Abstract
Objective: To evaluate the effect of operator experience on the treatment outcomes of radiofrequency ablation (RFA) for benign thyroid nodules (BTN).
Methods: Data from the 90 first RFA procedures of a single operator in treating benign thyroid nodules were prospectively collected and retrospectively analyzed. Patients were divided into 3 groups according to their chronological treatment rank: patients 1–30 (G1), 31–60 (G2) and 61–90 (G3). Clinical symptoms, volume reduction ratio (VRR), technique efficacy (TE) defined as a VRR > 50% and ablation ratio (AR) were compared between the three groups at 6 months follow-up. All complications and side effects were recorded.
Results: No significant difference was observed in improvement of clinical symptoms after the RFA procedure between the three groups, with higher satisfaction however for pressure symptoms than for esthetic complaints (complete resolution 87.5% and 52.6%, respectively). In groups 1, 2 and 3, TE was 60%, 93.3%, 76.7%, VRR 54%, 65%, 60% and AR 13.1%, 34%, 34.6%, respectively. Thus, all ultrasound efficacy parameters (TE, VRR, AR) improved significantly between G1 and G2, with no difference between G2 and G3. Solely did AR improve in nodules ≤ 30 mL between G2 and G3 to reach a median value of 94.4% in G3 versus 57.1% in G2 and 13.7% in G1. Maximum values of TE and VRR (95.6% and 68%, respectively) were seen in nodules ≤ 30 mL in G2 at 6 months follow-up, with no improvement in G3 (84.2% and 63%, respectively). Both baseline volume and energy per volume were independently associated with VRR and AR. Three minor complications were recorded which all recovered totally after conservative treatment.
Conclusion: There was a measurable learning curve in RFA for benign thyroid nodules regarding efficacy until 90 patients. VRR and AR can be used as proficiency markers. Only three transient complications occurred confirming the safety of the procedure.
Introduction
Thermal ablation (TA) procedures are minimally invasive ultrasound (US)-guided ablation therapies that can be used for treating benign thyroid nodules (BTN). Previous studies have suggested that radiofrequency ablation (RFA) is effective and safe [Citation1–8]. Korean and Italian guidelines have previously validated TA as a first-line treatment for BTN [Citation9,Citation10], and more recently the European Thyroid Association (ETA) guidelines recommended TA in adult patients with BTN that cause pressure symptoms and/or cosmetic concerns and decline surgery. The ETA considers TA as an alternative option to surgical treatment or observation alone [Citation11]. Although TA is generally described as a simple and safe technique, little attention has been paid to the importance of operator experience [Citation3–5,Citation12]. However, ETA guidelines state that ‘the operators performing thyroid TA need appropriate experience in cervical US anatomy and US-guided diagnostic procedures and a dedicated training in image-guided therapeutic procedures’ [Citation11]. Indeed, it is fairly certain that a learning curve exists and it could be considered as an obligation for the clinicians who are starting this new health technology in the treatment of patients to recognize and overcome it. Regarding surgery, learning curves are commonly used to plot the number of cases necessary to acquire skills and gain the mastery and proficiency of an experienced surgeon [Citation13]. Clinicians embarking on RFA treatment should master this learning curve before they can provide the optimum treatment to patients with low morbidity and a high ablation rate [Citation14]. Clinical effectiveness based on symptoms reduction and volume reduction ratio could be used as proficiency markers. However, because it can be technically difficult to completely ablate all the nodule margin from a single-session, it was hypothesized this could represent a supplementary difficulty in the RFA learning curve of benign thyroid nodules. Thus, the criterion called ablation ratio (AR) proposed by Sim et al. [Citation15,Citation16] and later studied by other authors with contrast-enhancement ultrasound (CEUS) [Citation17–19] could also be used to study the learning curve. The AR is the ratio of the ablated volume to the total remaining volume. The ablated volume is obtained after RFA and corresponds to the usually located central hypoechoic and avascular area.
