Abstract
Purpose
This study aimed to evaluate the incidence and severity of biliary complications after treating periductal hepatocellular carcinomas (HCCs) using either cryoablation (CA) or radiofrequency ablation (RFA) and assess independent risk factors for biliary complications after treatment.
Materials and methods
Between July 2008 and August 2018, 949 patients with treatment-naïve HCCs underwent either RFA or CA in our institution. Of these, patients with multiple HCCs, tumors equal to or larger than 3 cm or smaller than 1 cm, and tumors with non-periductal locations were excluded. Finally, 31 patients and 25 patients were included in the RFA group and the CA group, respectively. The incidence and severity of biliary complications were compared between the RFA and CA groups. The risk factors for biliary complications were assessed using univariable and multivariable logistic regression analyses using the following variables: age, sex, tumor size, Child–Pugh score, tumor location (peripheral duct versus central duct), ablation method (RFA versus CA), the number of applicators, ablation time, and ablation volume.
Results
The incidence and severity of biliary complications were significantly higher in the RFA group than in the CA group (p = 0.007 and p = 0.002, respectively). In univariable and multivariable analyses, the ablation method was an independent risk factor for biliary complications (p = 0.004 and 0.013, respectively).
Conclusions
The incidence and severity of biliary complications after treating HCCs abutting the bile duct are lower in CA than RFA, demonstrating that CA is safer than RFA for ablating small periductal HCCs.
Introduction
Percutaneous local ablation therapy is widely used as a curative treatment for very early- or early-stage hepatocellular carcinoma (HCC) patients who are not eligible for surgical resection or transplantation [Citation1]. Radiofrequency ablation (RFA) is a standard technique used for ablating small HCCs and has well-established safety and efficacy profiles [Citation2]. However, not all small HCCs can be managed with RFA because serious complications may occur depending on the tumor location [Citation3,Citation4].
Cryoablation (CA) has been used as a local ablation therapy and is as safe and effective as RFA [Citation5,Citation6]. The advantages of CA over RFA include the following: (a) less pain compared to heat-based ablation therapy; (b) the iceball is easily visualized with various guiding modalities, including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), which facilitates accurate monitoring of the ablation zone, reducing adjacent tissue injury during the procedure [Citation7]; and (c) less damage to adjacent structures such as the gallbladder and portal vein compared with RFA [Citation8–10].
Tumor location should be considered for the successful and safe local ablation of HCCs. The management of periductal HCCs by RFA is challenging because thermal injury to the bile duct can result in biliary complications such as bile duct stricture and hepatic biloma [Citation11–13]. In addition, central bile duct injury can severely impair liver function and lead to poor prognosis [Citation14]. Given that CA is safer than RFA for tumors close to critical structures [Citation8,Citation15], tumors near the bile duct can be treated with CA without serious biliary complications. Studies comparing CA and RFA have not found significant differences in the overall safety between the two ablative techniques for HCC; however, tumor location relative to the bile duct was not determined in these studies [Citation5,Citation6]. Therefore, this study aimed to evaluate the incidence and severity of biliary complications after treating HCCs abutting the bile duct using CA or RFA and assess independent risk factors for biliary complications after these procedures.
Materials and methods
Patients
This retrospective study was approved by the institutional review board of our institution, and the requirement for written informed consent was waived. RFA has been widely used in our institution for managing HCCs measuring <3 cm. Since 2015, our group has been using CA for periportal or periductal tumors because RFA may cause aggressive intrasegmental recurrence and biliary complications [Citation10,Citation16]. The treatment modality was chosen by a team of hepatologists, surgeons, oncologists, radiation oncologists, and interventional radiologists.
Between July 2008 and August 2018, a total of 912 HCC patients underwent RFA at our institution. Of these, multiple HCCs (n = 129), tumors equal to or larger than 3 cm or smaller than 1 cm (n = 70), and tumors with non-periductal location (n = 682) were sequentially excluded. In the same period, a total of 37 patients underwent CA. Of these, multiple HCCs (n = 5), tumors equal to or larger than 3 cm or smaller than 1 cm (n = 3), and tumors with non-periductal location (n = 4) were excluded. The patient selection diagram is shown in . Periductal HCCs were defined as tumors abutting the bile duct on pretreatment MRI (either T2-weighted or hepatobiliary phase images). HCC was diagnosed according to current guidelines [Citation17]. Finally, 31 patients and 25 patients were included in the RFA group and the CA group, respectively.
