?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the prevalence of pelvic adhesions in patients with uterine fibroids after high-intensity focused ultrasound (HIFU) treatment, then analyze the influencing factors of pelvic adhesions.
Materials and methods
From October 2010 to March 2020, a total of 2619 patients with uterine fibroids underwent either hysterectomy, myomectomy, or cesarean section in Suining Central Hospital of Sichuan province. Of the 2619 patients, 810 were excluded because of a documented history of either pelvic infections, endometriosis, prior abdominopelvic surgery, or gynecological malignancies; 1809 patients were enrolled and the data were retrospectively assessed for the prevalence and patterns of pelvic adhesions. Among them, 96 patients with uterine fibroids had had prior HIFU treatment (HIFU group), 1713 patients had not had HIFU or surgical treatments (control group).
Results
Among the 96 patients in the HIFU group, adhesions were detected in 42 patients, the incidence of pelvic adhesion being 43.75%; the 1713 patients in the control group, adhesions were detected in 619 patients, the prevalence of pelvic adhesion being 36.14%. No statistically significant difference in the incidence of adhesion between the two groups was observed (p = .132), no significant difference in location of pelvic adhesions between the two groups and no significant difference in the severity of adhesions between the two groups was observed (p > .05).
Conclusions
Based on our results with limited numbers, we concluded that HIFU treatment did not significantly increase the risk of pelvic adhesions.
Introduction
Pelvic adhesions are a common surgical complication. It refers to the connection of separated organs by fibrous tissue. Adhesions can be induced by mechanical injury, thermal injury, radiation, pelvic inflammatory disease, and endometriosis. Pelvic adhesions may cause chronic pelvic pain, infertility, and lead to reoperations. For women with prior open abdominopelvic surgery, the presence of adhesions could lead to added morbidity with an increased risk of bowel injury if further surgery is required. Moreover, the cost of management for pelvic adhesions is a heavy burden both on patients and society. According to the studies, in the United States of America, the annual cost of treatment for abdominal adhesions was over $2 billion. In the United Kingdom, the cost of hospitalization due to adhesions after surgeries was £2.42 million [Citation1,Citation2].
Uterine fibroids are the most common benign tumors in the reproductive system of reproductive women. Almost half of the patients present with heavy menstrual bleeding, lumbosacral pain, constipation, and other symptoms [Citation3]. Currently, surgery is still the treatment of choice for uterine fibroids and myomectomy is one of the most common gynecological procedures. However, post-surgical adhesions are of great concern. One study showed that 93.7% of patients undergoing open myomectomy form pelvic adhesions [Citation4]. Compared with open surgery, laparoscopy reduces peritoneal trauma and de novo adhesion formation; however, it may cause peritoneal inflammation due to pneumoperitoneum pressure and thermal injuries. Pelvic adhesions remain a major public health problem despite the development of laparoscopy [Citation5].
High intensity focused ultrasound (HIFU) is a novel local thermal ablation technique. The treatment is performed under the guidance of magnetic resonance imaging (MRI) or ultrasound, the therapeutic ultrasound beams penetrate the tissues of the body and are focused on the tumor to induce coagulative necrosis by mechanical effect, thermal effect, and cavitation effect. Over the last decade, many studies have shown that HIFU is safe and effective in the treatment of uterine fibroids [Citation6–9]. As a noninvasive treatment, HIFU has no peritoneal trauma, but thermal injuries may cause peritoneal inflammation. However, no study was performed to investigate if HIFU treatment for uterine fibroids results in pelvic adhesions. Therefore, the aim of this study was to investigate whether HIFU treatment was involved in the process of pelvic adhesions.
Materials and methods
This retrospective study was approved by the ethics committee at our institute. Before each procedure, the details of the treatment were discussed with all patients, who then signed consent.