Therefore, the primary goal of this study was to assess if a learning curve exists regarding the efficacy of RFA in treating BTN, using symptoms reduction, volume reduction and ablation ratios as main markers. The secondary goals were to identify technical factors that could explain the learning curve and help practitioners to gain time in this process, and to describe and explain the major and minor complications encountered, to determine how to gain safety.
Patients and methods
Our institutional review board (Clinical Thyroid Research Board from Sorbonne University GRC-16-SU) approved this retrospective study and waived the requirement for written informed consent, in accordance with French recommendations [Citation20]. All patients were advised that their data could be used for research. The terminology used in this report refers to the report by Mauri et al. [Citation21].
Patients
The inclusion period was from January 2016 to January 2020. The first 90 patients were treated by RFA by a single operator (GR), in a single referral thyroid center at La Pitie-Salpetriere Hospital. Patients were divided into 3 groups according to their chronological treatment rank: patients 1–30 (G1), 31–60 (G2) and 61–90 (G3). The operator was a radiologist with more than 20 years of experience in thyroid imaging, fine-needle aspirations, core-needle biopsies, percutaneous ethanol therapy and had performed 40 laser thyroid ablation procedures before starting RFA. Inclusion criteria were (i) patients complaining of pressure symptoms and/or with esthetic complaints and patients with no symptoms but having a nodule with a proven growth (more than 50% volume increase compared to the first available measure) and measuring at least 4 cm, (ii) EU-TIRADS 3 or 4 nodules, (iii) normal serum calcitonin, (iv) two benign cytological results (FNAC) and (v) a wish to avoid surgery. All indications for TA were validated prior to treatment by a multidisciplinary team consensus (including endocrine and ENT surgeons, endocrinologists and radiologists).
Exclusion criteria were: (i) patients under 18 years old, (ii) absence of symptoms and nodular size less than 4 cm, (iii) other thyroid nodule(s) >2 cm, (iv) EU-TIRADS 5 nodules, (v) indeterminate or malignant cytological results and (vi) no specific wish to avoid surgery.
In the case of TSH was ≤0.6 µIU/mL, an iodine scintigraphy (I123) was performed. If an autonomous nodule was found, iodine was the preferred modality of treatment. RFA was only performed in case of refusal of iodine and surgery by the patient.
Pre-ablation assessment
At enrollment, patients were asked to specify the kind of pressure symptoms they felt: dysphagia, foreign body sensation, coughing and the position, if any, that triggered the symptoms. Pressure symptoms and esthetic complaints were recorded as present or absent.
The following biochemical tests were performed: serum TSH (µIU/mL), fT3 (pg/mL), fT4 (ng/dL), anti-TPO antibodies (IU/L), serum calcitonin (pg/mL), blood calcium level (mg/L), blood count, and routine coagulation tests.
All examinations were performed with an Esaote MyLab and a LA533 linear 3-13 MHz probe. All nodules were measured in three dimensions, at least twice and the volume was calculated by the following formula: d1xd2xd3xπ/6. In case of discrepancy between the two measures, the maximum volume was retained and this was applied to all subsequent examinations during follow-up. Nodules were subdivided into small to medium ones (≤30 ml) and large ones (>30 ml). The size cutoff of 30 ml was in accordance with what was suggested by Mauri et al. [Citation21] and already applied by other authors [Citation2]. All nodules were scored according to the EU-TIRADS classification [Citation22]. The position of the vagus nerve, middle cervical sympathetic ganglion (if visible), anterior jugular vein(s) and the relation of the nodule and the theoretical location of the recurrent laryngeal nerve region (so-called ‘danger triangle’) were assessed. Vascularity was categorized by color flow Doppler and microvascular imaging (MicroV, Esaote) as absent, perinodular only, mild intranodular or intense intranodular. Stiffness was assessed with strain elastography as low, focally increased or diffusely increased.
