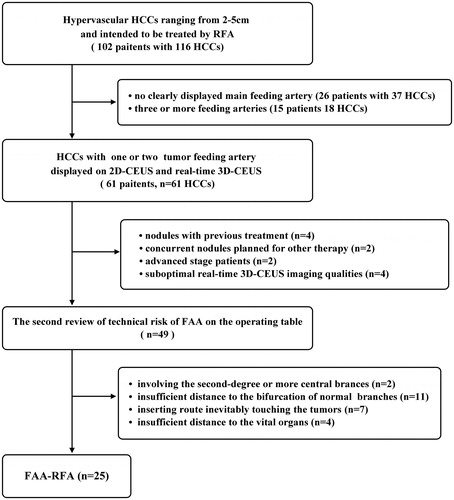
This prospective, non-randomized study was approved by the ethics committee of the First Affiliated Hospital of SYSU, and informed consent was obtained from all patients. It was registered at the Chinese Clinical Trial Registry (https://www.chictr.org.cn/) (No. ChiCTR2000029116). From June 2014 to August 2016, 102 patients with 116 hypervascular HCCs ranging from 2 to 5 cm in diameter were intended to be treated by RFA in our center. HCC diagnosis was based on the histopathology or on noninvasive criteria of the European Association for the Study of the Liver [Citation17]. The optimal treatment protocol was designed by the multidisciplinary team (MDT) including surgeons, radiologists, and oncologists. All cases were submitted to the regular MDT conference twice a week and CEUS images were reviewed, among which 61 patients with 61 target HCCs were consecutively selected as the potential candidates of FAA according to the following criteria: (a) hypervascular HCC; (b) moderate-sized nodules ranging from 2 to 5 cm in diameter; (c) 2D-CEUS and real-time 3D-CEUS displaying one or two tumor feeding arteries without complex branches; (d) Child-Pugh A or B; (e) platelet count > 50 × 109/L, and prothrombin time < 20 s. The exclusion criteria were: (a) nodules with any previous treatment; (b) presence of concurrent HCCs planned for combination therapy, such as Transarterial chemoembolization (TACE) or Sorafenib; (c) advanced-stage disease with major vascular invasion or extrahepatic metastases; and (d) suboptimal real-time 3D-CEUS imaging qualities. Twelve nodules were excluded for the following reasons: nodules with previous treatment (n = 4); nodules with concurrent intrahepatic foci planned for other combination therapy (n = 2); nodules in advanced-stage patients (n = 2); and suboptimal real-time 3D-CEUS imaging qualities (n = 4).
Among the remaining 49 candidates, the technical risk of FAA was reviewed again by 2 interventional radiologists by consensus according to the following criteria: (1) the target FAA point must be set in third-degree or greater distal arterial branches; (2) it must be more than 1 cm away from the bifurcation of non-feeding branches to prevent ischemia of normal liver parenchyma; (3) the inserting route must not touch the tumor to identify the independent effect of feeding artery occlusion; (4) it must be 1 cm away from the adjacent vital organs such as bowel, diaphragm, gallbladder and bile duct. Finally, 25 target nodules in 25 patients without technical risks were finally selected for FAA-RFA. FAA was considered risky in the following situations: the presumed FAA point involved the second-degree or more central vessel branches (n = 2); it was less than 1 cm to the bifurcation of non-feeding branches (n = 11); contact with the tumor (n = 7), or the adjacent vital organs was unavoidable (n = 4) ().