Subjects
From October 2010 to March 2020, a total of 2619 patients with uterine fibroids underwent either hysterectomy, myomectomy, or cesarean section in Suining Central Hospital of Sichuan province. According to the inclusion criteria and exclusion criteria below, 810 patients were excluded because 504 had a documented history of pelvic infections, 112 had endometriosis, 178 had prior abdominopelvic surgery, and 16 patients had gynecological malignancies. Therefore, 1809 patients were enrolled in this study and the data were retrospectively assessed for the prevalence and patterns of pelvic adhesions. Among them, 96 patients with uterine fibroids had prior HIFU treatment (HIFU group), 1713 patients had not had HIFU or surgical treatments (control group) ().
Figure 1. CONSORT flow diagram of the enrollment and data analysis of patients with uterine fibroids underwent surgical treatment.
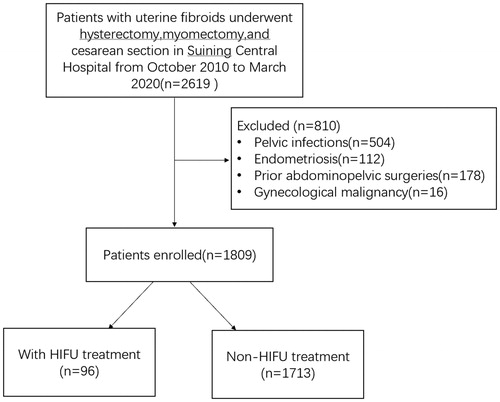
Inclusion criteria: all patients with uterine fibroids who underwent surgical treatments including hysterectomy, myomectomy, or cesarean section.
Exclusion criteria: (1) patients with any prior abdominopelvic surgeries; (2) patients with documented prior pelvic infections, endometriosis, and gynecological malignancy.
HIFU treatment
All patients received specific bowel and skin preparation prior to HIFU treatment. Bowel preparation included ingesting liquid food for 2 days before HIFU treatment, 12 h fasting before HIFU and a cleansing enema in the morning of the treatment day. Skin preparation included shaving, cleaning skin with alcohol solution and removing air bubbles off skin surface of the anterior abdominal wall from the umbilicus to the upper margin of the pubic symphysis. In order to optimize the therapeutic acoustic pathway, a urinary catheter was inserted into the bladder to control the size of the bladder by infusing normal saline before the HIFU procedure.
The procedure of HIFU ablation was performed under conscious sedation (fentanyl 0.8–1 μg/kg, administrated at 30–40 min intervals; midazolam hydrochloride, 0.02–0.03 mg/kg, administrated at 30–40 min intervals). Procedure was performed using JC200 Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co, Ltd, Chongqing, China). Real-time ultrasound imaging was provided to guide the treatment and monitor the response of uterine fibroids to HIFU. The patients were required to report any discomfort during the procedure and the vital signs such as heart rate, blood pressure, respiration, and oxygen saturation were monitored until the entire fibroid had been treated.
The patients were carefully positioned prone on the HIFU treatment table, and the lower abdomen was in contact with water in which air bubbles removed. A cold degassed water balloon was placed between the transducer and abdominal wall to compress and push away the bowels from the acoustic pathway. Treatment began from the posterior to anterior side of the fibroids, from feet side to head side and the focus was at least 1.5 cm away from the endometrium and 1 cm from the margin of the fibroids. During HIFU treatment, based on feedback from the patient and changes in gray scale on ultrasonographic imaging the ultrasonic energy is adjusted. This process was repeated on a section-by-section basis. Once the entire target fibroid has a hyperechoic appearance or the contrast-enhanced ultrasound showed no blood supply, then the treatment was terminated.
