Abstract
Purpose
To determine a novel quantitative index, residual vital ratio(RVR) by contrast-enhanced ultrasound(CEUS) with conventional Ultrasound(US), to early predict nodule regrowth after radiofrequency ablation (RFA)for benign thyroid nodules.
Methods
This retrospective study evaluated 186 patients with 206 benign thyroid nodules underwent RFA. Patients were followed at 1, 3, 6, 12 months and every 12 months thereafter by conventional US, CEUS and clinical evaluation. RVR was defined as the initial ratio of residual vital volume to the total volume calculated by CEUS and conventional US at the first follow-up period after RFA. The relationship between RVR and regrowth was investigated.
Results
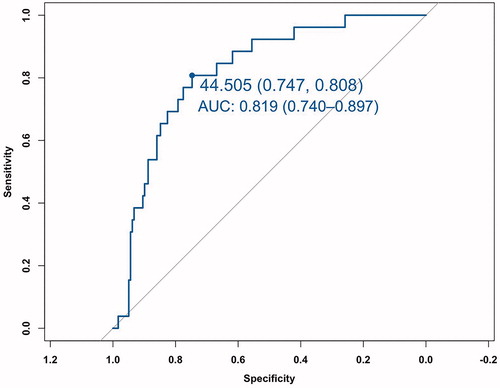
The mean volume of thyroid nodules was 10.09 ± 12.90 ml (range 0.40–71.39 ml), which decreased significantly to 2.33 ± 4.65 ml (range 0–36.75 ml) (p < .001) after a mean follow-up time of 22.50 ± 13.29 months (range 6–68 months) with a mean VRR as 85.26 ± 15.02% (range 32.23–100%). The overall incidence of regrowth was 12.62% (26/206) and the mean timing of regrowth was 20.77 ± 12.03 months (range 6–48 months). Multivariate logistic regression revealed that RVR (OR = 1.050, 95%CI 1.025–1.075), initial volume(OR = 1.033, 95%CI 1.000–1.066), location close to critical structures (OR = 5.967, 95%CI 1.898–18.760) and vascularity (OR = 2.216, 95%CI 1.185–4.143) were independent factors associated with regrowth. According to receiver-operating characteristic curve, the area under curve for RVR to regrowth was 0.819 (95% CI 0.740–0.897, p < .001) with the optimal cutoff value of 44.5% (sensitivity 80.8%, specificity 74.7%).
Conclusion
RVR was not only an independent factor but also an early quantitative predictor for regrowth. If RVR was larger than 44.5%, the nodule tended to regrowth in the follow-up.
Introduction
Thyroid nodules are common in the general population, occurring in 20–70% of individuals [Citation1]. Most asymptomatic thyroid nodules are benign and only need observation, but a minority require treatment because of progressive growth with compressive symptoms and cosmetic problems [Citation2]. Surgery is the standard treatment for thyroid nodules. However, it has several drawbacks, including general anesthesia, risk of complications and scar formation. Additionally, the partial or total removal of the thyroid tissue in the surgery results in a high incidence of hypoparathyroidism. Patients often need thyroid hormone supplementation, which is associated with adverse effects on bones and cardiovascular systems [Citation1,Citation3]. Thus, thermal ablation techniques, such as radiofrequency ablation (RFA), microwave ablation (MWA), laser ablation (LA) and high-intensity focused ultrasound(HIFU) have been introduced and have yielded good results [Citation4–10].
RFA and other thermal ablation techniques have been recommended as safe and effective treatments for benign thyroid nodules by guidelines [Citation3,Citation11–13]. Studies from multiple centers have reported a significant decrease in nodule volume with improvement of local symptoms or cosmetic problems after ablation [Citation14–18]. A meta-analysis showed that the volume reduction rate(VRR) at 6, 12 and 24 months after RFA was 68%, 75% and 87%, respectively [Citation8]. However, some studies reported that 4.1–37.5% of the treated nodule occurred regrowth after 2 to 3 years of ablation [Citation19–25]. Regrowth was defined as an increase in nodule volume 50% over the previously recorded smallest volume [Citation26], which was usually observed at the untreated peripheral area and needed additional treatment [Citation19–23]. Recently, Sim et al. [Citation20] proposed a method of monitoring the nodule volume after ablation by dividing it into the total volume(Vt), ablated volume(Va) and vital volume(Vv). They found Vv increase tended to occur about 1 year earlier than regrowth. However, the volume measurements were only based on conventional US, which could not accurately differentiate the vital area from the ablated nodule even combined with color/power Doppler [Citation27]. Moreover, Vv increase was observed at 27.5 ± 18.5 months after RFA, which was not really an early predictor for regrowth. To our best knowledge, no early predictor of nodule regrowth has been identified. In addition, little is known about the evaluation of vital area after ablation by contrast-enhancement ultrasound (CEUS).