RFA technique and procedure
All treatments were performed on an outpatient basis. After insertion of a venous catheter, patients received prior to treatment oral administration of 1 g paracetamol and 0.5 to 1 mg of alprazolam. A local anesthetic patch was applied about one hour before beginning the procedure. All treatments were performed under local anesthesia, using 10–20 ml of 2% lidocaine infiltrated in the sterno-thyroid muscle under US guidance. All patients were consecutively treated by the same operator. RFA treatment was performed under US guidance with free-hand technique using a generator (VIVA RF generator, STARmed, Gyeonggi, Korea) and an internally cooled 18-gauge electrode, 7 cm length with an active 10-mm tip (VIVA, STARmed, Gyeonggi, Korea). The moving shot technique was applied with a trans-isthmic approach [Citation23]. Ablation began with 30 W of radiofrequency power. If a transient hyperechoic zone did not appear at the electrode tip within 10 s, RFA power was increased up to 50 W in 10 W steps. If the patient did not tolerate pain during ablation, the power was reduced or turned off for several seconds.
Per-ablation assessment
Power output, ablation duration, total applied energy in Joules (J) and energy/nodular volume in J/mL were recorded. Patients were asked periodically what their pain level was, on a verbal analogue scale from 1 to 10 and the maximum pain level was noted down. Voice was tested by asking the patient to talk regularly and any modification was recorded. All other complications (voice problem, hematomas, Horner’s syndrome, skin burn, etc.) and side effects were also recorded and graded according to Mauri et al. [Citation21]. At the end of the procedure, total disappearance of the vascularity of the nodule was systematically checked with color Doppler and microvascular imaging.
Post-ablation assessment
All patients were followed-up at 6 months after treatment. Clinical success on pressure symptoms and esthetic complaints was rated as absent, partial or complete. Technique efficacy (TE) was defined as a VRR ≥50% [Citation21]. The volume reduction ratio (VRR) was calculated by applying the following formula: (initial volume – final volume) × 100/initial volume. Ablated volume was defined as the area become hypoechoic, totally avascular on color Doppler and microvascular Imaging, even when increasing the gain at a level when noise appeared, and with diffuse increased stiffness. The ablation ratio (AR) was the ratio of the ablated volume to the total remaining volume [Citation15,Citation16]. It was calculated using a general volume calculation formula: V=π×w × d × l/6 (w is the width, d is the depth, and l is the length). However, in some cases, measuring these distances was difficult because the margins of the ablated area were not well-defined or were irregular. In those cases, the closest approximation was measured to estimate the correct volume, and the mean value was used by repeating measurements more than twice.
The 90 cases were ordered chronologically, from the earliest to the latest procedure date. Patients were divided into 3 groups according to their chronological treatment rank: patients 1–30 (G1), 31–60 (G2) and 61–90 (G3). Patients of subgroups G2 and G3 were further subdivided according to nodular volume (≤30 ml and >30 ml).
Statistical analysis
Continuous variables were expressed as median with interquartile range. Median values were compared using Mood’s test. Differences between groups 1, 2 and 3 in clinical symptoms reduction, TE, VRR and AR were assessed. Differences in continuous variables were evaluated by the Mann–Whitney U-test when comparing two groups and the Kruskal–Wallis test when comparing the three groups simultaneously. Differences in all categorical variables between 3 groups were compared with Fisher’s exact test. To search for possible explicative factors, Spearman’s correlation test was performed between on the one hand technique efficacy, volume reduction ratio, ablation ratio and on the other hand energy applied per volume. A multivariable analysis of factors predicting volume reduction ratio (VRR) and ablation ratio (AR) was performed to identify factors that were independently predictive of efficacy. Variables entered into the model included age, sex, pretreatment nodule volume and mean delivered energy per mL. p values ≤.05 were considered to indicate statistically significant differences. The analysis was performed by using Excel® Data Analysis Package and the R Project package®.