Pelvic adhesion scoring system
During surgery, the abdominal cavity was inspected for the presence of adhesions. The degree and scope of adhesion between the ovary fallopian tube and the surrounding tissue, the obliteration of the pouch of Douglas, and the degree of patency of the fallopian tube were explored and quantified (). The pelvic adhesion score system includes five parts: adhesion degree (1 point: membranous or loose; 2 points: dense; 3 points: severe dense), adhesion range (1 point: 2-6 cm; 2 points: >6-10cm; 3 points: >10cm), ovarian involvement (1 point: unilateral; 2 points: bilateral), fallopian tube involvement (1 point: unilateral; 2 points: bilateral), obliterated pouch of Douglas (1 point: partly; 2 points: entirely). According to the above adhesion score system, a score of 2–5 points indicates mild pelvic adhesion, a score of 6–9 points indicates moderate pelvic adhesion, and a score of 10–14 points indicates severe pelvic adhesion [Citation10]. The severity of pelvic adhesions was graded, and the factors that may cause pelvic adhesions were stratified and analyzed in two groups.
Statistical analysis
The data were analyzed using SPSS software (SPSS17.0, IBM Company, Chicago, IL). The normally distributed data are reported using mean ± standard deviation; the skewed data distribution was reported using the median and the interquartile range. One-way ANOVA analysis was applied when comparing variables between the two groups. The Wilcoxon test and chi-square test were applied for the analysis of the quantitative and enumerated data. A p value of less than .05 was defined as statistically significant.
Results
Baseline characteristics of the patients
The mean age of the patients in the HIFU group was 39.15 ± 5.46 years; and 42.18 ± 5.67 years in the control group. The median menstruation volume was 80.55 ± 4.96 ml in the HIFU group; and 81.12 ± 3.48 ml in the control group. The average menstrual period was 7.89 ± 1.08 days in the HIFU group; and 8.65 ± 1.14 in the control group. The average size of the fibroids in the HIFU group was 4.82 ± 0.56 cm in diameter; and 5.57 ± 0.82 in the control group. The median number of abortions in both the HIFU group and the control group was 2; the median number of deliveries was 1 in both the HIFU group and the control group. No significant difference was observed in baseline characteristics between the two groups ().
Table 1. Baseline characteristics of the patients in the two groups.
Comparison of the incidence rate of pelvic adhesion between patients with and without prior HIFU
Of the 96 patients in the HIFU group, 27 patients underwent hysterectomy due to local recurrence or de novo fibroids; 38 patients had myomectomy due to recurrence in 31 cases, and due to psychological factors in 7 cases; 19 patients had laparoscopic surgery due to ovarian diseases; and 12 patients had cesarean section. In the control group, 450 patients underwent hysterectomy, 1049 had myomectomy, and 214 had cesarean section. Pelvic adhesions were detected in 42 (43.75%) patients; of the 1713 patients in the control group, adhesions were detected in 619 (36.14%) patients. No statistically significant difference in the incidence of adhesion between the two groups was observed (p = .132) (). shows the location of adhesions in the two groups. There was no significant difference in location of pelvic adhesions between the two groups (p > .05). As shown in , there was no significant difference in the severity of adhesions between the two groups (p > .05).
Table 2. Comparison of the incidence of pelvic adhesions in the two groups.
Table 3. Location of adhesions in the two groups.
Table 4. Comparison of the severity of pelvic adhesions between the two groups [n (%)].
Evaluation of the independent factors that affect pelvic adhesions
In the HIFU group, the results of the Chi-square test showed a significant difference in the past history of the number of abortions, the number of deliveries, and the size of uterine fibroids between the patients with and without adhesions (p < .05) (). It indicated that these factors might relate to pelvic adhesions. The stratified analysis further showed that patients with more than 2 abortions and lesion size larger than 4 cm in diameter were more likely to have pelvic adhesions (p < .05) (). However, the multi-factor correlation analysis revealed that these factors were not the independent factors for pelvic adhesions (p > .05) (). We further compared the therapeutic parameters of HIFU in patients with and without pelvic adhesions. The results showed significant differences in nonperfused volume (NPV) ratio, total energy used for ablating the fibroid, sonication time, and the energy efficiency factor (EEF) between the patients with and without adhesions (p < .05) (). In the control group, the results of Chi-square test showed a significant difference in age, the number of abortions, and the size of uterine fibroids between the patients with and without adhesions (p < .05) (). The stratified analysis further showed that if patients were older than 35 years, had had more than 2 abortions, and had fibroids of 3.5 cm to 4 cm, then the incidence of pelvic adhesion was higher than the other subgroups (p < .05) (). However, the multi-factor correlation analysis did not reveal any significant relationship between these factors and pelvic adhesions, it revealed that these factors were not the independent factors of pelvic adhesions (p > .05) ().