Therefore, the purpose of this study was to determine a novel quantitative index, residual vital ratio (RVR) by CEUS with conventional US, to early predict nodule regrowth after RFA for benign thyroid nodules.
Materials and methods
The Institutional Review Board of our institutional approved this retrospective study (Approval number: S2019-211-01). All the patients were provided written information consent before RFA and CEUS.
Patients
All the enrolled patients fulfilled these inclusion criteria [Citation1]: confirmation of benign nodule status on two separate fine-needle aspiration (FNA) or core-needle biopsy (CNB) [Citation2]; no suspicious malignant features on US examination [Citation3,Citation28,Citation3] solid (≤10% of fluid component) or predominantly solid nodules(11–50% of fluid component) [Citation4]; report of cosmetic and/or symptomatic problems or concern of nodules growing rapidly or malignant transformation [Citation5]; serum thyroid hormone and thyrotropin levels within normal ranges [Citation6]; refusal or ineligibility for surgery [Citation7]; follow-up time ≥6 months [Citation8]; underwent CEUS during the follow-up. Exclusion criteria were [Citation1]: follicular neoplasm or malignancy findings on FNA or CNB [Citation2]; nodules with benign result on FNA or CNB had suspicious of malignancy in US, including marked hypoechoic, ill-defined margins, taller-than-wide shape or microcalcifications [Citation3]; patients with cystic or predominantly cystic nodules [Citation4]; patients with contra-lateral vocal cord paralysis [Citation5]; previous radiation to the head and neck [Citation6]; follow-up time < 6 months [Citation7]; refused CEUS during the follow-up.
From August 2014 to December 2018, 786 patients with benign thyroid nodules underwent RFA in this institution. Among them, patients with cystic/predominantly cystic nodules (N = 215) or refused CEUS during the follow-up (N = 295) or follow-up time less than 6 months (N = 87) were excluded. At last, 189 patients with 206 benign thyroid nodules were evaluated in this study. The flowchart of patient enrollment is shown in .
Pre-ablation assessment
Before treatment, each nodule underwent conventional US to evaluate the size, location, component, margin, shape, echogenicity, calcification and vascularity. The volume of thyroid nodules was calculated with the equations: V = πabc/6 (V is the volume, while a is the largest diameter, b and c are the other two perpendicular diameters). According to the volume, thyroid nodules were categorized into three subgroups, which were the small subgroup (<10ml), the medium subgroup (10–30ml) and the large subgroup (>30ml) [Citation26]. Nodule location was classified as follows: normal location and close to critical structures, including trachea, cervical artery, jugular vein, esophagus and recurrent laryngeal nerve. Nodule component was classified as follows [Citation26]: solid (≤10% of fluid component) and predominantly solid (11-50% of fluid component). Nodule vascularity was scored as follows [Citation11]: grade 1, no vascularity; grade 2, peripheral vascularity; grade 3, intra-nodular vascularity <50%; grade 4, intra-nodular vascularity >50%. Symptom score was self-measured by patients using a 10-cm visual analogue scale (grade 0–10) [Citation11]. The cosmetic score was assessed by a physician (1, no palpable mass; 2, no cosmetic problem but palpable mass; 3, a cosmetic problem on swallowing only; and 4, a readily detected cosmetic problem) [Citation11].
CEUS was used to evaluate the ablated area of the nodule immediately after RFA and in the follow-up. Sulfur hexafluoride (SonoVueR) was used as ultrasound contrast agent. CEUS was performed after bolus injection of SonoVue (2.4 ml), followed by a 5 ml of normal saline flush.
