Abstract
Aim
To investigate the antitumor efficacy of microwave ablation combined with dendritic cell-derived exosomes (Dex) or dendritic cells (DC) in treating hepatocellular carcinoma using a tumor-bearing mouse model.
Methods
We used a bilateral tumor-bearing mouse model treated with MWA, MWA + DC (DC-combined group) or MWA + Dex (Dex-combined group). Following tumor ablation on one side, the tumor volume on the contralateral side was monitored. The proportions of CD8+ (cytotoxic) T cells and regulatory T (Treg) cells in the spleen were analyzed by flow cytometry, and the number of CD8+ T cells and Treg cells in tumor sites was detected by immunohistochemistry. The concentration of interleukin-10 and interferon-γ in plasma was identified using enzyme-linked immunosorbent assay.
Results
The combination therapy significantly inhibited tumor growth compared with MWA monotherapy. In addition, the tumor immune microenvironment was significantly improved in HCC mice in the combination therapy groups compared to MWA group demonstrated by an increased number of CD8+ T cells and a decreased number of Treg cells in tumor sites. A lower proportion of Treg cells were observed in the spleen in the combination therapy groups compared to MWA group. Moreover, the concentration of plasma IFN-γ increased, and the concentration of plasma IL-10 decreased in the combination therapy groups compared to the MWA group. However, there was no statistical difference between the Dex-combined group and the DC-combined group in the comparisons mentioned above.
Conclusions
Our results provide evidence that MWA combined with Dex can significantly inhibit tumor growth and improve the immune microenvironment compared to MWA alone. Furthermore, the immune-enhancing effect of Dex and DC was equivalent in our combination therapy strategy.
Graphical Abstract
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent malignancy worldwide, and was the fourth most common cause of cancer-related death in 2018 [Citation1]. Microwave ablation (MWA), a form of thermal ablation, is widely used in the treatment of HCC, and offers advantages including high efficiency, minimal invasion, and limited complications [Citation2,Citation3]. Yet, there remains a high recurrence rate of HCC following thermal ablation, and the 5-year progression rate is up to 50% [Citation4].
It is well established that the occurrence and progression of HCC is inextricably related to the continuous immunosuppressive state in vivo. Dendritic cell (DC)-based immunotherapy is a method of improving the local and systemic antitumor immune environment in vivo and has become prominent in HCC therapy. Studies have shown that thermal ablation can not only inactivate tumors, but can also reduce the immunosuppression state in vivo by exposing the tumor-related antigens, which might help to overcome tumor immunotolerance [Citation5]. So, in theory, thermal ablation combined with DC immunotherapy can synergistically enhance anti-tumor immune responses in multiple tumor models, including the HCC mouse model [Citation6–8]. However, there are some limitations to DC immunotherapy, which may hinder its broader clinical application. For instance, the patient-derived DC has a short life cycle and the preparation process is costly and tedious [Citation9]. Furthermore, DC vaccines can be converted to tolerogenic DC by immunosuppressive cytokines or exosomes secreted by tumor cells in vivo [Citation10,Citation11]. Moreover, live DC is difficult to store and lacks adequate quality control [Citation12]. Using DC-derived exosomes (Dex) as an alternative to DC may be a viable way to overcome the challenges described above.
Exosome are extracellular vesicles (approximately 50–150 nm in diameter) that are secreted by a variety of cell types, including DC. DC-derived exosomes (Dex) inherit many molecules from their parental cells that contribute to antigen presentation, such as the major histocompatibility complex (MHC) and costimulatory molecules, and have a powerful anti-tumor immune activation effect [Citation13]. Lu et al. [Citation14] have shown that Dex can induce anti-tumor immune responses in a HCC tumor mouse model.
We have previously demonstrated that MWA combined with a DC vaccine could synergistically enhance anti-tumor immune responses [Citation8]. However, there is still no research on the efficacy of MWA combined with Dex in the treatment of HCC. It remains unknown whether Dex can achieve an equivalent antitumor immune-enhancing effect to DC in combination therapy.
Considering that the inhibition of progress and metastasis of HCC following thermal ablation still remains a challenge in clinics, the purpose of our present study was to investigate the antitumor efficacy of the MWA combined with Dex in the treatment of HCC using the hepa1-6 tumor-bearing mouse model.