Ultrasound examination
An interventional radiologist with over ten years of experience in abdominal US and ablation performed all scans using a US imaging system (Aplio 500, Toshiba, Tokyo, Japan), with a convex array probe (PVT-375BT, 1.0–4.0 MHz) and a convex-volume probe (PVT-675MVT, 2.0–8.0 MHz). The number, size, and location of the tumor were identified by grayscale US. The vascular profile of the target tumor was roughly evaluated by color Doppler flow imaging and in detail by 2 D-CEUS and real-time 3D-CEUS, focusing on the origin, continuity, numbers, and structure of the feeding artery. 2 D-CEUS and real-time 3 D-CEUS were performed in our center as previously described [Citation16]. Briefly, 2D-CEUS was performed using contrast harmonic imaging after a bolus of 2.4 ml of ultrasound contrast agent (SonoVue, Barcco, Milan, Italy) was injected into the antecubital vein and followed by a flush of 5 ml of normal saline. Continuous scanning began immediately after SonoVue administration, focusing on the arterial phase (7–30 s). At least 5 min later, real-time 3D-CEUS was performed using an automated volume scanning mode under the same injection method and equipment settings, but without saline flush in order to prolong the arterial phase for better observation of the feeding artery [Citation16]. The feeding artery was defined as the branch of the hepatic artery that was connected to the enhanced areas of the tumor [Citation18]. The enhancing thickness of the target artery during the early arterial phase (10–15 s) was measured as the feeding artery diameter on 2D-CEUS. The highest systolic peak flow velocities were measured by pulsed-wave Doppler mode imaging.
Feeding vessel ablation (FAA)
The practical technique of FAA as an add on to conventional RFA is expressed in . FAA was performed by a Cool-tip ablation system (Valleylab, Covidien, Boulder, USA) with a maximum power of 200 W. After administration of analgesia (0.05–0.1 mg of fentanyl) as well as local anesthesia (5–15 ml of 1% lidocaine) and under the guidance of 2D-CEUS, a 3-cm active tip, unipolar electrode (ACT 2030) was percutaneously inserted into the target vessel. The system provided two energy application modes: one was Standard Ablation Mode for tumor ablation, and the other was Cauterization Mode for needle track ablation, which had the characteristic of fast temperature rise and concentrated energy distribution [Citation19]. As to the Cauterization mode of FAA, the output power was turned to maximum initially and then the electrode tip was manually maintained at 85–90 °C [Citation19]; as with the Standard Ablation mode of FAA, the output power was manually turned to maximum initially, and then applied by an automated pulsing algorithm based on the tissue impedance. The decisions regarding the ablation mode and energy application time were generally based on the feeding artery diameter and blood velocity [Citation20]: if the vessel diameter was > 3 mm and the highest peak systolic peak velocity was > 80 cm/s, the standard ablation mode was used for 1–2 cycles at 2 min per cycle; or else, the cauterization mode was used for 1–2 cycles at 4 min per cycle. If the cauterization mode failed to occlude the feeding vessel after 2 cycles, the standard ablation mode was considered as an addition, but the total ablation time was no more than 12 min. This strategy aimed for a small ablation zone to occlude the feeding artery and to cause minimum damage to the liver; in addition, the smaller the range of echogenic shadow created in the first step, the less the obstacle of repositioning in the second step (). 2 D-CEUS scan was carried out after each cycle for immediate evaluation. The technical success of FAA was defined as the disappearance of the target artery distal to the FAA point during the arterial phase. Complete perfusion response to FAA referred to absolute non-enhancement of the entire tumor, while partial perfusion response was defined as tumor enhancement defect or reduced maximum intensity during the arterial phase [Citation21].
Figure 2. The practical technique of FAA as add on to RFA. FAA was performed by ablating the target feeding artery but sparing the non-feeding branches, and after that RFA was performed to ablate the tumor. FAA: feeding artery ablation.