Radiofrequency ablation
RFA was performed by one of the five interventional radiologists (K. D. S., T. W. K., M. W. L., H. R., or H. K. L.) with >3 years of experience in local ablation therapy for hepatic tumors. Before RFA, US was performed on an outpatient basis to evaluate whether local ablation therapy was feasible. Treatments were performed on an inpatient basis under conscious sedation or monitored anesthesia care; the latter was performed by anesthesiologists. Given the long study period, various RFA systems, including the VIVA RFA (STARmed, Goyang-si, South Korea), Jet-tip RFA (RF Medical, Seoul, South Korea), and Cool-tip RF Ablation (Medtronic, Minneapolis, MN) were used according to the operator’s preference and temporal availability. Real-time US or fusion imaging (LOGIQ E9; GE Healthcare, Chicago, IL or RS80A; Samsung Medison, Chicago, IL) of real-time US and CT/MRI were used for guidance and monitoring the procedures [Citation18]. Contrast-enhanced US (Sonazoid®; GE Healthcare, Chicago, IL) was applied in cases in which lesion conspicuity was insufficient for accurate needle placement under fusion imaging guidance [Citation19]. Artificial ascites was introduced when needed to enhance sonographic window or avoid collateral thermal damage. The aim of RFA was to eradicate the tumor and achieve at least a 0.5-cm ablative margin around the tumor, except for the perivascular or subscapular portion of the tumors. Additional ablation after repositioning the electrode was performed when necessary. After the RFA procedure, tract ablation was performed to avoid tract bleeding or tumor seeding.
Cryoablation
CA procedures were performed by one of the five interventional radiologists. After local anesthesia with or without conscious sedation, cryoprobes (IceSphere1.5® needle, straight type; Galil Medical, Yokneam, Israel) were percutaneously placed to the index tumor under US or fusion imaging guidance. One to three cryoprobes were used depending on the tumor size and morphology. According to the manufacturers’ recommendation, two CA cycles, including freezing (10 min), thawing (7 min), refreezing (10 min), and rethawing (3 min), were routinely used. The aim of CA and the guiding method for CA were the same as those for RFA. During the procedure, the ice-ball formation was continuously monitored with the real-time US. Therefore, the operators could decide whether the ablative margin was insufficient as the ice-ball was clearly demarcated on US. In cases in which the ablative margin was insufficient, additional CA was performed after needle repositioning. Procedures were completed when the iceball induced by CA on US was large enough to cover the entire tumor and the surrounding liver [Citation10]. During the procedure, warm saline-soaked gauze was used to protect the overlying skin from frostbite. After the procedure, cryoprobes were gently removed as tract ablation was not applicable to a cryoprobe.
Post-procedure follow-up
Immediately after the ablation procedures, contrast-enhanced CT was performed to evaluate the therapeutic response and possible complications. In cases where residual enhancing tumor was detected, an additional treatment session was attempted within 24 h after the procedures.
The diameters and volume of the ablation zone were evaluated based on an immediate post-procedure CT scan. Maximum (Dmx), minimum (Dmi), and vertical (Dv) diameter were measured on the axial and coronal images of the portal phase. The volume of the non-enhancing ablation zone was calculated with the assumption of the ablation zone as an ellipsoid using the following formula: π(Dv × Dmx × Dmi)/6 [Citation20,Citation21].
All patients underwent follow-up contrast-enhanced CT or MRI, chest radiography, and laboratory blood tests, including serum alpha-fetoprotein and liver function test, 1 month after initial treatment, every 3 months during the first 2 years, and every 4–6 months subsequently.
Assessment of treatment outcomes
Local tumor progression (LTP) was defined as the appearance of enhancing tumor foci at the margin of the ablation zone after at least one follow-up CT/MRI demonstrated the absence of viable tumor in the ablation zone [Citation22].
The presence of complications was assessed in consensus by two radiologists (S. E. K. and M. W. L.) based on immediate post-ablation and follow-up CT/MRI findings. These examiners were blinded to the type of ablation therapy used in each patient. Asymptomatic biliary stricture was defined as upstream bile duct dilatation in the ablation zone without symptoms or signs [Citation14]. Abscess or biloma was defined as fluid accumulation in the ablation zone with or without adjacent hyperemia, and symptoms and signs of infection. A major complication was defined as an event that led to substantial morbidity and disability and increased the level of care, resulted in hospital admission, or substantially prolonged hospital stay. All other complications were regarded as minor [Citation22].