Materials and methods
Animals
We used wild-type male C57BL/6 J mice (6–8 weeks old). All of the animal experiments were carried out in the animal unit of Tianjin Third Central Hospital (Tianjin, China) according to the procedures authorized by the institutional ethics committee.
Cell lines
DC2.4 (referred to as DC) was the murine dendritic cell line used in this study, and was kindly provided by Dr. Shaokai Sun, Tianjin Medical University, CN. The DC were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 1% antibiotics, 1% glutamine (Gln), and 10% fetal bovine serum (FBS, BD). Murine HCC cell line hepa1-6 (derived from C57L mice) was purchased from the Academy of Military Medical Sciences and was cultured in DMEM with 10% FBS (BD, USA).
Collection of antigen-pulsed DC and Dex
Hepa1-6 cell lysates (LYS) were obtained using an ultrasonic cell crusher. They were subsequently centrifuged (at 3,000 g for 10 min) twice and filtered with a 0.22-um filter to remove debris. The concentration was quantified using a Bradford assay (BCA Protein Assay Kit, Thermo) and saved at −80 °C. Antigen-pulsed mature DC (mDC) were obtained by co-culturing DC with LYS overnight. The isolation protocol of mDC-derived exosomes (Dex) was completed as previously described [Citation15]. Briefly, DC were pulsed with 100 μg/mL LYS overnight, and then the culture medium supernatant was replaced with fresh depleted FBS (1,20,000 g over 18 h) medium. The supernatant was collected after another 24 h of culturing and was successively centrifuged at 1000 g for 10 min and 10,000 g for 30 min to move cells and cell debris, and was filtered with a 0.45 μm and a 0.22 μm filter (Millipore, US). This was followed by ultracentrifugation at 1,20,000 g for 1.5 h twice to pellet exosomes. The protein concentration of Dex was quantified using a bicinchoninic acid (BCA) assay and saved at −80 °C.
Exosomes characterization
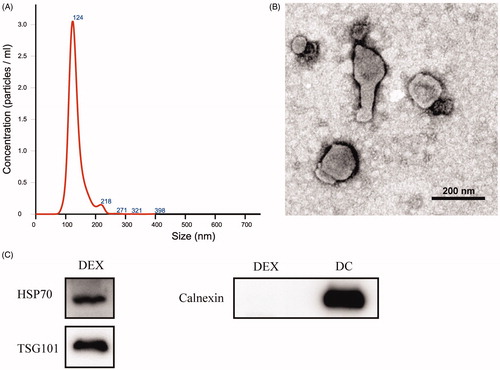
The size distribution of Dex was measured by nanoparticle tracking analysis (NTA). Samples were diluted in phosphate buffered saline (PBS) for a particle count of between 1 × 109 and 3 × 109 particles/ml. The camera focus was adjusted to make the particles appear as sharp dots. A transmission electron microscope (TEM) was used to examine the morphology of Dex. Western blot analysis was employed to confirm the exosome-positive marker protein tumor susceptibility gene (Tsg) 101 (Abcam, UK), heat shock protein (Hsp) 70 (Abcam, UK), and negative protein calnexin (Abcam, UK).
Animal model
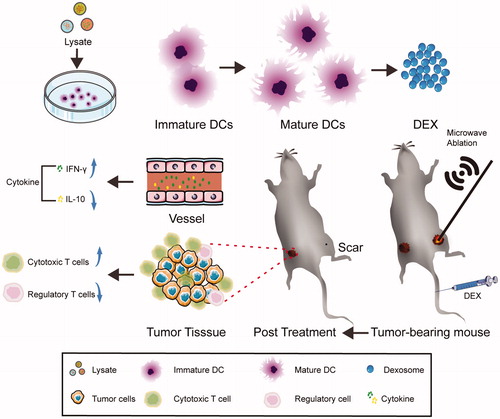
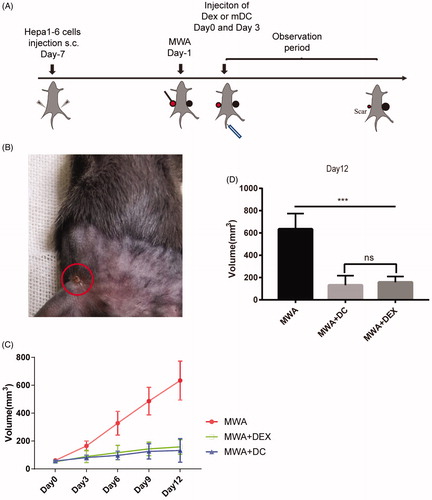
Bilateral sides of the mice were subcutaneously injected with 1 × 107 hepa1-6 cells. After the tumor had grown to 6–8 mm in long diameter, one side of the tumor was treated with MWA (Day 1) and 80 μg Dex or 1 × 106 mDC was injected via the tail vein twice (Day 0 and Day 3) (). The volume of the tumor on the untreated side was measured every 3 days, over a 12-day period. Tumor volume was calculated using the following formula: tumor volume (mm3) = (longest diameter) × (shortest diameter)2/2. On Day 12, the mice were euthanized and the spleen, plasma, and tumor tissue were collected for further analysis.