Table 5. The related factor analysis of pelvic adhesions in the HIFU group [n(%)].
Table 6. The multi-factor correlation analysis of pelvic adhesions in the HIFU group.
Table 7. The HIFU treatment results between the patients with and without adhesions in HIFU group.
Table 8. The related factor analysis of pelvic adhesions in the control group [n(%)].
Table 9. The multi-factor correlation analysis of pelvic adhesions in the control group.
Discussion
Previous studies have shown that adhesions occurred in 60–90% of patients after abdominopelvic surgeries [Citation11,Citation12]. After excluding those patients with documented prior pelvic infections, endometriosis, prior abdominopelvic surgery, and gynecological malignancy, we found 42 patients in the HIFU group had adhesions, thus the prevalence of adhesions was 43.75% (42/96), which was lower than that in the previous studies of patients after abdominopelvic surgery [Citation11,Citation12]. Among these 42 patients who had pelvic adhesions after HIFU, 26 had multiple uterine fibroids, 16 had a solitary fibroid. The average size of the fibroids was 4.21 ± 0.36 cm in diameter. In the other 54 patients who did not have pelvic adhesions, 24 had multiple uterine fibroids, 30 had a solitary fibroid. The average size of the fibroids was 3.84 ± 0.47 cm in diameter. We did not find any significant difference in size and numbers of fibroids between the two groups. However, the HIFU therapeutic results showed significant differences in NPV ratio, the total energy used for fibroid ablation, the treatment time and sonication time, and the energy efficiency factor (EEF) (p < .05) (). The total energy used for fibroid ablation, the treatment time, the sonication time, and EEF were significantly higher in patients with adhesions than that in patients without adhesions; while the NPV ratio was significantly lower than that in patients without pelvic adhesions. Therefore, the risk of pelvic adhesions after HIFU seems related to the delivery ultrasound energy. In the control group, we found 619 patients had adhesions, thus the prevalence of adhesions in the control group was 36.14% (619/1713). No significant difference was observed in the prevalence of adhesions between the patients in the HIFU group and the control group. The observed prevalence of adhesions in either the HIFU group or the control group in this study was also in accordance with a previous study seen in women without previous abdominopelvic surgery or pelvic inflammatory disease [Citation13]. We further compared the prevalence of adhesions between the HIFU group and the control group in the following sites: uterus (19.8 vs. 18.6%), ovaries (3.1 vs. 1.6%), fallopian tubes (4.2 vs. 6.9%), pouch of Douglas (5.2 vs. 2.4%), bladder (4.2 vs. 2.6%), and bowel (7.3 vs. 4.0%) and found no significant difference in the prevalence of adhesions between the two groups. We also compared the severity of pelvic adhesions between the two groups, the results showed no significant difference. Therefore, HIFU treatment seems did not significantly increase the prevalence of pelvic adhesions. In addition to abdominopelvic surgery, many other factors may also cause pelvic adhesions. In this study, the prevalence of adhesions in patients without documented previous surgery, pelvic infections, and endometriosis was 36.54% (661/1809). Some hidden factors may cause pelvic adhesions. Therefore, we first analyzed the factors that might relate to pelvic adhesions in the HIFU group. The results showed that the number of abortions (p = .001), the number of deliveries (p = .012), and the size of uterine fibroids (p = .004) were related to pelvic adhesions (). However, the multi-factor correlation analysis showed that these factors were not independent factors (p > .05) (). We then analyzed the factors that might cause pelvic adhesions in the control group, the results showed that age, the number of abortions and the size of uterine fibroids were the factors related to pelvic adhesion (p < .05) (), but the multiple factor analysis showed that these factors were not independent factors (p < .05) ().