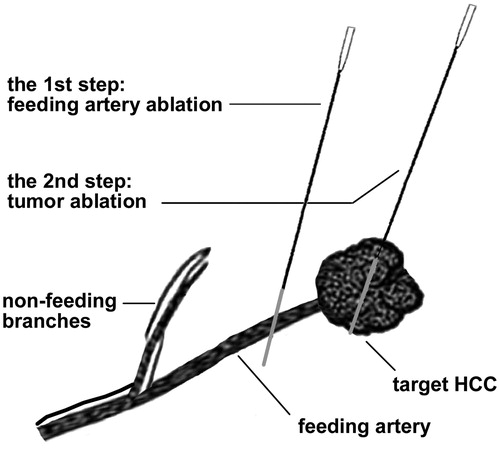
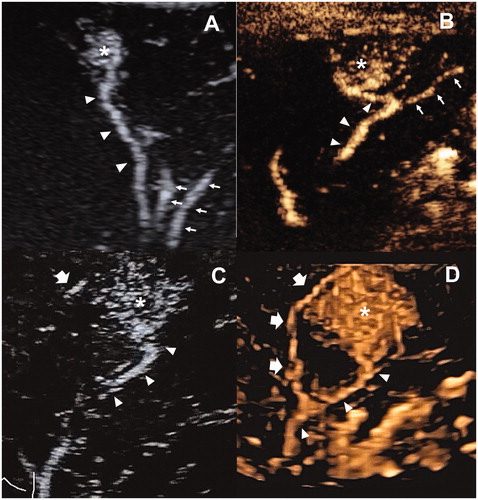
Figure 3. The examples illustrated the selection of target feeding arteries. (A) One subsegmental feeding artery (arrowhead) of the tumor (asterisk) was clearly depicted in the early arterial phase of 2D-CEUS, which is typically suitable for FAA. The principle of both sparing the non-feeding branches (fine arrow) and non-contact to the tumor could be achieved. (B) The tumor (asterisk) was not selected for FAA because of insufficient distance to the bifurcation of feeding (arrowhead) and non-feeding branches (arrow). (C) two subsegmental feeding arteries (arrowhead and thick arrow) of the tumor (asterisk) were selected as the targets of FAA, which were partly visible on 2D-CEUS through back-and-forth breathing movement. (D) Real-time 3D-CEUS depicted the two feeding arteries (arrowhead and thick arrow) in the reconstructed image with good continuity and contributed to feasibility determination. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
Tumor ablation
RFA was performed by the same ablation system and electrode as above. The decision regarding the number and arrangement of electrodes was based on the tumor size. Two electrodes with a switch controller (Valleylab, Covidien, Boulder, USA) were routinely used. If tumors were larger than 3.0 cm in diameter, three or more overlapping ablations were performed. The distances between the electrodes were 1.5–2.0 cm [Citation22]. The number of electrodes, overlapping cycles and ablation time were modulated according to the tumor location, perfusion response after FAA and the real-time monitoring of the ablation process. An ablative margin of more than 5 mm was considered to be safe. The concurrent non-target tumors, if any, were also to be ablated through a conventional method.
Patient follow-up
Therapeutic efficacy and complications were reported according to standardized terminology and criteria of image-guided tumor ablation [Citation1]. 2D-CEUS was performed in 30 min of RFA to assess the technical success of the procedure and the occurrence of procedure-related complications. In cases of technical failure, a repeat procedure of RFA was performed to achieve complete ablation immediately.
Technique efficacy was evaluated based on 1-month contrast-enhanced CT/MRI. Residual tumor, if any, was retreated with a second RFA session. If complete ablation of the tumor could not be achieved within two sessions, the treatment was considered unsuccessful. The ablation area was measured in the greatest dimension of the transverse (long- and short-axial diameter) and coronal planes (perpendicular-axial diameter). In addition, the ablation volume was calculated by carefully tracing the ablation margin on all axially acquired images using a commercially available PACS workstation (UniSight, DJ Health Union, Shanghai, China), as previously described [Citation5]. The pre- and 1-month portal venous phase CT/MRI images were reviewed side by side to calculate the ablative margin based on anatomic landmarks in two perpendicular planes [Citation23].
The follow-up interval was 3–4 months for the first 2 years and was then increased to 6 months. At each follow-up visit, 2D-CEUS, serum liver function tests and AFP were carried out. CT/MRI was performed when clinically indicated. A patient was considered lost-to-follow-up if the last information available was more than 6 months old.
Study endpoints
The primary endpoint was the cumulative LTP rate. LTP was defined as the appearance of tumor foci at the edge of the ablation after having achieved complete ablation. Secondary endpoints were the comparison of overall survival (OS), recurrence-free survival (RFS) and complications. Recurrence included LTP, intrahepatic and extrahepatic recurrence.