Statistical analysis
Study patients’ demographics and clinical characteristics were compared between the two study groups using a Student t-test, and the Mann–Whitney test for numeric variables and Chi-square test and Fisher’s exact test for categorical variables.
The incidence of biliary complications (asymptomatic biliary stricture, biloma, or abscess) was compared between the groups using the Chi-square test or Fisher’s exact test. The risk factors for biliary complications after treatment were assessed by univariate and multivariate logistic regression analyses using the following variables: age, sex, tumor size, Child–Pugh score, albumin–bilirubin (ALBI) grade, tumor location (peripheral duct versus central duct), ablation method (RFA versus CA), the number of applicators and ablation time, and ablation volume. For multivariate analysis, variables with p-values < 0.1 were included for logistic regression analysis. Multicollinearity among the variables was checked using variance influence factor (VIF), and VIF > 4 were considered multicollinear.
The cumulative LTP rate in each group was estimated using the Kaplan–Meier method and the log-rank test. The follow-up period was significantly different between the two groups because CA was initiated later than RFA. Therefore, based on the longest follow-up period (58 months) in the CA group, patients in the RFA group were censored to assess LTP at this time point. To investigate factors affecting LTP, a Cox proportional hazards model was used to accommodate covariates, including age, gender, tumor size, Child–Pugh score, ALBI grade, tumor location (peripheral duct versus central duct), ablation method (RFA versus CA), the occurrence of biliary complications, the number of applicators, ablation time, and ablation volume. The proportional hazard (PH) assumption was tested using correlation analysis of Schoenfeld residuals and time to LTP. Time-dependent Cox regression was performed to determine variables that did not satisfy the PH assumption. Before multivariate analysis, multicollinearity among the study variables was checked using variance influence factor (VIF), and variables with VIF >4 were considered multicollinear. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS Statistics software package version 25.0 (SPSS, Chicago, IL). A two-sided p-value of <0.05 was considered to indicate a statistically significant difference.
Results
Patients
Thirty-one patients (21 men and 10 women; mean age, 59.6 years; age range, 43–83 years) and 25 patients (21 men and four women; mean age, 60.9 years; age range, 41–78 years) were included in the RFA and CA group, respectively. The median follow-up period in the CA and RFA group was 47 months (range, 16–58 months) and 62 months (range, 8–118 months), respectively. Patient and tumor characteristics and each procedure-related factors are shown in . Segmental tumor location and the proportion of tumors abutting the bile duct (central or peripheral duct) were not significantly different between the CA and RFA group (p = 0.438 and p = 0.571, respectively). Compared to the RFA group, the CA group had a significantly smaller tumor size (p < 0.001), a significantly lower ALBI grade (p = 0.016), and a significantly higher platelet count (p = 0.009).
Table 1. Demographic and clinical characteristics of study patients.
Procedure-related factors
The number of applicators was higher in the CA group than RFA group (median, 2; range, 1–3 versus median, 1; range, 1–3), and ablation time was also longer in the CA group than RFA group (median, 30 min; range, 30–47 versus median, 14 min; range, 6–25) (p < 0.001, both) (). However, ablation volume measured on immediate post-ablation CT was slightly smaller in the CA group than in the RFA group, which did not reach statistical significance (median, 10.5 cm3; range, 1.6–39.4 versus median, 13.9 cm3; range, 3.2–48.1) (p = 0.154).
Complications after the procedure
Procedure-related complications are summarized in . All complications were found within 3 months of the ablation procedures. The overall incidence of complications was significantly higher in the RFA group than in the CA group (67.7% [22/31] versus 28% [7/25]; p = 0.007). However, asymptomatic biliary strictures were more common than abscess or biloma in both groups. Abscess or biloma was found in 9.7% (3/31) patients in the RFA group; however, abscess or biloma was not found in the CA group (p = 0.245) ( and ).