Microwave ablation
MWA procedures were performed by using a microwave generator (WB-3100AI, Baoxing, China), which has a frequency of 2,450 MHz and a maximum output power of 99 w. Mice were anesthetized by intraperitoneal injection with 4% chloral hydrate (1 ml/kg) and the skin was sterilized. Small gauzes were used to protect the normal tissue around the tumor. The microwave ablation electrode needle was inserted into the tumor and worked at a power of 5 w for 3 min.
Detection of Dex- or mDC-induced killing effect on HCC cells in vitro
The mice were subcutaneously injected with 1 × 107 hepa1-6 cells. After the tumor had grown to 6–8 mm in long diameter, the tumor was treated with MWA. Mice were sacrificed 1 day after MWA. The spleen was harvested and processed into a single cell suspension. Red blood cell lysis buffer was used to remove red blood cells in the splenic cell suspension. After being co-cultured with Dex (40, 60, 80 μg) or mDC (1 × 106) for 48 h, spleen cells (1 × 107) were harvested as effector cells. Hepa1-6 cells served as target cells. After co-culturing the effector cells with the target cells at a ratio of 10:1 overnight, the supernatant was collected. The release of lactate dehydrogenase (LDH) was measured using a LDH release assay (Beyotime, China) according to the manufacturer’s instructions. The killing effect was measured according to the following formula: killing effect = (experimental group - effector cell release control - target cell release control + cell culture medium control)/(target cell max release control - target cell release control). Four repeat wells were calculated for each group.
Flow cytometry
DC were stained with phycoerythrin (PE)-labeled cluster of differentiation (CD) 80 (eBioscience, US), CD83 (eBioscience, US), MHC class I (MHC-I) (eBioscience, US) mouse monoclonal antibody, and related isotype control antibodies (eBioscience, US) according to the manufacturer’s instructions, and analyzed using a fluorescence-activated cell sorting (FACS) Canto II flow cytometer (BD Biosciences). The splenocytes were collected on Day 12 and stained with anti-CD3 antibodies (eBioscience, US), anti-CD8 antibodies (eBioscience, US), anti-CD4 antibodies (eBioscience, US), anti-CD25 antibodies (eBioscience, US), and anti-Forkhead box protein (Foxp3) antibodies (eBioscience, US) according to the manufacturer’s instructions. The stained samples were analyzed using a Canto II flow cytometer (BD Biosciences).
Immunohistochemical analysis
Subcutaneous tumor tissues were fixed in a 4% formalin solution and embedded in paraffin. Paraffin sections were stained with anti-mouse CD8 (Abcam, UK) and anti-Fxop3 (Abcam, UK) at a dilution of 1:500 respectively, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. Nuclei were stained with hematoxylin. The positive cells were quantified and averaged by counting five randomly selected fields at a magnification of ×400.
Enzyme-linked immunosorbent assay (ELISA) assay
The mice were euthanized on Day 12 and eyeballs were excised to harvest blood. The plasma was separated by centrifugation (1000 g, 10 min) and stored at −80 °C for further study. Plasma IL-10 and IFN-γ level were measured using ELISA (eBioscience, US) according to the manufacturer’s instructions.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for statistical analysis. Student’s t-test or one-way analysis of variance (ANOVA) was used for inter-group comparison. All statistics were performed by SPSS 22.0 software and a p-value <0.05 was considered statistically significant.