Statistical analysis
The statistical analyses were performed using the IBM SPSS 22.0 Statistics software (Armonk, NY: IBM Corp). Categorical data were compared using the χ2 test or Fisher’s exact test. Continuous data were compared using Student’s t-test or Mann–Whitney test. The cumulative LTP rates and survival curves were constructed by the Kaplan–Meier method. The risk factors associated with survival were identified using a Cox model. First, the factors used in the univariate analysis were age, AFP level, platelet count, tumor type, number, size, and perfusion response. Then, variables with a p value < 0.10 were entered into a multivariate Cox model. A p value < 0.05 was considered significant.
Results
FAA effectiveness and tumor perfusion response
The feeding artery could be visualized on CEUS in 65.5% (76/116) of the hypervascular HCCs ranging from 2 to 5 cm in diameter, but only 52.6% (61/116) of which met the inclusion criterion with one or two feeding arteries without complex branches. Finally, 41.0% (25/61) of the lesions underwent FAA before the conventional RFA (). The demographic and baseline clinical characteristics of patients are listed in . The diameter of the target feeding artery was 2.8 ± 0.4 (2.0–3.6) mm, with a systolic arterial flow rate of 60.0 ± 30.0 (16.6–128.2) cm/s. Cauterization mode was used alone for 18 feeding branches, and standard ablation was used alone for 5, and a combination of the two modes was used for 6. The number of electrode insertions per vessel was 1.1 ± 0.3 (1–2), and the number of ablation cycles per vessel was 1.9 ± 0.7 (1–3). The time of energy application per procedure was 6.5 ± 1.9(4–10)min (). The technical success rate of FAA reached 100%, as all the target feeding arteries disappeared after FAA. However, only 13/25 (52.0%) target tumors were evaluated as complete perfusion response (, Supplementary Video 1 and Video 2), while 12/25 (48.0%) were evaluated as partial perfusion response ().
Figure 4. Images in a 41-year-old male with recurrent HCC measured 3.2 cm in diameter. (A) one feeding artery (arrowhead) of the tumor (asterisk) was depicted in the early arterial phase of 2D-CEUS. (B) FAA created a small ablation zone (arrowhead) using 1 cycle and 4 min cauterization mode. The target tumor was generally intact (asterisk). The arrow showed the electrode inserting path. (C) Immediate 2D-CEUS after FAA showed complete non-enhancement within the contour of the tumor in the arterial phase, which was identified as a complete perfusion response to FAA. (D) Hyperechoic area covered the tumor (arrowhead) after RFA, using two electrodes with a switch controller, 16 min in total. (E) 2D-CEUS performed in 30 min after RFA showed a large ablation zone measuring 5.2 cm × 4.2 cm without residue. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
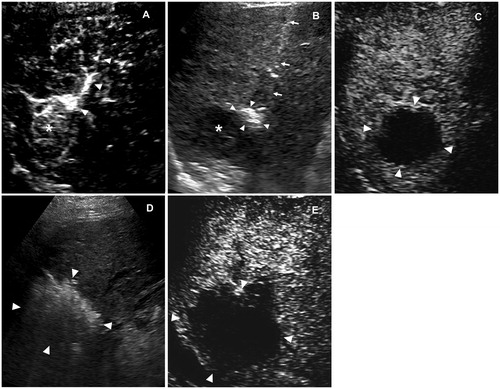
Figure 5. Images in a 43-year-old male with recurrent HCC measured 2.8 cm in diameter. (A) one feeding artery (arrowhead) of the tumor (cross) was depicted in the early arterial phase of 2D-CEUS. (B) Before FAA, 2D-CEUS showed hyperenhancement of the entire tumor at 23 s. (C) FAA created a small ablation zone (arrowhead) using 2 cycles and 8 min cauterization mode. The target tumor was intact (cross). The arrow showed the electrode inserting path. (D,E) After FAA, dual imaging showed intratumoral enhancement defect of the tumor at 23 s, which identified as a partial perfusion response to FAA. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
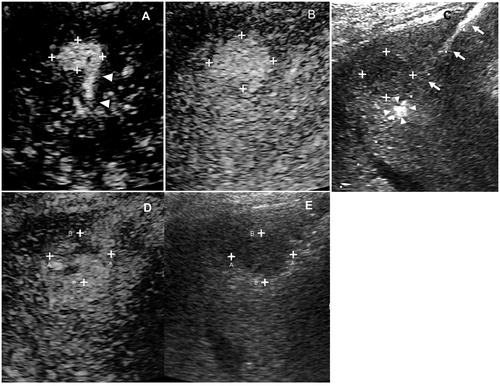
Table 1. Demographic and Baseline Clinical Characteristics of Patients.