Ablation procedure
All RFA procedures were performed by an experienced US physician with more than 20-year experience in thyroid US and interventional US (Y.K.L). A bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18–gauge bipolar RF electrodes with 0.9 cm active tip were used (CelonProSurge micro 100-T09, Olympus Surgical Technologies Europe) in this study.
Patients lay on an operating table in the supine position with the neck extended. Local anesthesia with 1% lidocaine was administered. If the distance between the tumor and critical cervical structures (trachea, cervical artery, jugular vein, esophagus and recurrent laryngeal nerve) was less than 5 mm, hydrodissection technique was used. Normal saline was injected using another needle to form at least 1 cm distance between the nodule and the critical structure in order to prevent thermal injury. RFA was performed using the trans-isthmic approach and moving-shot technique. The RFA power was 3 W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the radiofrequency power was increased to 5–9 W. CEUS was performed immediately after the RFA procedure to evaluate the ablation area. If any enhancement existed, a complementary ablation could be performed.
During the procedure, special attention was given to the protection of critical cervical structures in order to prevent significant complications such as hematoma or nerve injury. Each patient was observed for 1–2 h in the hospital while any adverse event including complication and side effect occurring during and immediately after ablation were carefully evaluated according to the clinical signs and symptoms [Citation26].
Post-ablation assessment
After RFA, Vt of the treated nodule was divided into 2 parts, which was Va and Vv [Citation20]. Vt was the total volume, which was measured and calculated by conventional US. Va was defined as the volume of ablated area which usually located in the central zone. It was measured and calculated by CEUS, which presented as a non-enhancement area within the treated nodule during both the arterial phase and venous phase on CEUS. Vv was defined as the incompletely treated vital volume which located peripherally. It was calculated by the following equation: Vv = Vt-Va [Citation20]. RVR was the initial ratio of residual vital volume to the total volume after RFA and the equation was as follows: RVR = Vv/Vt× 100%. The immediate data were not used because the immediate range of the ablated area could not represent the final necrotic volume caused by ablation [Citation1]. Therefore, RVR was calculated by the measurements at the first follow-up period. The first follow-up was done between 1 and 3 months after RFA.
After RFA, patients were followed up at 13,612 months and every 12 months thereafter by conventional US, CEUS and clinical evaluation. Three types of nodule volumes, VRR, cosmetic and symptom scores were evaluated during the follow-up period. The volume reduction was calculated as follows: VRR = ([initial volume-final volume] × 100%)/initial volume. Technique efficacy was defined as a > 50% volume reduction at a predefined time point [Citation26], and thus technique efficacy at 1 year and at last follow-up were reported. Regrowth was defined as an increase in total volume 50% over the previously recorded smallest volume [Citation26] and Vv increased was defined as a more than 50% increase compared to the previously reported smallest vital volume [Citation20]. All the nodules were divided into two groups depending on whether regrowth occurred, which was the regrowth group and the non-regrowth group.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software(Version 25.0) and R software version 3.6.2 (www.r-project.org). Continuous data were expressed as mean ± SD (range). Wilcoxon signed rank tests were used to compare volume, symptom and cosmetic scores before RFA and at last period after RFA. Kruskal–Wallis H test was used to compare the RVR and VRR in subgroups at each follow-up period. Multivariate logistic regression analysis was performed to assess independent factors associated with regrowth, using the factors screened by univariate logistic regression analysis, with p < .05. Odds ratios (OR) and 95% confidence intervals (CI) were reported. A receiver-operating characteristic (ROC) analysis was used to determine the optimal cutoff value of RVR for regrowth and the area under the curve (AUC) were calculated. A difference with p < .05 was considered as statistically significant.
Results
Clinical characteristics of patients are presented in . A total of 189 patients (168 females, 21 males, mean age 46.56 ± 11.88 years, range 18–79 years) with 206 benign thyroid nodules (initial volume 10.09 ± 12.90 ml, range 0.40–71.39 ml) were enrolled in this study. There were 139 nodules in the small subgroup, 50 in the medium subgroup and 17 in the large subgroup, respectively.