Figure 2. A 76-year-old woman with autoimmune hepatitis. (A) Hepatobiliary phase magnetic resonance image shows a 1-cm HCC (arrow) abutting the right bile duct in segment 7. The bile duct is visualized as a tubular structure with high-signal intensity (arrowheads) because of contrast excretion. The tumor is also close to the right main portal vein (anterior side) and right hepatic vein (posterior side). (B) Portal-phase CT scan obtained immediately after cryoablation show an oval-shaped ablation zone covering the entire tumor; the ablation zone size is small because a single cryoprobe was used. There is no bile duct dilatation, suggesting the absence of severe bile duct damage. In addition, the adjacent portal vein and hepatic vein seem to be patent, and there is no perfusion abnormality in the liver. (C) Hepatobiliary phase magnetic resonance image obtained 4 months after the procedure shows the intact right bile duct. There is no upstream bile duct dilatation (not shown). No local tumor progression was noted during the 29-month follow-up period.
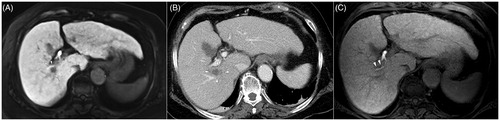
Figure 3. A 48-year-old man with liver cirrhosis due to chronic hepatitis B viral infection. (A) Hepatobiliary phase magnetic resonance image shows a 2.1-cm hepatocellular carcinoma (arrow) abutting the right anterior portal vein and right anterior bile duct (arrowheads) in segment 8. (B) Portal-phase computed tomography scan obtained immediately after radiofrequency ablation shows that the tumor is completely ablated with no immediate procedure-related complications. (C) Additional computed tomography (CT) scan obtained 4 days after the procedure because of high fever and abdominal pain. Portal-phase CT image shows air bubbles (arrowhead) in the radiofrequency ablation (RFA) zone and fat stranding in the perihepatic space near the RFA zone, indicating possible abscess and inflammation. The patient’s condition recovered after administration of empirical antibiotics.
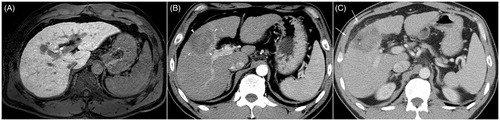
Table 2. Complications after treatment of hepatocellular carcinoma.
With regard to non-biliary complications, two patients in the RFA group presented portal vein thrombosis that required conservative management and longer hospital stay. One patient presented post-ablation tract bleeding after RFA and required blood transfusion ().
The severity of complications was higher in the RFA group than in the CA group (p = 0.002). The subanalysis of tumors abutting the central bile duct indicated that the incidence and severity of biliary complications were not significantly different between the CA and RFA groups (p = 0.074 and p = 0.052, respectively).
Risk factors for biliary complications
The ablation method and ablation time were risk factors for biliary complications in the univariable analysis (p = 0.004 and 0.019, respectively). In subsequent multivariable analysis, the ablation method was an independent risk factor for developing biliary complications (p = 0.013). The multicollinearity between ablation time and ablation method was considered to be present and thus ablation time was not included in multivariable analysis ().
Table 3. Univariable and multivariable analyses of risk factors for biliary complications (biliary stricture, biloma, or abscess).
Other factors such as age, sex, tumor size, Child–Pugh score, ALBI grade, tumor location (peripheral or central duct), the number of applicators, and ablation volume were not risk factors for biliary complications.
Local tumor progression
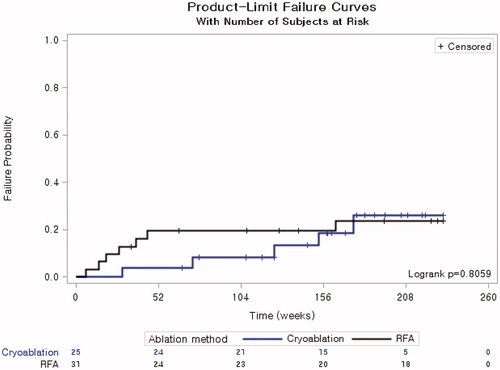
The cumulative LTP rate in 1, 2, 3 and 4 years was 4.0%, 8.2%, 18.7%, and 26.1% in the CA group and 19.6%, 19.6%, 19.6%, and 23.6% in the RFA group, respectively. These values were not significantly different between the two groups (p = 0.806) ().
Figure 4. Cumulative local tumor progression rate of cryoablation and radiofrequency ablation. * Patients in the radiofrequency ablation group were censored to assess local tumor progression at 58 months based on the longest follow-up period for the cryoablation group.
The univariable analysis indicated that tumor abutting the bile duct was a significant risk factor for LTP (p = 0.034). However, multivariable analysis revealed that it was not a significant risk factor for LTP (p = 0.067) ().
Table 4. Univariable and multivariable analyses of risk factors for local tumor progression.