Table 2. Technical details of FAA as add-on to RFA in 25 tumors with 29 target feeding arteries.
Ablation sizes
The number of electrode insertions per procedure was 2.0 ± 0.7 (1–4). Total ablation time per procedure was 16.6 ± 6.5 min (8–36 min) (). The diameters of ablation zone evaluated immediately by 2 -CEUS and 1 month later by CT/MRI is shown in . The ablation volume was 41.9 ± 17.5 cm3 (14.9–78.2 cm3) and the ablative safety margin was 8.2 ± 1.9 mm (4–12 mm) at the 1-month evaluation. However, there was no significant difference in these data between the subgroups of complete and partial perfusion response (all p > 0.05, ).
Table 3. Comparison of ablation sizes according to perfusion reduction (complete vs. partial response).
Therapeutic efficacy and local tumor progression
The follow-up period was 34.0 ± 10.8 months (range, 6.8–50.0 months). At 3-years follow-up, 2/25 (8.0%) patients were lost to follow-up. The follow-up duration of these lost-to-follow-up patients was 6.8 and 22.3 months.
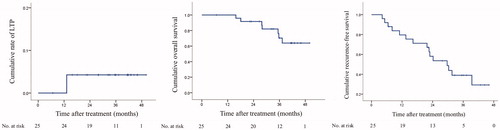
At the 1-month evaluation, no residual tumor was found. At 3-year follow-up, LTP occurred in 4.0% (1/25) of the patients, who was evaluated as a partial response after FAA. This patient was successfully managed with another session of RFA against the LTP foci. The cumulative rates of LTP at 1-, 2-, and 3-year follow-ups were 0.0%, 4.2% and 4.2%, respectively ().
Survival
On the day of the endpoint of the study, 7/25 (28.0%) patients had died. Causes of death were: HCC progression 5/25 (20.0%), and liver failure with stable tumor 2/25 (8.0%). The 1-, 2-, and 3-year overall survival (OS) was 100%, 91.7%, and 70.3% (). In the multivariate Cox model (), 2 variables were associated with worse OS: more than one nodule HR = 7.307 (95% CI: 1.123–47.545, p = 0.037), and AFP > 20 ng/mL HR = 16.501 (1.724–157.902, p = 0.015).
Table 4. Univariate and multivariate cox analysis of the predictors of overall survival.
The 1-, 2-, and 3-year recurrence-free survival (RFS) was 79.6%, 58.1%, and 39.0% (). In the multivariate Cox model (), no variables were found to be associated with RFS.
Table 5. Univariate and multivariate Cox analysis of the predictors for recurrence-free survival.
Complications
There were no treatment-related deaths. Sepsis and consequent liver decompensation occurred in one patient, who finally recovered in 1 week after administration of antibiotics and other supportive treatment (Grade D). Tumor perfusion after FAA was evaluated as a partial response in this patient. No complications related to injury of the portal vein or bile duct occurred, such as widespread liver ischemia, needle track bleeding and bile duct dilation, or stricture. As a result, the major complication rate was 4.0% (1/25).
Discussion
In this study, it was shown to be possible to totally block tumor perfusion using percutaneous ultrasound-guided FAA in 13/25 (52.0%) of target tumors, while in the other 12/25 (48.0%) partial perfusion remained. To the best of our knowledge, this was the first report about the immediate change of tumor perfusion after FAA. By means of a modified ablative technique, in spite of a very small ablation size of FAA, a large final ablation size and ablative safety margin were achieved without adding more electrodes or other devices. The cumulative rates of LTP at 1-, 2-, and 3-year follow-ups were 0.0%, 4.2% and 4.2%, respectively. These results indicated that FAA may add extra benefits to conventional RFA in carefully selected HCCs.