Table 1. Clinical characteristics of patients before RFA.
During RFA, power of 3 W-5W was used in 60 nodules; 6–8 W was used in 114 nodules; 9 W–10W was used in 32 nodules. The mean energy was 2394.96 ± 1970.47J (range 85–10,200 J) and energy applied per volume was 520.64 ± 567.20J/ml (range 8.89–3897.79J/ml).
Efficacy
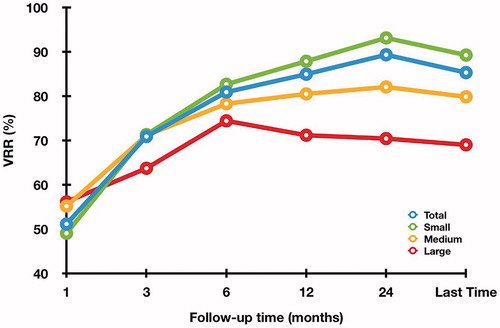
The mean follow-up time was 22.50 ± 13.29 months (range 6–68 months). The mean volume decreased significantly form 10.09 ± 12.90 ml (range 0.4–71.39 ml) to 2.33 ± 4.65 ml (range 0–36.65 ml) (p < .001) with a mean VRR of 85.26 ± 15.02% (range 32.23–100%). The changes of VRR in subgroups at each follow-up period after RFA are shown in . The VRR was 89.21 ± 12.74%, 79.80 ± 15.11% and 68.96 ± 16.79% in the small, medium, and large subgroups, respectively (). The VRR in small subgroup was significantly higher than in the other two subgroups at 12 months, 24 months and the last follow-up period. A total of 33 nodules (16.02%) disappeared during the follow-up, which were all in small subgroup. At 1 year after RFA, the technique efficacy rate was 97.01% (162/167). At last follow-up, technique efficacy rate was 96.60% (199/206). Symptom score significantly decreased from 2.74 ± 2.18 to 0.98 ± 1.18 (p < .001). Cosmetic score significantly decreased from 2.47 ± 1.21 to 1.34 ± 0.59 (p < .001).
Table 2. Changes of VRR in the subgroups at each follow-up period after RFA.
The changes of Vt, Va and Vv in the subgroups at each follow-up period after RFA are summarized in and . In the small subgroup, Vt, Va and Vv were all decreased during the follow-up. In the medium subgroup, Vv and Vt began to enlarge at 12 and 24 months, respectively. In the large subgroup, Vv increase and Vt enlarged both occurred at 12 months. The mean timing of Vv increase was at 15.95 ± 7.49 months (range 3–24 months) and the mean timing of Vt regrowth was at 20.77 ± 12.03 months (range 6–48 months).
Figure 3. The changes of Vt, Va and Vv in each subgroup at each follow-up point after RFA (A: small subgroup; B: medium subgroup; C: large subgroup).
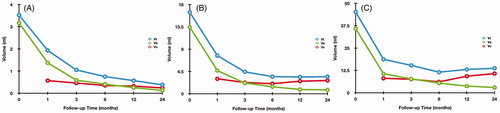
Table 3. Changes of Vt, Va and Vv in the subgroups at each follow-up period after RFA.
Regrowth and RVR
The overall incidence of regrowth was 12.62% (26/206), which all occurred in the untreated peripheral area. The number of regrowth nodules in each subgroup were as follows: 12 (8.63%) in the small subgroup, 10 (20.00%) in the medium subgroup and 4 (23.53%) in the large subgroup. All the regrowth nodules underwent CNB to exclude neoplastic transformation, and then underwent additional RFA.
RVR in the regrowth group was significantly larger than in non-regrowth group(55.76 ± 16.72% vs 31.24 ± 21.75%, p < .001). The regrowth rate in patient with a 12-month VRR ≥ 50% was 3.70% (6/162) and in patients with a 12-month VRR < 50% was 60%(3/5). There were significant differences in regrowth rate between nodules with a 12-months VRR < 50% and those with a 12-month VRR ≥ 50% (p < .001). RVR was 31.04 ± 21.31%, 39.66 ± 23.95%, and 46.47 ± 24.17% in the small, medium, and large subgroup, respectively. There were significant differences of RVR between the small subgroup and the large subgroup (p = .026), but no significant differences were found in the medium subgroup and large subgroup (p = .826) or the medium subgroup and small subgroup (p = .065).
