Abstract
Objective
To investigate the safety and efficacy of high-intensity focused ultrasound (HIFU) treatment for diffuse uterine leiomyomatosis (DUL).
Methods
Eight patients with DUL were admitted to the Department of Gynecology of Shanghai First Maternity and Infant Hospital and underwent HIFU treatment. MRI was performed before and one day after HIFU treatment for the evaluation of lesion ablation. The uterine size was measured at 3-8 months after HIFU ablation. The menstrual volume score and serum levels of hemoglobin and CA-125 were measured pre-HIFU ablation and 12-36 months post-HIFU ablation.
Results
After an average of 5.9 months of follow-up after HIFU treatment, an average uterine volume reduction of 67.6% was observed. Menstruation returned to normal in all patients, and their serum HGB and CA-125 levels also returned to normal after an average of 19.1 months of clinical follow-up. The quality of life of all patients improved significantly.
Conclusion
HIFU treatment is safe and effective in the treatment of patients with DUL.
1. Introduction
Diffuse uterine leiomyomatosis (DUL) is a benign and extremely rare condition that mainly affects reproductive age women [Citation1]. To the best of our knowledge, only sporadic cases have been reported, and the etiology remains unclear. The uterus is symmetrically enlarged with innumerable, ill-defined smooth muscle nodules that are usually less than 30 mm in diameter [Citation2–4]. The typical symptoms include severe menorrhagia, dysmenorrhea, pelvis pressure and infertility. DUL is often misdiagnosed as multiple uterine myomas or adenomyosis [Citation5]. Histologically, leiomyomatosis comprises proliferating smooth muscle cells and an increased nuclear cytoplasmic ratio, but the nucleus lacks atypia and mitotic activity. Therefore, DUL has low invasive capacity and rarely metastasizes to other tissues [Citation6]. Furthermore, the nodules develop from separate clones because they are genetically dissimilar [Citation7]. Hysterectomy has been suggested as the most effective management of DUL, especially for patients without pregnancy desire. However, hysterectomy is not suitable for patients who wish to remain fertile. As a novel noninvasive treatment, high-intensity focused ultrasound (HIFU) ablation has proven to be a safe and effective treatment in the management of multiple uterus leiomyomas. However, only one case of DUL treated with HIFU has been reported [Citation8]. In this study, we reported the results of eight DUL patients treated with HIFU.
2. Materials and methods
This study was approved by the Patient Care and Protection Committee of Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine. All patients provided a written informed consent.
2.1. Patients
Between October 2016 and October 2018, fifteen patients diagnosed with DUL by magnetic resonance imaging (MRI) were admitted to Shanghai First Maternity and Infant Hospital. Eight of them who desired fertility and refused to undergo hysterectomy or myomectomy were treated with HIFU (). Seven patients received one session of HIFU treatment, and 1 patient received two sessions of HIFU. The uterine size was then measured at 3–8 months after HIFU ablation on MRI. The menstrual volume score and serum levels of hemoglobin and CA-125 were measured pre- and post-HIFU ablation. The median clinical follow-up time was 19.1 (range: 12–36) months.
2.2. MRI examination
All patients underwent MRI (1.5 T SignaHD/HDx; GE Healthcare, Waukesha, WI, USA) of the pelvis before and 3–8 months after HIFU ablation. Transverse fast spin echo T1 and T2, diffusion weighted imaging (DWI), sagittal T2WI, fat suppressed T2WI, and dynamic multiphase fast contrast-enhanced T1WI were performed in all cases. Sagittal, transverse and coronal enhanced T1WI was performed immediately after 10 ml of gadolinium (Dotarem, Guerbet) administration intravenously. Two radiologists reviewed the MRI and made the diagnosis of DUL. The length, height and width of the uterus were measured to calculate the uterine volume with the following equation: V = 0.5233 × length × width × height. Necrosis was defined as the absence of contrast enhancement on T1WI.
2.3. Hifu treatment
HIFU ablation was performed with the Mode-JC 200 HIFU Tumor Therapy System (Haifu Medical Technology Co., Chongqing, China). Before treatment, routine bowel preparation was performed as follows: the patients were suggested to ingest a semi-liquid diet on day 1 and liquid food on day 2 and had to fast 12 h before HIFU. In addition, an enema was also performed before the HIFU procedure. On the morning of the treatment day, the skin from the umbilicus level to the upper margin of the pubic symphysis must be carefully shaved, degreased and degassed to reduce the risk of skin burn. A urinary catheter was inserted to control the bladder volume with normal saline infusion. Patients lay prone on the HIFU table, and the abdominal wall was in contact with degassed water. A water balloon was placed between the transducer and the abdominal wall to push the bowel away from the acoustic pathway. Sonication started from the posterior to the anterior part and from the inferior to the superior part of the uterus, and the focal point was kept at least 1.5 cm away from the endometrium and the serosa. Contrast-enhanced ultrasound was performed to evaluate the nonperfusion range of the lesion at the end of HIFU treatment.