Discussion
In this study, we evaluated the incidence of biliary complications after RFA or CA for periductal HCCs and found that the incidence was significantly higher with RFA than with CA. Moreover, the severity of complications was higher after RFA than after CA. Univariable and multivariable analyses showed that RFA was an independent risk factor for the development of biliary complications. Other complications, such as portal vein thrombosis or tract bleeding, were only observed in the RFA group. No independent factor affected LTP after treatment. These results indicate that CA is safe for ablating small HCCs abutting the bile duct, and local tumor control efficacy of CA is similar to that of RFA.
Previous studies have reported that the percutaneous thermal ablation of tumors close to the bile duct (within 10 mm) cause bile duct injury [Citation12,Citation13]. In one study [Citation14], bile duct dilatation after RFA worsened prognosis as patients with severe dilatation in two or more bile duct segments showed lower survival and higher HCC recurrence than patients without this complication. Hence, central bile duct injury after ablation therapy should be avoided as much as possible.
Some studies attempted to avoid this problem by performing intraductal cooling of the bile ducts using either endoscopic nasobiliary drainage or percutaneous transhepatic cholangial drainage [Citation4,Citation23]. In these studies, cooling reduced the risk of complications during the thermal ablation of tumors close to the central bile duct. However, this approach is moderately invasive and may cause procedure-related complications such as pancreatitis and bleeding [Citation23].
The European Association for the Study of the Liver clinical practice guidelines on the management of HCC state that ethanol injection is useful in cases in which thermal ablation is not technically feasible, especially for HCCs measuring <2 cm [Citation24]. However, ethanol injection therapy for periductal HCCs adjacent to the central bile duct may cause major biliary complications [Citation25]. Although the pathogenesis of bile duct stricture after ethanol injection therapy is not fully understood, the inflammation of tissues adjacent to tumors, including the bile duct, may occur after injection therapy. In addition, alcohol diffusion may cause bile duct injury [Citation25]. Therefore, ethanol injection therapy is not safe for periductal HCCs.
Irreversible electroporation (IRE), a non-thermal ablation technique, causes cell death using pulsating direct currents. This technique is known to be highly safe for ablating hepatic tumors close to central bile ducts [Citation26,Citation27] as it does not damage the architectural integrity of the bile duct [Citation28]. Therefore, it could be used as an alternative treatment of RFA for ablating periductal tumors, although it requires general anesthesia and higher cost compared to thermal ablation.
A previous study treated 443 tumors with CT fluoroscopic-guided percutaneous cryotherapy and reported that there were no serious biliary complications despite the central location of tumors; however, the total number of tumors close to the central bile duct and the distance between the tumors and the bile duct were not reported [Citation15]. The safety of CA for periductal HCCs was confirmed in the present study. The absence of significant differences in the incidence and severity of biliary complications between the two groups after treating tumors abutting the central bile duct (p = 0.074 and p = 0.052, respectively) may be attributed to the small sample size. In a previous study, percutaneous CA of hepatic tumors close to the gallbladder was safe and effective; postprocedural gallbladder thickening was common, but it was self-limited and clinically irrelevant, even when the iceball extended into the gallbladder lumen [Citation9]. Another study has shown that CA is safe and effective for periportal HCCs and has a very low risk of causing vascular complications [Citation10]. These results indicate that CA is relatively safe and less damaging to adjacent structures and can be useful for ablating tumors in high-risk locations. Moreover, contrary to our expectation, tract bleeding was not observed in the CA group even though tract ablation was not applicable. Although earlier studies reported that serious complications such as cryo-shock, liver crack, or excessive bleeding could occur after cryoablation for treating large HCCs with an old version of cryoprobes with thick shaft [Citation15,Citation29], recent studies rarely reported this kind of serious complication [Citation5,Citation30]. Therefore, cryoablation using the latest model seems to be relatively safe for treating small HCCs, which was also confirmed in the present study.
This study had some limitations. First, this was a retrospective and single-center study. Second, the number of participants was small due to the specific location of the tumors. Third, the follow-up period was longer in the RFA group than in the CA group because CA was initiated later than RFA. Therefore, the difference in the cumulative LTP rate between the two treatments after 58 months is unknown. In addition, the real performance of RFA in local tumor control may have been underestimated because the RFA technology may have evolved during the long study period. Fourth, several variables, including tumor size, were different between the two groups, limiting direct comparisons. Nonetheless, multiple logistic regression analysis revealed that the ablation method was an independent risk factor for biliary complications after local ablation therapy. Our results indicate that CA can be a useful treatment option, particularly in patients with periductal HCCs not suitable for surgical resection.