The univariate and multivariate logistic regression analyses for regrowth are shown in . According to univariate logistic regression, RVR, initial volume, location close to critical structures and vascularity (all p < .05) were characterized as factors related to regrowth, whereas age, female, solid and energy per volume (all p > .05) were not significantly associated with regrowth. Multivariate logistic regression revealed RVR (OR = 1.050, 95%CI 1.025–1.075), initial volume (OR = 1.033, 95%CI 1.000–1.066), location close to critical structures (OR = 5.967, 95%CI 1.898–18.760) and vascularity (OR = 2.216, 95%CI 1.185–4.143) were independent factors associated with regrowth.
Table 4. Univariate and multivariate logistic regression analyses for nodule regrowth.
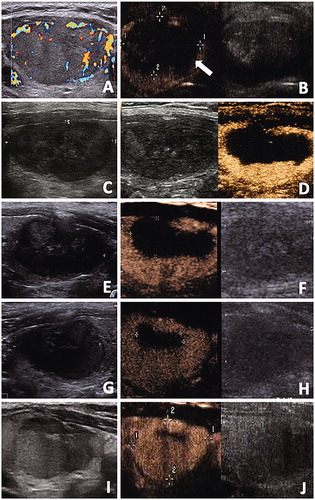
ROC analysis revealed that the AUC for RVR to predict regrowth was 0.819 (95%CI 0.740–0.897, p < .001) with the optimal cutoff value of 44.5% (sensitivity 80.8%, specificity 74.7%) (). A representative case of regrowth before and after RFA is shown in .
Figure 5. The conventional US and CEUS images of a 48-year-old male with a benign thyroid nodule. (A) Before RFA, a solid thyroid nodule located in the right thyroid lobe with an initial volume of 22.27 ml. Color Doppler showed the vascularity was grade 3. (B) CEUS performed immediately after RFA showed a lack of enhancement on the treated area(arrow). (C, D) At one months after RFA, conventional US showed the treated nodule began to shrink and total volume(Vt) was 5.28ml. CEUS showed the treated nodule was divided into the centrally non-enhancement area(ablated volume, Va) and peripheral iso-enhancement area(vital volume, Vv). Va was 2.59ml and Vv was 2.69ml. RVR was 50.95%. (E, F) At 3 months after RFA, conventional US showed Vt was 3.48ml and the echogenicity of nodule was heterogeneous. Va decreased to 1.11ml on CEUS and Vv was 2.37ml. (G, H) At 6 months after RFA, conventional US and CEUS showed Vt decreased to 2.89 ml and Va decreased to 0.27ml, respectively. However, Vv began to enlarge to 2.62ml.(I, J) At 12 months after RFA, conventional US showed nodule regrowth and Vt was 4.34ml. Va decreased to 0.20ml on CEUS and thus Vv increase also occurred and Vv was 4.13ml.
Safety
All the patients were tolerable to the RFA procedure. Side effect like local pain and discomfort occurred in 19 patients (9.22%) and resolved spontaneously within 3 days. No complications occurred during or after RFA. No patients had side effect or delayed complication related to CEUS.
Discussion
No early predictor of nodule regrowth has been identified. This study demonstrated that RVR calculated by CEUS with conventional US, as well as initial volume, location close to critical structures and vascularity were the independent factors associated with nodule regrowth after RFA. Furthermore, RVR calculated at first follow-up period, was a much earlier predictor for regrowth than Vv increase which occurred at 15.95 ± 7.49 months after RFA. The AUC for RVR to predict regrowth was 0.819 with a sensitivity of 80.8% and a specificity of 74.7%. We also showed that the optimal cutoff value of RVR was 44.5% by ROC analysis, that was, when RVR was larger than 44.5%, the nodule tended to regrowth in the follow-up.