Results
Characterization of DC phenotype
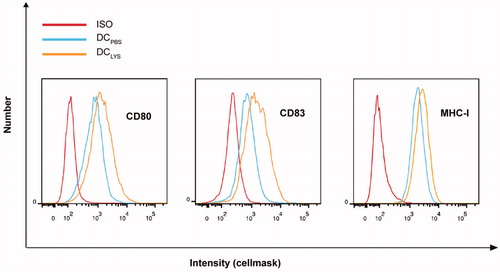
To obtain the mDC, we pulsed DC with LSY overnight and used flow cytometry to confirm the change of DC phenotype. A highly matured and activated phenotype of mDC was demonstrated by the elevated levels of MHC-I, CD80, and CD83 compared with the unpulsed DC (), indicating that mDC was successfully produced.
Size, morphology, and exosomal surface protein expression of Dex
To characterize the Dex obtained, we detected the size distribution curve of Dex using the NS300 Nanoparticle Analyzer. The results showed that the samples had a peak size of 124 nm () and 1 μg Dex sample contained about 3.2 × 108 particles. TEM was used to observe the morphology of Dex, and the results showed that Dex had an oval-biconcave-shaped structure (). Finally, Western blot analysis showed that Dex expressed exosome-positive markers such as Hsp70 and TSG101 without the expression of negative marker calnexin ().
Figure 2. Exosome characterization. (A) The size distribution of Dex was analyzed by NS300 Nanoparticle Analyzer. (B) The morphology of Dex was observed by transmission electron microscope. (C) The surface makers of Dex were detected by Western blot. A total of 12 μg protein was loaded for all lanes.
Dex or mDC-induced cytotoxicity against HCC cells in vitro
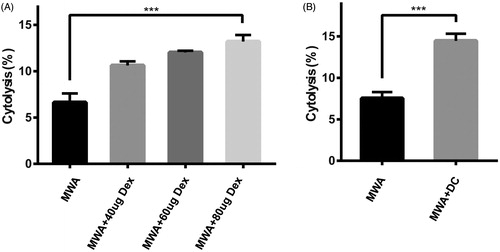
To examine the cytolytic effect of Dex against hepa1-6 cells in a lymphocyte-dependent manner, different amounts (40, 60, and 80 μg) of Dex were co-cultured with same number spleen cells (1 × 107) respectively for 48 h. A dose-dependent cytolysis trend was established and 80 μg Dex induced nearly 2-fold cytolysis effect compared to the control group (). In addition, mDC (1 × 106) were co-cultured with the spleen cells (1 × 107) at a ratio of 1:10, and the results showed that the spleen cells presented an almost 2-fold cytotoxicity effect to hepa1-6 cells compared to the control group ().
Effect of MWA in combination with the injection of Dex or mDC on tumor growth
Our experimental scheme is shown in . Briefly, 18 mice were randomly divided into three groups (MWA group, DC-combined group, and Dex-combined group). Dex (80 μg) or mDC (1 × 106) were injected into the mice via the tail vein (on Day 0 and Day 3) after MWA treatment (Day 1). Tumor volume was monitored every 3 days. Approximately 10 days after treatment with MWA, the tumor had disappeared and was replaced by a small scar (). As for the unablated side, tumor growth was significantly inhibited in the combination group compared to the MWA group (). However, there was no statistical difference in tumor growth inhibition between the DC-combined group and the Dex-combined group ().
Figure 4. The treatment effect of MWA combined with Dex or mDC in murine hepa1-6 subcutaneous tumor models. (A) Time schedule outlines for the treatments in this study; 80 μg or 1 × 106 mDC were injected into the tail vein of the mice (Day 0, Day 3) after MWA; the unablation side tumor was monitored for 12 days. (B) The ablation side tumor was replaced by a small scar (Day 9). (C, D) Tumor volume was monitored and compared among three groups: (1) MWA, (2) MWA + DC, (3), and MWA + Dex. ***p<.001; NS: not significant (n = 18, six mice were used per group).