Although 100% of the target feeding arteries disappeared after FAA, only 13/25 (52.0%) of the target tumors were determined to be complete perfusion response and the remaining ones were deemed to be partial perfusion response. Partial perfusion response could suggest the presence of other feeding branches not displayed by CEUS due to a high BMI, fatty liver, tumor location with acoustic shadow or artifact, as well as small crossover blood supply and extrahepatic collateral pathways that were difficult to detect [Citation24]. However, the classification of perfusion reduction did not influence treatment outcomes, such as LTP rate, OS and RFS. Possible reasons might include comparable ablation size and safety margin, which was similar in both subgroups and contributed greatly to local effectiveness. As the effect of the addition of FAA to RFA was similar to that of TACE or TAE, the perfusion change identified by intraprocedural CEUS was also similar to the reported complete response (CR) rate after one session of TACE, which ranged from 48% to 57% in 1 or 2 months analyses [Citation25–27]. Although TACE seemed to have higher CR rate theoretically, most embolization agents remained in the tumor only for a few weeks [Citation28] and led to reperfusion of the tumor which might be construed as CR immediately after embolization. Based on this reason, some authors suggested Transcatheter Arterial Embolization (TAE) or TACE should be followed by RFA as quickly as possible to create a larger ablation volume [Citation29,Citation30]. However, it was not practical to apply combined treatment with RFA and TACE in most clinical settings. In fact, the time interval between TACE and RFA was 3 days to 4 weeks [Citation8,Citation9]. In the present study, RFA could be performed immediately after FAA without the need for additional devices or specialists, which was also cost-effective for patients.
In the present study, 72% (18/25) of FAA was performed using cauterization mode, which only created an ablation volume of about 1.0–1.5 cm3 in 2-4 min [Citation19]. Cauterization Mode is usually used for needle track ablation because of its rapidly rising temperature and small but focused ablation area [Citation19]. Here it was used first to ablate the feeding artery and showed its practicability. Because the additional ablation zone created by FAA was very small, we reasonably believed that FAA pretreatment enlarged the ablation size through decreasing perfusion-mediated cooling, rather than simply adding a tiny ablation zone. This result was similar to the findings of a previous report [Citation15]. However, the previous researcher ablated the feeding artery as well as part of the tumor initially, which made it difficult to judge the independent effect of FAA on tumor perfusion and the ablation area.
It was observed that 41.0% (25/61) of patients can benefit from FAA before RFA of the main tumor. From the technical point of view, the most common reason precluding FAA was that the presumed FAA point was less than 1 cm from the bifurcation into non-feeding branches. The feeding vessel structure of HCC varies; however, the previous researchers paid little attention to the origin, continuity, numbers and structure of the feeding artery, partly because of the limitation of less advanced ultrasound systems and techniques. For example, real-time 3 D-CEUS could provide richer and more intuitive information on feeding arteries [Citation16], which facilitated the procedure of FAA. Some researchers inserted the electrodes to the area where the arteries surrounded or entered the tumor [Citation11–13,Citation15], and another even punctured the nearby blood vessel directly [Citation14]. In fact, this kind of manipulation has a risk of vessel damage, causing occlusion, thrombus, bleeding, or fatal liver infarction [Citation31,Citation32]. The incidence of liver infarction after thermal ablation reported in the literature was 0–1.8% [Citation3,Citation33]. Although heat injury to the vessels was rare because of the cooling effect of the blood flow, there was probably a higher risk if the energy had been directly transferred to the vessel during FAA, especially for a tumor located centrally, close to the portal vein and the hepatic artery. One study reported the occurrence of thrombosis within the right portal vein and right hepatic artery soon after RFA, which led to serious liver infarction [Citation34]. Therefore, in this study, we set the target FAA point only in third-degree or more distal branches and carefully avoided non-feeding branches to prevent serious vascular complications. In addition, the non-contact approach is an important prerequisite of FAA because of possible tumoral tract seeding [Citation35].