In conclusion, the incidence and severity of biliary complications after treating HCCs abutting the bile duct are lower with CA than with RFA, demonstrating that CA is safer than RFA for managing periductal HCCs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853.
- Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol. 2009;20(7 Suppl):S342–S347.
- Kasugai H, Osaki Y, Oka H, Osaka Liver Cancer Study Group, et al. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72(Suppl 1):72–75.
- Koda M, Murawaki Y, Hirooka Y, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42(11):1058–1064.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61(5):1579–1590.
- Dunne RM, Shyn PB, Sung JC, et al. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. Eur J Radiol. 2014;83(4):632–638.
- Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(4):509–515.
- Kim GM, Won JY, Kim MD, et al. Cryoablation of hepatocellular carcinoma with high-risk for percutaneous ablation: safety and efficacy. Cardiovasc Intervent Radiol. 2016;39(10):1447–1454.
- Fairchild AH, Tatli S, Dunne RM, et al. Percutaneous cryoablation of hepatic tumors adjacent to the gallbladder: assessment of safety and effectiveness. J Vasc Interv Radiol. 2014;25(9):1449–1455.
- Kim R, Kang TW, Cha DI, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur Radiol. 2019;29(2):654–662.
- Raman SS, Aziz D, Chang X, et al. Minimizing central bile duct injury during radiofrequency ablation: use of intraductal chilled saline perfusion–initial observations from a study in pigs. Radiology. 2004;232(1):154–159.
- Lin MX, Ye JY, Tian WS, et al. Risk factors for bile duct injury after percutaneous thermal ablation of malignant liver tumors: a retrospective case-control study. Dig Dis Sci. 2017;62(4):1086–1094.
- Liu J, Wu Y, Xu E, et al. Risk factors of intrahepatic biloma and secondary infection after thermal ablation for malignant hepatic tumors. Int J Hyperthermia. 2019;36(1):980–985.
- Kondo Y, Shiina S, Tateishi R, et al. Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: impact on patient's prognosis. Liver Int. 2011;31(2):197–205.
- Littrup PJ, Aoun HD, Adam B, et al. Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series. Abdom Radiol. 2016;41(4):767–780.
- Kang TW, Lim HK, Lee MW, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276(1):274–285.
- Korean Liver Cancer Study G, National Cancer Center K. 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16(3):465–522.
- Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33(4):227–239.
- Song KD, Lee MW, Rhim H, et al. Percutaneous US/MRI fusion-guided radiofrequency ablation for recurrent subcentimeter hepatocellular carcinoma: technical feasibility and therapeutic outcomes. Radiology. 2018;288(3):878–886.
- Chang W, Lee JM, Lee DH, et al. Comparison of switching bipolar ablation with multiple cooled wet electrodes and switching monopolar ablation with separable clustered electrode in treatment of small hepatocellular carcinoma: a randomized controlled trial. PLoS One. 2018;13(2):e0192173.
- Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42(10):676–683.
- Ahmed M, Solbiati L, Brace CL, Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Ge N, Huang J, Shi Z, et al. Safety and efficacy of microwave ablation for periductal hepatocellular carcinoma with intraductal cooling of the central bile ducts through a percutaneous transhepatic cholangial drainage tube. J Interv Med. 2019;2(2):84–90.
- European Association for the Study of the Liver. Electronic address [email protected], European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Cha DI, Lee MW, Rhim H, et al. Therapeutic efficacy and safety of percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas in high risk locations. Korean J Radiol. 2013;14(2):240–247.
- Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118.
- Dollinger M, Zeman F, Niessen C, et al. Bile duct injury after irreversible electroporation of hepatic malignancies: evaluation of MR imaging findings and laboratory values. J Vasc Interv Radiol. 2016;27(1):96–103.
- Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48.
- Sohn RL, Carlin AM, Steffes C, et al. The extent of cryosurgery increases the complication rate after hepatic cryoablation. Am Surg. 2003;69(4):317–323.
- Cha SY, Kang TW, Min JH, et al. RF ablation versus cryoablation for small perivascular hepatocellular carcinoma: propensity score analyses of mid-term outcomes. Cardiovasc Intervent Radiol. 2020;43(3):434–444.