2.4. Clinical evaluation and follow-up
The post-HIFU MRI examinations were performed 1 day and 3-8 (average 5.9) months after HIFU ablation to assess the nonperfusion volume of the lesions and the shrinkage of the uterine volume. The menstrual volume, hemoglobin and serum levels of CA-125 were measured before and every 6 months after HIFU ablation for 12–36 (average 19.1) months. The menstrual volume score and the uterine volume were assessed as previously described [Citation9]. All patients were followed up to October 2019 in the outpatient department.
2.5. Adverse effects
Adverse effects, including skin burn, abdominal pain, buttock pain, leg pain, abnormal vaginal discharge and fever, were evaluated according to the SIR classification system [Citation10].
2.6. Statistical analysis
Statistical analysis was performed with Prism 5.0 GraphPad Software Inc. (La Jolla, CA, USA). Normally distributed data are depicted as the mean ± standard deviation; skewed distributed data are depicted as the median and interquartile range (IQR). p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of patients
As shown in , the age of patients diagnosed with DUL ranged from 27 to 41 years. All the patients presented with menorrhagia and one patient also complained of dysmenorrhea. As shown in , the uterine size was equivalent to 12–20 gestational weeks, and the mean uterine volume was 617872.05 (range: 289248.43–901547.90) mm3. Six patients previously underwent hysteromyomectomy, and one patient received transabdominal myomectomy twice. Five patients had never given birth before, and all eight patients wished to remain fertile. All of the patients had anemia, and the mean pretreatment serum hemoglobin was 81.55 g/L when they were admitted to the hospital. One patient also had an elevated serum CA-125 level of 69.2 U/ml.
Table 1. Baseline characteristics of DUL patients.
Table 2. Uterine vol and laboratory values.
3.2. Evaluation of HIFU treatment
All eight patients completed ultrasound-guided HIFU ablation successfully, and one patient received 2 sessions of HIFU treatment. The average treatment time was 129.11 (range: 42.0–285.0) minutes, the mean sonication time was 1423.56 (range: 660–2222) seconds, the average exposure energy was 663616.67 (range: 261000–1681800) J, and the mean treatment volume was 7870 (range: 4500–22880) mm3 (Supplementary Table 1). The dynamic enhanced MRI examination was performed 1 day after HIFU treatment. As shown in , numerous fibroid nodules were found in each layer of the uterus, while a nonperfusion area was observed in all the treated lesions (). The treated tumors shrank, and the uterus returned to normal anatomical structure 6 months after HIFU treatment ().
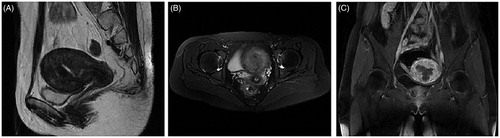
Figure 2. Magnetic resonance imaging (MRI) results obtained from a 28-year-old patient with diffuse uterine leiomyomatosis (DUL).
The sagittal (A), transverse (B) and coronal (C) T2-weighted images revealed an enlarged uterus fulfilled with innumerable fibroid nodules in each layer of the uterus before HIFU treatment. Each uterine myoma was less than 30 mm in dilation and displayed a low signal on T2WI.
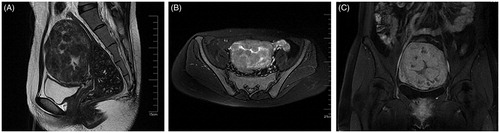
Figure 3. Enhanced MRI image obtained from the patient with DUL 1 day after HIFU treatment. The sagittal (A), transverse (B) and coronal (C) contrast-enhanced T1-weighted MRI showed nonperfusion areas within the uterus, indicating complete myoma nodule ablation one day after HIFU treatment.
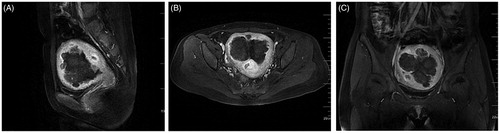
Figure 4. MRI image obtained from the patient with DUL 6 months after HIFU treatment. The sagittal (A), transverse (B) and coronal (C) T2WI revealed a significant uterine volume reduction and a uterus with nearly normal anatomical structure 6 months after HIFU.
After HIFU treatment, three patients reported a small volume of yellow vaginal discharge for 1 week. No other complications, including skin burn, abdominal pain, buttock pain, and leg pain were observed.