The cumulative LTP rate was estimated as 4.2% at 3 years in our study. This result was comparable to the previous FAA study using CEUS guidance (8.5%) [Citation15], and superior to others using Doppler guidance (13.5–17.6%) [Citation12–14]. In general, cumulative LTP rates at 3 years ranged from 10.0% to 38.6% in conventional RFA of HCC [Citation36–38]. It seemed that FAA-RFA offered better local tumor control than older methods, especially facilitated by CEUS. Similar to the previous studies, OS was more relevant to the personal characteristics, such as AFP levels and tumor number in the present study. In addition, no risk factor was found for RFS in this study.
This study had several limitations. First, it was a single-arm study. As the technical risk was finally determined by the radiologist through a dynamic and detailed scan on the operating table, it is difficult to randomize and set a fair control in this clinical setting. Second, the intraprocedural tumor perfusion response was estimated subjectively by two radiologists using visual observation. Therefore, this parameter might be determined more accurately using quantitative CEUS analysis. Finally, on a technical level, it may be quite demanding to target a feeding artery quickly on the arterial phase of CEUS. Although the ablation zone of FAA was small, it still covered a certain area and the central temperature was very high [Citation19]. If the electrode is inserted as close as possible to the feeding artery, there is still a high probability of achieving a successful FAA. Considering the fact that the feeding artery is clear on CEUS and the enhancing diameter can be 2–3 mm, an experienced interventional radiologist might be able to master the skill after sufficient practice.
In conclusion, the present study provided additional information on the technical aspects of FAA, including feeding artery selection, intraprocedural perfusion evaluation and ablation strategy. This add-on measure safely enlarged the ablation area and delineated the potential to reduce LTP after RFA.
Supplemental Material
Download MP4 Video (5.9 MB)Supplemental Material
Download MP4 Video (5.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.e1694.
- Kim YS, Rhim H, Cho OK, et al. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59(3):432–441.
- Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vascular Interventional Radiol. 1998;9(1):101–111.
- Fukuda H, Numata K, Moriya S, et al. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation–propensity score matching analysis. Radiology. 2014;272(2):598–604.
- Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: Increased tumor destruction with adjuvant liposomal doxorubicin therapy. Am J Roentgenol. 2002;179(1):93–101.
- Sudheendra D, Neeman Z, Kam A, et al. Intermittent hepatic vein balloon occlusion during radiofrequency ablation in the liver. Cardiovasc Intervent Radiol. 2006;29(6):1088–1092.
- Yamasaki T, Kimura T, Kurokawa F, et al. Percutaneous radiofrequency ablation with cooled electrodes combined with hepatic arterial balloon occlusion in hepatocellular carcinoma. J Gastroenterol. 2005;40(2):171–178.
- Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432.
- Frich L, Mala T, Gladhaug IP. Hepatic radiofrequency ablation using perfusion electrodes in a pig model: effect of the Pringle manoeuvre. Eur J Surg Oncol. 2006;32(5):527–532.
- Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33(4):428–436.
- Cheng YT, Jeng WJ, Lin CC, et al. Percutaneous radiofrequency ablation of tumor feeding artery before target tumor ablation may reduce local tumor progression in hepatocellular carcinoma. Biomed J. 2016;39(6):400–406.
- Hou YB, Chen MH, Yan K, et al. Adjuvant percutaneous radiofrequency ablation of feeding artery of hepatocellular carcinoma before treatment. World J Gastroenterol. 2009;15(21):2638–2643.
- Santambrogio R, Costa M, Barabino M, et al. Laparoscopic radiofrequency of hepatocellular carcinoma using ultrasound-guided selective intrahepatic vascular occlusion. Surg Endosc. 2008;22(9):2051–2055.
- Zhang ZY, Lee JC, Yang W, et al. Percutaneous ablation of the tumor feeding artery for hypervascular hepatocellular carcinoma before tumor ablation. Int J Hyperthermia. 2018;35(1):133–139.