Dex or mDC improved the immune microenvironment of MWA-treated mice
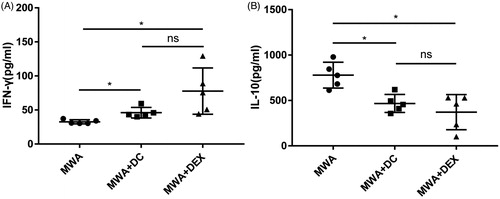
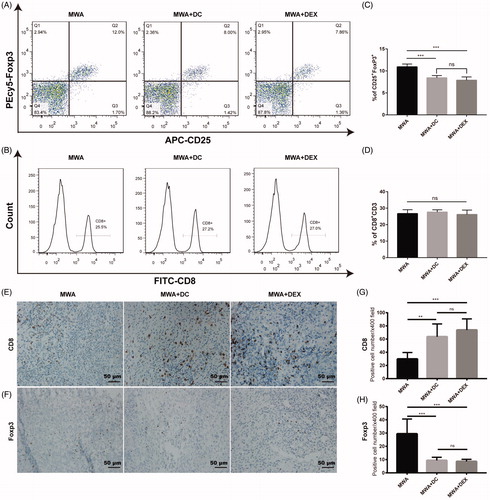
We used flow cytometry to detect the proportion of Treg cells and CD8+ T cells in the spleen of the mice on day 12. The proportion of Treg cells in the spleen showed some decline in the Dex or the DC-combined group compared to the MWA group (). However, the proportion of CD8+ T cells showed no difference among the three groups (). Furthermore, we examined the number of tumor-infiltrating T cells in the non-ablated tumor by immunohistochemistry (IHC). IHC results showed that there were less FoxP3+ cells () and more CD8+ T cells () in the Dex or the DC-combined group compared to the MWA group. We also analyzed the levels of certain cytokines in plasma in the treated mice on day 12; results showed significantly increased levels of IFN-γ and reduced levels of IL-10 in the combination group compared to the control group ().
Figure 5. Evaluation of the immune milieu in mice. (A, B, C, D) On Day 12, splenocytes were stained with anti-CD3, anti-CD8, anti-CD4, anti-CD25, and anti-FoxP3 antibodies and analyzed by flow cytometry. Six mice were used in each group. (E, F, G, H) The number CD8+ and FoxP3+ cells in untreated tumors were counted in five randomly chosen areas at 400-fold magnification. Five mice were used in each group. ***p<.001; **p<.01; NS: not significant.
Discussion
In this study, we established a bilateral tumor-bearing mouse model and used MWA to destroy the tumor on one side followed by intravenous injections of Dex or mDC. The results showed there was a significant inhibition of tumor growth and an improvement of the immune microenvironment in the combination group (MWA + Dex/MWA + DC) compared to the MWA group.
Previous studies have demonstrated that thermal ablation can not only directly disrupt tumor tissue, but also help expose tumor-related antigens which can trigger an antitumor immune response in vivo [Citation16]. On the basis of this theory, Nakagawa et al. [Citation6] used a bilateral tumor-bearing mouse model to demonstrate that tumor thermal ablation on one side followed by intravenous injection of DC vaccine can significantly inhibit the growth of the nonablated tumor. Consistent with the results of Nakagawa et al., our results showed that MWA combined with a Dex or DC vaccine could significantly inhibit the growth of a tumor on the non-ablated side compared to MWA alone. Furthermore, there was no statistical difference in tumor inhibition between the Dex-combined group (MWA + Dex) and the DC-combined group (MWA + DC), which indicated that Dex could achieve an equivalent tumor-inhibition effect to DC in our study. It is noteworthy that since the primary objective of our study was to investigate the antitumor efficacy of Dex in HCC after MWA therapy, no Dex group or DC group was set in this study.