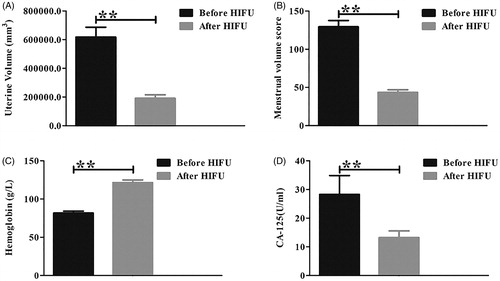
The follow-up MRI at 3–8 (average 5.9) months after HIFU treatment showed that seven patients had significant uterine volume shrinkage. The other patient developed recurrent disease 8 months after HIFU ablation and received HIFU treatment again and underwent another MRI 8 months later. As shown in and , the average uterine volume of the patients was 189945.76 (range: 96697.78–288246.77) mm3, which was an average 67.6% (range: 55.4–85.9%) reduction compared with the uterine volume before treatment (). After a mean clinical follow-up of 19.1 (range: 12–36) months, all eight patients resumed normal menstruation (), and their anemia was improved. The mean serum HGB level was 121.4 g/L and the CA-125 value was within the normal range in every patient ( and ).
Figure 5. The uterine volume, menstrual volume score, serum hemoglobin and CA-125 level were compared before and after HIFU treatment. (A,B), The uterine volume (A) and menstrual volume score (B) decreased significantly after HIFU treatment (p < 0.05). (C,D) The serum hemoglobin level increased (C), while the CA-125 level decreased (D) following HIFU treatment (p < 0.05). ** refers to p < 0.05.
Discussion
DUL is a rare benign disease, but the treatment for patients with DUL who wish to remain fertile is a challenge. Several groups have tried to explore the possibility of treating patients with novel modalities to preserve the uterus, but there is still a lack of consensus on optimized treatment.
Myomectomy is almost impossible in of the treatment of DUL because it carries great risk of recurrence [Citation11]. Tumors with ill-defined margins can even merged together, leading to a high level of difficulty and trauma during the operation [Citation2, Citation12]. Yen CF et al. performed a hysteroscopic operation on 5 patients to remove the submucous myomas to achieve a normal-looking endometrial cavity. In that study, 3 patients successfully conceived and delivered babies at term, 2 had recurring menorrhagia, and 1 underwent treatment for adhesion [Citation13]. Recently, Zhao et al. reported 8 patients treated using the improved hysteroscopic technology called the Hysteroscopy Endo Operative System (HEOS); 6 patients had full-term pregnancies, while 2 patients had synechia, and 6 patients underwent 2-3 myomectomies in this therapeutic option [Citation14]. However, the leiomyomas were found in each layer of the uterus, while the hysteroscopic management only resected the submucous lesions, resulting in unavoidable recurrence and repeat treatment. Masato et al. performed hysteroscopic surgery in 2 DUL patients, but the myomas recurred and neither of the two patients conceived, revealing that the effectiveness of hysteroscopy is controversial [Citation12]. Uterine artery embolization (UAE) is another choice for patients with DUL. Scheurig et al. reported 6 patients with DUL treated by UAE, and clinical success was achieved in 5 patients, but hysteroscopic surgery was followed in 4 patients to remove the partially expelled fibroid tissue [Citation11]. Koh et al. reported 7 DUL cases treated by UAE, and the uterine size was reduced by 50.1% in 5 patients 3 months after UAE [Citation15]. However, pregnancy after UAE is still controversial [Citation16]. Gonadotrophin releasing hormone agonists (GnRHa) are widely used to reduce the size of uterine leiomyomas and improve fibroid-related symptoms [Citation17]. In addition to the so-called ovarian defect symptoms, the deficiencies of GnRHa also include minimal regression in large and multiple lesions and fibroids recurrence after drug withdrawal [Citation18]. HIFU has been widely used in the treatment of uterine fibroids, and it has proven its effectiveness and safety in previous studies [Citation19–20]. In 2015, Chen et al. first reported a case of DUL treated with HIFU [Citation8]. After 4 sessions of HIFU ablation treatment, the patient reported that the symptoms were completely relieved, and a 44% uterine size reduction was achieved. In this study, we successfully treated eight DUL patients with HIFU. Our patients were young (mean age: 32.7 years old) and five of them were nullipara. These patients had a great desire to preserve the uterus. Fortunately, all the patients completed the treatment without any complications. The mean treatment time was 129.11 min, much shorter than other minimally invasive therapies. In these patients, the pre-HIFU MRI showed that the size of the fibroids was small, and most of them appeared hypointense on T2-weighted images with poor vascular perfusion on T1WI contrast enhancement images. Therefore, the lesions were very sensitive to HIFU, and the shortest treatment time was 42 min. After HIFU treatment, the lesions were absorbed gradually; thus, the uterine structure returned to normal conditions. The average uterine size reduction rate reached to 67.6% at 5.9 months after HIFU treatment. After an average of 19.1 months of follow-up, the symptoms of the patients were relieved, and subsequent anemia was greatly improved. In this study, we only had three patients who reported a small volume of vaginal discharge, and no other complications were observed.