Results
Pre-ablation assessment
Ninety nodules in 90 patients underwent a first session of RFA between January 2017 and January 2020. Patient’s demographic, clinical and US characteristics are summarized in . There was no significant difference in nodular median initial volume between the three groups (p = .95). TSH was ≤0.6 µIU/mL in 13 patients (none with a TSH <0.1) and iodine scintigraphy showed an autonomous nodule in two cases. In three patients (number 5, 7 and 3 of groups 1, 2 and 3, respectively) there were neither pressure symptoms, nor esthetic complaints but a nodule measuring more than 4 cm with a proven growth on US. These patients had been referred to surgery and refused it. There were 9 large nodules (>30 ml) in group 1, 7 in group 2 and 11 in group 3.
Table 1. Patient’s demographic, clinical and US characteristics.
Per-ablation assessment
Median energy, median delivered energy per mL, maximum power output and ablation duration were significantly higher in group 2 versus group 1, but did not differ between groups 2 and 3. On the contrary, there was no significant difference in vascularity suppression at the end of the procedure between the three groups ().
Table 2. Per ablation assessment.
Post-ablation assessment
Clinical efficacy
No significant differences were observed between the three treated groups for pressure symptoms and esthetic complaints reduction (). Most patients (87.5%) experienced complete reduction of their pressure symptoms, but only half of them for esthetic complaints.
Table 3. Post ablation assessment.
Effectiveness based on US criteria
In the whole series, TE was reached in 78.9% of cases, median VRR and AR were 61% and 28%, respectively and mean VRR and AR 61 ± 14.4% and 45.7 ± 35.1% ( and ). A significant improvement in TE, VRR and AR was found between groups 1 and 2, but not between groups 2 and 3. In the subgroup of small and medium nodules (≤30 ml), the same observations were made in TE and VRR, but AR did continue to improve between groups 2 and 3 (). In large nodules, TE only improved between groups 1 and 2, whereas it also improved between groups 1 and 3 in VRR and AR. Of note, in group 3 AR reached a median value of 94.4% in small and medium nodules, but did not exceed 34.6% in large ones. TE, VRR and AR were significantly higher in small to medium nodules than in large ones (p = .03, p = .0001 and p = .01, respectively).
Figure 1. Six months follow-up volume reduction ratio (VRR) (vertical axis in %) against case number (horizontal axis), showing moderate increase of VRR over time.
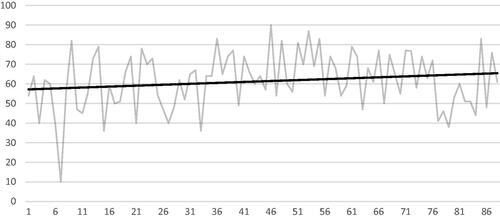
Figure 2. Six months follow-up of ablation ratio (AR) (vertical axis in %) against case number (horizontal axis), showing marked increase of AR over time.
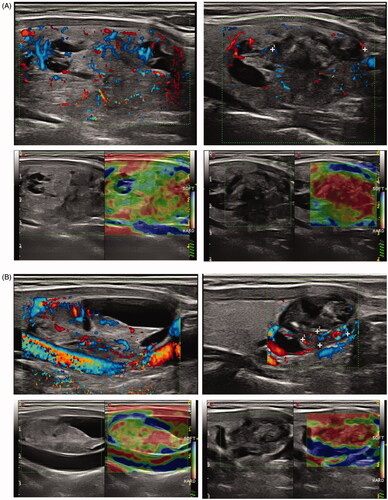
Figure 3. (A) Example of incomplete ablation in longitudinal plane. Left part of picture shows nodule before treatment (upper picture: color Doppler, lower picture: strain elastography) with moderate central vascularity and low heterogeneous stiffness. The right part of the picture shows the same nodule after treatment (same techniques). The upper and inferior part of the nodule has turned hypoechoic, avascular and with high stiffness, corresponding to the ablated volume, representing only a fraction of the remaining volume. (B) Example of nearly complete ablation in longitudinal plane. Left part of picture shows nodule before treatment (upper picture: color Doppler, lower picture: strain elastography) with moderate central vascularity and low heterogeneous stiffness. The right part of the picture shows the same nodule after treatment (same techniques). Nearly the entire nodule has turned hypoechoic, avascular and with high stiffness, corresponding to the ablated volume, representing the main fraction of the remaining volume.