To further analyze changes in the immune microenvironment among the three groups, we measured the immune cells in the mice on Day 12. Results showed that there were more tumor-infiltrating CD8+ T cells in the combination therapy groups (MWA + Dex/MWA + DC) compared to the MWA group. CD8+ T cells are among the most important effector cells and play a pivotal role in antitumor immune responses [Citation17]. The high CD8+ T cells infiltration into the tumor is thought to be the key factor in tumor regression [Citation18]. In addition, numerous studies have demonstrated that thermal ablation combined with immunotherapy can not only enhance the tumor infiltration of CD8+ T cells, but can also significantly increase the proportion of CD8+ T cells in the spleen [Citation6,Citation19,Citation20]. However, in our study, there were no statistical differences in the proportion of CD8+ T cells in the spleen among the three groups. Treg cells are a type of CD4+ T cell subset and play an important role in tumor immune tolerance [Citation21]. Research has found that Treg cells can promote tumor progression by suppressing the antitumor effect of immune cells [Citation22]. Therefore, the decrease in Treg cells may contribute to enhancing antitumor immune responses in vivo [Citation21]. Our previous study found that MWA combined with a DC vaccine could cause a reduction in splenic Treg cells compared to the MWA group [Citation8]. In the present study, a similar trend was observed in both the Dex-combined group (MWA + Dex) and DC-combined group (MWA + DC). Furthermore, we found that there were much lower tumor-infiltrating Treg cells in the combination groups (MWA + Dex/MWA + DC) compared to the MWA group. Lee et al. [Citation23] found that the number of intratumoral Treg cells in HCC patients was positively correlated with the tumor size, which corresponds to our findings. Kobayashi et al. [Citation24] determined that the occurrence and development of HCC are accompanied by an increase in intratumoral Treg cells and a decrease in intratumoral CD8+ T cells. Interestingly, the injection of Dex or DC could induce an increase of intratumoral CD8+ T cells and a reduction of intratumoral Treg cells in MWA-treated mice in our study, indicating that MWA combined with Dex or DC is an effective treatment for HCC.
Besides the immune cells, the levels of particular cytokines in plasma can also reflect the anti-tumor immune state in vivo, and may even be the indicator for predicting the prognosis of cancer patients [Citation25]. IFN-γ, a cytokine long-established as being important in immunoregulation, predominantly plays a significant role in antitumor immunity [Citation26]. IL-10, another immune-regulation cytokine, is upregulated in a variety of cancer patients and contributes to the formation of immune suppression and immune tolerance [Citation27]. A high level of IL-10 in plasma is usually positively correlated with tumor progression and recurrence [Citation27]. Botella-Estrada et al. [Citation28] found that there are more IL-10-secreting lymphocytes and less IFN-γ-secreting lymphocytes in the blood of melanoma patients compared to healthy individuals. Rao et al. [Citation29] and Lu et al. [Citation14] have demonstrated that a Dex or DC vaccine can improve the immune microenvironment in HCC mice, shown by an increase in plasma IFN-γ and a reduction in plasma IL-10. Consistent with these findings, our results showed that there was a significantly higher level of plasma IFN-γ and a lower level of plasma IL-10 in the combination groups (MWA + Dex/MWA + DC) compared to the MWA group. Put simply, the injection of Dex or DC could significantly improve the immune microenvironment in HCC mice following MWA, which may be the critical factor leading to the inhibition of tumor growth on the non-ablated side in our combination groups. In addition, our study found no statistical differences between the Dex-combined group (MWA + Dex) and the DC-combined group (MWA + DC) in the comparison aspects mentioned above.
It should be noted that the dosage of Dex and DC injected into the mice must be carefully considered. Rao et al. [Citation29] and Lu et al. [Citation14] have demonstrated that DC or Dex can induce a dose-dependent immune response by a splenic cell activation experiment in vitro. Thery et al. [Citation30] pointed out that cell numbers, MHC molecules, or total protein amounts would essentially determine whether Dex was less, equally, or more efficient than DC for T cell priming in vivo. They also found the T cell-activation effect induced by 10–22 μg of Dex was of a similar level to that induced by 3 × 105–5 × 105 of DCs based on MHC molecules (since 1 μg Dex has the same amount of MHC II molecules as 0.19 × 106 DC).
As is well known, Dex can induce an immune response in vivo via direct or indirect interaction with immune cells. It is also well established that the spleen is the largest secondary lymphoid organ, is rich in immune cells, and can participate in local and systemic immune regulation [Citation31]. Thus, in order to obtain more reliable results, we co-cultured Dex or DC with spleen cells derived from the tumor-bearing mice after MWA, and then measured the cytotoxic effect of the spleen cells on hepa1-6 cells using a LDH assay. The results showed that 80 μg of Dex had a similar immune cell-activation effect to 1 × 106 of DC. In our study, the average amount of exosomal proteins derived from DC was 0.22–0.38 μg/1 × 106 cells/24h, which is similar to the results of Thery et al.