It is well known that patients may have a dysfunctional uterus after multiple myomectomy or uterine artery embolization, and it is often suggested that the patients wait at least 1 year to conceive. Recently, Zou et al. revealed that HIFU ablation might inactivate the fibroids in the site without damaging to the normal myometrium. Some patients conceived within 6 months after HIFU treatment for multiple uterine fibroids and delivered babies at terms without any complications. In this study, we did not have the post-HIFU pregnancy outcomes from these DUL patients because six of them were unmarried and the other 2 did not have a pregnancy plan recently. Ultrasound-guided HIFU can precisely ablate fibroids without thermal injury to normal adjacent tissues. Therefore, HIFU treatment could prominently save the pregnancy preparation time and benefit fertility.
In conclusion, HIFU treatment is safe, effective and reliable for patients with DUL. Based on our results, HIFU is suitable for young women with DUL and may lead to normal menstruation and improved quality of life.
Supplemental Material
Download MS Excel (9.8 KB)Acknowledgements
The authors thank Professor Xiaoping Wan for providing grant support during the research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Thomas EO, Gordon J, Smith-Thomas S, et al. Diffuse uterine leiomyomatosis with uterine rupture and benign metastatic lesions of the bone. Obstet Gynecol. 2007;109(2 Pt2):528–530.
- Fedele L, Zamberletti D, Carinelli S, et al. Diffuse uterine leiomyomatosis. Acta Eur Fertil. 1982;13(3):125–131.
- Clement PB, Young RH. Diffuse leiomyomatosis of the uterus: a report of four cases. Int J Gynecol Pathol. 1987;6(4):322–330.
- Mulvany NJ, Ostor AG, Ross I. Diffuse leiomyomatosis of the uterus. Histopathology. 1995;27(2):175–179.
- Fedele L, Bianchi S, Zanconato G, et al. Conservative treatment of diffuse uterine leiomyomatosis. Fertil Steril. 2004;82(2):450–453.
- Coskun A, Ozdemir O, Vardar MA, et al. A case with diffuse uterine leiomyomatosis and review of the literature. Clin Exp Obstet Gynecol. 2008;35(3):227–230.
- Baschinsky DY, Isa A, Niemann TH, et al. Diffuse leiomyomatosis of the uterus: a case report with clonality analysis. Hum Pathol. 2000;31(11):1429–1432.
- Chen L, Xiao X, Wang Q, et al. High-intensity focused ultrasound ablation for diffuse uterine leiomyomatosis: A case report. Ultrason Sonochem. 2015;27:717–721.
- Yang X, Zhang X, Lin B, et al. Combined therapeutic effects of HIFU, GnRH-a and LNG-IUS for the treatment of severe adenomyosis. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. Int J Hyperthermia. 2019;36(1):486–492.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: Two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Scheurig C, Islam T, Zimmermann E, et al. Uterine artery embolization in patients with symptomatic diffuse leiomyomatosis of the uterus. J Vascular Interventional Radiol. 2008;19(2):279–284.
- Nishida M, Ichikawa R, Arai Y, et al. New myomectomy technique for diffuse uterine leiomyomatosis. J Obstet Gynaecol Res. 2014;40(6):1689–1694.
- Yen CF, Lee CL, Wang CJ, et al. Successful pregnancies in women with diffuse uterine leiomyomatosis after hysteroscopic management. Fertil Steril. 2007;88(6):1667–1673.
- Zhao H, Yang B, Li H, et al. Successful Pregnancies in Women with Diffuse Uterine Leiomyomatosis after Hysteroscopic Management Using the Hysteroscopy Endo Operative System. J Minim Invasive Gynecol. 2019;26(5):960–967.
- Koh J, Kim MD, Jung DC, et al. Uterine artery embolization (UAE) for diffuse leiomyomatosis of the uterus: clinical and imaging results. Eur J Radiol. 2012;81(10):2726–2729.
- Lefebvre G, Vilos G, Allaire C, et al. The management of uterine leiomyomas. J Obstetr Gynaecol Canada 2003;25(5):396–418.
- Zhang Y, Sun L, Guo Y, et al. The impact of preoperative gonadotropin-releasing hormone agonist treatment on women with uterine fibroids: a meta-analysis. Obstet Gynecol Surv. 2014;69(2):100–108.
- Sankaran S, Manyonda IT. Medical management of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):655–676.
- Liu X, Xue L, Wang Y, et al. Vaginal delivery outcomes of pregnancies following ultrasound-guided high-intensity focused ultrasound ablation treatment for uterine fibroids. Int J Hyperthermia. 2018;35(1):510–517.
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181–1188.