As alternatives to surgery, the long-term efficacy of thermal ablation techniques has attracted research attention. Sim et al. [Citation20] found that the nodule regrowth began at 12 months and tended to be prominent after 2 years of follow-up with an incidence of 4.1–37.5% [Citation19–24]. Recently, a multicenter study found that regrowth occurred in 20% of patients who were followed for 5 years after a single session of RFA [Citation25]. Similar results were found in this study. The incidence of regrowth was 12.62% in this study and all the regrowth occurred at the incompletely treated peripheral area. Although the primary purpose of RFA for benign thyroid nodules is to improve symptoms rather than to obtain complete ablation, incomplete and insufficient ablation could lead to regrowth and affect the efficacy of treatment. Therefore, it is necessary to evaluate and predict regrowth for the follow-up management and the planning of additional treatment.
There are several parameters to evaluate the efficacy of RFA, such as VRR, technique efficacy, cosmetic score and symptom score, but none of them is available to early predict regrowth. Recently, Sim et al. [Citation20] found that Vv increase tended to occur one year earlier than Vt regrowth and suggested that measuring Vv separately was advantageous for identifying the early sign of regrowth. However, Sim et al. [Citation20] reported that Vv increase was observed at 27.5 ± 18.5 months after RFA, which was not a very early predictor for regrowth. More importantly, they only used conventional US for volume measurement and evaluation. Because the margin of the ablated area was ill-defined and the presence of a color signal in the vital area was not constant [Citation29], measurement via conventional US could be difficult and even incorrect, thus limiting its reliability for volume measurement [Citation27].
Accurate detection and measurements of the true volume was essential for successful evaluation [Citation27], thus CEUS was performed in this study. CEUS is a contrast harmonic imaging technique that allows detection and characterization focal lesions by assessing the micro-vascularization with second contrast agent, thus overcoming the limitations of conventional US and enabling the display of parenchymal microvasculature [Citation27,Citation30,Citation31]. It has been recommended for monitoring ablation treatment and periprocedural assessment of treatment response after ablation for liver and thyroid lesions [Citation31,Citation32]. Recently, Schiaffino et al. [Citation33] showed CEUS had higher reproducibility and inter- and intra-observer agreement compared to conventional US in the assessment of Va measurement after RFA for benign thyroid nodules. In this study, the size and margins of Va were successfully differentiated from the ablated nodule using CEUS, supporting its usefulness for the accurate calculation of the true Vv. Therefore, the changes of three types of volumes in each subgroup after RFA could be demonstrated clearly. We found that Vv decreased in all the subgroups during the early stage of follow-up and began to enlarge in size one year later in medium and large subgroup. It was probably because the vital area usually located in the peripheral portion of the heated ablation area, which underwent indirect heat injury leading to microvascular damage, cellular apoptosis, altered cytokine release and inflammatory cells activation [Citation34–36]. When the phagocytosis of apoptosis tissues finished, Vv stopped to decrease and then started to enlarge. Moreover, in the large subgroup, Vv increase was observed at 12 months. It suggested that for nodules with an initial volume larger than 30 ml, the appropriate timing of additional ablation was at 12 months after initial ablation.
In this study, a novel quantitative parameter RVR was determined by CEUS with conventional US at the first follow-up period. Compared with Vv increase which was observed at 15.95 ± 7.49 months in this study, RVR was a much earlier parameter. Multivariate logistic regression analysis revealed that RVR was one of the independent factors related to regrowth. Moreover, ROC curve analysis showed that the AUC for RVR to predict regrowth was 0.819 with the optimal cutoff value of 44.5%. The large RVR indicated the presence of an amount of peripheral vital tissue around the central ablated area. When RVR was larger than 45%, the treated nodule was likely to regrowth during the follow-up. Such patients should be fully informed the outcomes and recommended the close follow-up. As Vv increase was observed, it was the appropriate timing to perform additional treatment. The reason why the immediate data were not used for RVR was because the immediate range of the ablated area could not represent the final necrotic volume caused by ablation [Citation1]. Studies indicated that the immediate post-procedural necrotic volume on CEUS were smaller than those measured 24 h or 7 days later [Citation37,Citation38]. It was probably because of the fact that a certain amount of sub-lethally injured tissues at the periphery were expanded and become necrotic, which were evident at 1 to 7 days after ablation [Citation35–37]. Then the volume decreased slightly during the subsequent weeks as a result of the breakdown of and removal of necrotic tissue by inflammatory cells( [Citation35,Citation39].