Several limitations of our study should be noted. First, Lemdani et al. [Citation32] reported that MWA combined with immunotherapy did not increase the proportion of CD8+ T cells in the spleen compared to the MWA group, although they did observe an elevated expression of TNF-alpha and IFN-γ by CD8+T cells. However, we did not detect the expression of IFN-γ and TNF-alpha by CD8+T cells. Second, a subcutaneous HCC mouse model is not an ideal model for our in vivo study because the orthotopic tumor microenvironment is crucial to hepatocellular carcinoma. Third, it remains unclear about the anti-tumor immune effect of Dex or DC alone in this study. Last, the underlying mechanism of the anti-tumor immune response of Dex or DC has not yet been investigated.
In conclusion, our study demonstrated that MWA combined with the Dex or DC could significantly inhibit the tumor growth and improve the tumor immune microenvironment in HCC mouse models compared to MWA alone. Furthermore, there were no statistical differences in tumor growth inhibition or in immune regulation between the Dex-combined group and DC-combined group, indicating that Dex can induce an equivalent immune-enhancing effect to DC in our combination therapy strategy.
Acknowledgments
We gratefully acknowledge Dr Shaokai Sun for providing the murine dendritic cell line. We gratefully acknowledge technical assistances by Artificial Cell Engineering Technology Research Center of Public Health Ministry in Tianjin third Central Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424.
- Crocetti L, Lencioni R. Thermal ablation of hepatocellular carcinoma. Cancer Imag. 2008;8(1):19–26.
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255.
- Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection-propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919.
- Li X, Liang P. Immunotherapy for hepatocellular carcinoma following thermal ablation. J Buon. 2014;19(4):867–871.
- Nakagawa H, Mizukoshi E, Iida N, et al. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol Immunother. 2014;63(4):347–356.
- Liu Q, Zhai B, Yang W, et al. Abrogation of local cancer recurrence after radiofrequency ablation by dendritic cell-based hyperthermic tumor vaccine Mol Ther. 2009;17(12):2049–2057.
- Zhou Y, Jiang J, Ding J, et al. Microwave ablation combined with dendritic cell vaccine: a potential synergistic therapy for hepatocellular carcinoma. Int J Clin Exp Med. 2019;12(9):11257–11264.
- Palmer DH, Midgley RS, Mirza N, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma Hepatology. 2009;49(1):124–132.
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature Rev Cancer. 2012;12(4):265–277.
- Han Q, Zhao H, Jiang Y, et al. HCC-derived exosomes: critical player and target for cancer immune escape. Cells. 2019;8(6):558.
- Pitt JM, Andre F, Amigorena S, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Investig. 2016;126(4):1224–1232.
- Mignot G, Roux S, Thery C, et al. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med. 2006;10(2):376–388.
- Lu Z, Zuo B, Jing R, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–748.
- Cho JA, Yeo DJ, Son HY, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114(4):613–622.
- Slovak R, Ludwig JM, Gettinger SN, et al. Immuno-thermal ablations – boosting the anticancer immune response. J Immunother Cancer. 2017;5(1):78.
- Mami-Chouaib F, Blanc C, Corgnac S, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6(1):10.
- Mukai S, Kjaergaard J, Shu SY, et al. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999;59(20):5245–5249.
- Li L, Wang W, Pan H, et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J Transl Med. 2017;15(1):23.
- Zhu J, Yu M, Chen L, et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: a pre-clinical study in a murine model. Diagn Interventional Imaging. 2018;99(3):135–142.
- Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118.
- Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102(2):419–424.
- Lee WC, Wu TJ, Chou HS, et al. The impact of CD4+ CD25+ T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery. 2012;151(2):213–222.
- Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3(+) regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–911.
- Kurzrock R. Cytokine deregulation in cancer. Biomed Pharmacother. 2001;55(9–10):543–547.
- Beatty GL, Paterson Y. Regulation of tumor growth by IFN-gamma in cancer immunotherapy. IR. 2001;24(2):201–210.
- Sato T, Terai M, Tamura Y, et al. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51(2-3):170–182.
- Botella-Estrada R, Escudero M, O'Connor JE, et al. Cytokine production by peripheral lymphocytes in melanoma. Eur Cytokine Network. 2005;16(1):47–55.
- Rao Q, Zuo B, Lu Z, et al. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456–472.
- Thery C, Duban L, Segura E, et al. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162.
- Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806–818.
- Lemdani K, Mignet N, Boudy V, et al. Local immunomodulation combined to radiofrequency ablation results in a complete cure of local and distant colorectal carcinoma. Oncoimmunology. 2019;8(3):1550342.