The impact of pelvic adhesions on patients has been a concern for all surgeons. Pelvic adhesions can lead to chronic pelvic pain, infertility, intestinal obstruction, and might increase the risk of postoperative chemotherapy failure. A recent survey of adhesion awareness among gynecological surgeons in European hospitals concluded that antiadhesion agents were too expensive to be used routinely in gynecological surgery [Citation14]. Over the last decades, HIFU has been widely used to treat different types of solid tumors, especially uterine fibroids [Citation15–17]. As a noninvasive treatment, this technique can be used precisely to ablate tumors, thus decreasing the risk of pelvic adhesions in clinical practice.
This study is limited because it is a retrospective study and the surgery was performed by different doctors, thus some bias may occur. This study is also limited because the number of patients in the HIFU group was small and the number of patients in the control group was large, this bias may have also affected the statistical power. Therefore, future studies with more patients with uterine fibroids who had HIFU treatment from multiple centers are needed to clarify the findings from this study.
Conclusions
Pelvic adhesions are the most common surgical complication. Based on our results from the limited number of patients, HIFU treatment for uterine fibroids does not significantly increase the risk of pelvic adhesions. However, future studies with a large sample size of patients with uterine fibroids treated with HIFU are needed to clarify the findings from this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sikirica V, Bapat B, Candrilli SD, et al. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11(1):13–13.
- Wilson MS, Menzies D, Knight AD, et al. Demonstrating the clinical and cost effectiveness of adhesion reduction strategies. Colorectal Dis. 2002;4(5):355–360.
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043
- Tulandi T, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993;82(2):213–215.
- Mais V. Peritoneal adhesions after laparoscopic gastrointestinal surgery. World J Gastroenterol. 2014;20(17):4917–4925.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Chen J, Li Y, Wang Z, Committee of the Clinical Trial of HIFU versus Surgical Treatment for Fibroids, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG: Int J Obstet Gy. 2018;125(3):354–364.
- Zhang W, He M, Huang G, et al. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int J Hyperthermia. 2016;32(6):623–629.
- Liu Y, Zhang W, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018; 35(1):56–61.
- Sun AJ, Huang J, Zhou YZ, et al. Evaluation of the diagnosis and treatment of infertility caused by pelvic adhesions by uterine tube iodine oil radiography and laparoscopy[J]. Chin J Practical Gynecol Obestetr. 2008;24(5):369–371.
- Hirschelmann A, Tchartchian G, Wallwiener M, et al. A review of the problematic adhesion prophylaxis in gynaecological surgery. Arch Gynecol Obstet. 2012;285(4):1089–1097.
- Tulandi T, Al-Shahrani A. Adhesion prevention in gynecologic surgery. Curr Opin Obstet Gynecol. 2005;17(4):395–398.
- Ikechebelu JI, Eleje GU, Joe-Ikechebelu NN, et al. Akintobi AONiger J Comparison of the prevalence of adhesions at the time of diagnostic laparoscopy for infertility between patient who had open myomectomy and those who had no previous pelvic-abdominal surgery or pelvic inflammatory disease. Clin Pract. 2018;21(11):1415–1421.
- Wallwiener M, Koninckx PR, Hackethal A, for The Anti-Adhesions in Gynecology Expert Panel (ANGEL), et al. A European survey on awareness of post-surgical adhesions among gynaecological surgeons . Gynecol Surg. 2014;11(2):105–112.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview[J]. Ultrason Sonochem. 2004;11(3-4):149–154.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4(3):294–302.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327.