RVR defined as the initial ratio of residual vital volume to the total volume, could also represent the performance of the RFA procedure, that is, how well the physician performed the ablation procedure. It is known that the experience of the physician is an important factor related to the ablation efficacy. Some studies defined an experienced physician as one who successfully completed more than 50 cases of thyroid ablation [Citation40–42]. However, no objective corresponding indicator have been defined yet. As a quantitative parameter of how much nodular tissue was ablated after the ablation procedure, RVR could not only indicate whether a complete ablation was performed, but also be helpful for physicians to improve the experience and skills
Besides RVR, initial volume, location close to critical structures and vascularity were also independent factors related to nodule regrowth by multivariate logistic regression analysis. It was well known that initial volume was the most common factor for regrowth [Citation19,Citation20,Citation43]. In this study, the initial volume in the regrowth group was significantly larger than in the non-regrowth group. We also found that RVR in the large subgroup was 46.47 ± 24.17%. These results indicated the large nodule required more treatment sessions to achieve satisfactory efficacy, because it was technically difficult to completely ablate all the nodule margin from a single session. Lim et al. [Citation19] suggested moving-shot technique was a suitable solution for peripheral ablation, which could enlarge the area of ablation by moving the energy source. During the procedure, the target nodule was virtually divided into multiple small ablation units, and the electrode tip was moved unit-by-unit to allow the entire nodule to be treated safely [Citation44].
For nodules located close to the critical structures, such as trachea, cervical artery, jugular vein, esophagus and recurrent laryngeal nerve, the possibility of incomplete ablation also existed. In this study, a significant higher percentage of nodules located to the critical structures was observed in the regrowth group than in the non-regrowth group, which was consistent with previous studies [Citation23,Citation45]. Fears of complications and structure damage could result in incomplete treatment and subsequent regrowth. Although leaving the nodule margin was for safe consideration, leaving too much could lead to regrowth. In this case, hydrodissection technique was recommended [Citation44]. By using this technique, fluid of normal saline was injected to separate the nodule from adjacent critical structures, which served as a protective thermal barrier to adjacent critical structures and limit damages [Citation45]. Because the injected fluid could spread rapidly along the longitudinally arranged neck muscle plane, fluid should be injected sufficiently and continuously during the procedure [Citation44,Citation45].
Vascularity was another factor associated with regrowth in this study. The vascularity score in the regrowth group was significantly higher than in the non-regrowth group. Similar results were also found in previous studies [Citation21,Citation23]. The blood vessels could act as a heat-sink to dissipate the hyperthermia, thereby decreased RFA efficacy [Citation34,Citation46]. To complete ablation, the vasculature in the thyroid nodules should be totally damaged during the ablation. Park et al. [Citation44] introduced vascular ablation techniques to solve this problem, which were artery-first ablation technique and the marginal venous ablation technique. The artery-first ablation technique ablated the tumor-feeding artery first to decrease the heat-sink effect and to limit the hemorrhage risk during the procedure. Then the marginal venous ablation technique could ablate the draining vein along the nodule margin and lead to complete ablation the nodule margin, which was particularly helpful to prevent regrowth.
There were some limitations in this study. First, it was a single-center retrospective study. Second, the sample size was relatively small, partially in the regrowth group, resulting in a small number for events per variable in logistic regression analysis. However, the overall incidence of nodule regrowth was only 4.1 to 37.5% [Citation19–25]. It was very difficult to collect enough data for events per variable. Moreover, ROC curve analysis showed the AUC for RVR to predict regrowth was 0.819, which suggested RVR had a high predictive value for nodule regrowth. Third, the follow-up time was relative short. Sim et al. [Citation20] found after ablation, there were two peaks of nodule regrowth. First peak began at 12 months after ablation and tended to be prominent at 2 years, and the second one appeared later than 5 years. Because the follow-up time of this study is 22.50 ± 13.29 months, the role of RVR on second peak of regrowth is needed further investigation. Fourth, because all the RFA procedure were performed by a single US physician, the results in this study could not account for the variations in experience and skill, which was obviously an important factor related to the nodule regrowth.