- Lu Y, Liu B, Zheng Y, et al. Application of real-time three-dimensional contrast-enhanced ultrasound using SonoVue for the evaluation of focal liver lesions: a prospective single-center study. Am J Transl Res. 2018;10(5):1469–1480.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- Ohta K, Shimohira M, Hashizume T, et al. Identification of the feeding arteries of hepatocellular carcinomas by performing dual arterial phase CT during pre-transarterial chemoembolization angiography. Abdom Radiol. 2019;44(6):2276–2282.
- Li XJu, Lin MXia, Xie XYan, et al. Different heating modes of Cool-tip on coagulation zone and thermal distribution: in vitro and in vivo experiment. Chin J Ultrasonogr. 2016;25(6):72–77.
- Numata K, Tanaka K, Mitsui K, et al. Flow characteristics of hepatic tumors at color Doppler sonography: correlation with arteriographic findings. Am J Roentgenol. 1993;160(3):515–521.
- Wang X, Erinjeri JP, Jia X, et al. Pattern of retained contrast on immediate postprocedure computed tomography (CT) after particle embolization of liver tumors predicts subsequent treatment response. Cardiovasc Intervent Radiol. 2013;36(4):1030–1038.
- Woo S, Lee JM, Yoon JH, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268(2):589–600.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Miyayama S, Yamashiro M, Shibata Y, et al. Variations in feeding arteries of hepatocellular carcinoma located in the left hepatic lobe. Jpn J Radiol. 2012;30(6):471–479.
- de Korompay N, Alshammari M, Klass D, et al. Intraprocedural parenchymal blood volume is a predictor of treatment response for chemoembolization in hepatocellular carcinoma: results of a prospective study. J Vasc Interv Radiol. 2018;29(7):928–935.
- Kaufmann S, Schulze M, Spira D, et al. Comparison of volume perfusion computed tomography and contrast-enhanced ultrasound for assessment of therapeutic effect of transarterial chemoembolization in patients with hepatocellular carcinoma: a preliminary report. Acta Radiol. 2016;57(1):8–12.
- Ippolito D, Trattenero C, Talei Franzesi C, et al. Dynamic contrast-enhanced magnetic resonance imaging with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid for quantitative assessment of vascular effects on hepatocellular-carcinoma lesions treated by transarterial chemoembolization or radiofrequency ablation. J Comput Assisted Tomogr. 2016;40(5):692–700.
- Lewandowski RJ, Geschwind JF, Liapi E, et al. Transcatheter intraarterial therapies: rationale and overview. Radiology. 2011;259(3):641–657.
- Guang C, Kawai N, Sato M, et al. Effect of interval between transcatheter hepatic arterial embolization and radiofrequency ablation on ablated lesion size in a swine model. Jpn J Radiol. 2011;29(9):649–655.
- Lee IJ, Kim YI, Kim KW, et al. Radiofrequency ablation combined with transcatheter arterial embolisation in rabbit liver: investigation of the ablation zone according to the time interval between the two therapies. Br J Radiol. 2012;85(1019):e987–e994.
- Chuang CH, Chen CY, Tsai HM. Hepatic infarction and hepatic artery pseudoaneurysm with peritoneal bleeding after radiofrequency ablation for hepatoma. Clin Gastroenterol Hepatol. 2005;3(11):A23.
- Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surgery. 2004;239(4):450–458.
- Kim YS, Rhim H, Lim HK, et al. Hepatic infarction after radiofrequency ablation of hepatocellular carcinoma with an internally cooled electrode. J Vasc Interv Radiol. 2007;18(9):1126–1133.
- Poggi G, Teragni C, Gazzaruso C, et al. Massive hepatic infarction complicating ultrasound-guided percutaneous radiofrequency thermal ablation. Liver Int. 2004;24(6):704–705.
- Jaskolka JD, Asch MR, Kachura JR, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16(4):485–491.
- Mohkam K, Dumont PN, Manichon AF, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68(6):1172–1180.
- Francica G, Saviano A, De Sio I, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Digestive Liver Dis. 2013;45(4):336–341.
- Ito T, Tanaka S, Iwai S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case-control study with propensity score matching. Hepatol Res. 2016;46(6):565–574.