In conclusion, RVR calculated by CEUS with conventional US, as well as initial volume, location close to critical structures and vascularity were independent factors associated with regrowth. Moreover, RVR represented how well the ablation procedure was performed, was also a very early quantitative predictor for nodule regrowth. If RVR was larger than 44.5%, the treated nodule tended to regrowth in the follow-up.
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Funding
References
- Gharib H, Papini E, Paschke R, AACE/AME/ETA Task Force on Thyroid Nodules, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010;33(5):287–250.
- Durante C, Grani G, Lamartina L, et al. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319(9):914–924.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(1):11–22.
- Bernardi S, Stacul F, Zecchin M, et al. Radiofrequency ablation for benign thyroid nodules. J Endocrinol Invest. 2016;39(9):1003–1013.
- Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;104(9):3751–3756.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Khanh HQ, Hung NQ, Vinh VH, et al. Efficacy of microwave ablation in the treatment of large (≥3 cm) benign thyroid nodules. World J Surg. 2020;44(7):2272–2279.
- Lang BHH, Woo YC, Chiu KW. Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol. 2019;29(1):93–101.
- Kim JH, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25(8):890–896.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Guang Y, He W, Luo Y, et al. Patient satisfaction of radiofrequency ablation for symptomatic benign solid thyroid nodules: our experience for 2-year follow up. BMC Cancer. 2019;19(1):147.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a mayo clinic case series. Mayo Clin Proc. 2018;93(8):1018–1025.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33(8):905–910.
- Døssing H, Bennedbaek FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165(1):123–128.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Wang B, Han ZY, Yu J, et al. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia. 2017;33(4):459–464.
- Negro R, Greco G, Deandrea M, et al. Twelve-month volume reduction ratio predicts regrowth and time to regrowth in thyroid nodules submitted to laser ablation: a 5-year follow-up retrospective study. Korean J Radiol. 2020;21(6):764–772.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the italian minimally invasive treatments of the thyroid group. Thyroid. 2020. DOI:10.1089/thy.2020.0202. Online ahead of print.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Albrecht T, Blomley M, Bolondi L, EFSUMB Study Group, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25(4):249–256.
- Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Ma JJ, Ding H, Xu BH, et al. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014;24(2):355–363.
- Claudon M, Dietrich CF, Choi BI, European Federation of Societies for Ultrasound, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39(2):187–210.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis And Management Of Thyroid Nodules – 2016 update. Endocrine Practice. 2016;22(Supplement 1):1–60.
- Schiaffino S, Serpi F, Rossi D, et al. Reproducibility of ablated volume measurement is higher with contrast-enhanced ultrasound than with B-mode ultrasound after benign thyroid nodule radiofrequency ablation-a preliminary study. J Clin Med. 2020;9(5):1054.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127(2):208–223.
- Wu F. Heat-based tumor ablation: role of the immune response. Adv Exp Med Biol. 2016;880:131–153.
- Meloni MF, Andreano A, Franza E, et al. Contrast enhanced ultrasound: should it play a role in immediate evaluation of liver tumors following thermal ablation? Eur J Radiol. 2012;81(8):e897-902–e902.
- Zhang L, Zhou W, Zhan W, et al. Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg. 2018;42(8):2476–2484.
- Ohno T, Kawano K, Sasaki A, et al. Expansion of an ablated site and induction of apoptosis after microwave coagulation therapy in rat liver. J Hepatobiliary Pancreat Surg. 2001;8(4):360–366.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Baek JH. Factors related to the recurrence of benign thyroid nodules after thermal ablation. Int J Hyperthermia. 2017;33(8):957–958.
- Sim JS, Baek JH. Long-term outcomes following thermal ablation of benign thyroid nodules as an alternative to surgery: the importance of controlling regrowth. Endocrinol Metab. 2019;34(2):117–123.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Zhao CK, Xu HX, Lu F, Sun LP, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. CH. 2017;65(4):393–405.
- Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.