Possible explicative factors
Energy applied per volume was positively correlated to VRR and AR (correlation coefficient 0.28 and 0.37 and p value .007 and .003, respectively) but not to TE (p = .1) ( and ). Ablation duration was associated with a greater reduction of AR (correlation coefficient 0.38 and p = .0002), but there was no significant correlation with TE and VRR (p = .3 and p = .29, respectively). Results of the multivariable analysis are summarized in . Two factors were independently associated with VRR and AR. Nodular baseline volume was negatively associated with these two criteria and energy delivered per ml positively associated. Time trend correlation showed a significant increase in delivered energy per volume and ablation duration with experience (correlation coefficient 0.52 and 0.57, respectively and p value <.001 for both criteria) ().
Figure 4. Correlation analysis between delivered energy per volume (horizontal axis in J/mL) and volume reduction ratio (VRR in %), showing moderate increase of VRR with delivered energy.
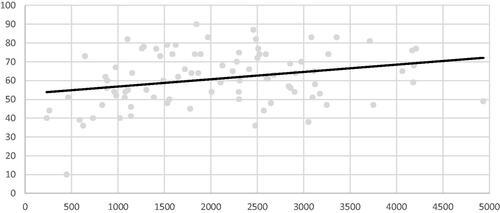
Figure 5. Correlation analysis between delivered energy per volume (horizontal axis in J/ml) and ablation ratio (AR) at 6 months follow-up in %, showing marked increase of AR with delivered energy per volume.
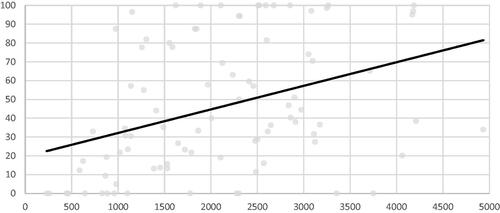
Figure 6. Time tendency curves of energy delivered per mL (red curves, left scale in J/mL) and RFA application time (blue curves, right scale in min) showing increasing values of these two parameters with time-experience. Horizontal axis: case number.
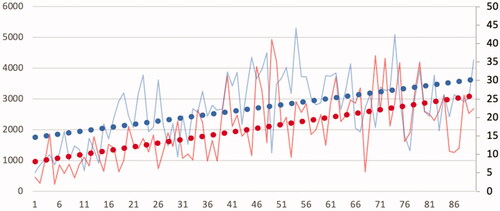
Table 4. Multivariable analysis of factors predicting volume reduction ratio (VRR) and ablation ratio (AR).
Complications
Three minor complications were observed. A transient vocal cord palsy was recorded in patient n°79 (group 3), due to recurrent laryngeal nerve injury. The complication occurred at the end of the procedure and the electrode was near the danger zone. It was immediately treated by hydrodissection with rapid voice improvement. The nodule occupied the whole right thyroid lobe and measured 65 ml. Power used was 50 W. Vocal cord palsy was proven by vocal cord examination. The patient recovered totally at the 4-month follow-up visit, as assessed clinically and by vocal cord examination. The second minor complication was a nodular rupture (patient n°41, group 2) which was detected one month after RFA as a red cervical lump with a fluid collection issued from the nodule through the muscle plane up to the skin on US, with no fever or biological disorders. It recovered totally and spontaneously after 4 months. Retrospective assessment lead to suppose that the electrode tip went across the nodular capsule during ablation. The third minor complication was a vaso-vagal reaction forcing to interrupt the procedure (patient no. 73, group 3), treated with atropine. There were side effects in 12 cases consisting of 11 small hematomas (2 in group 1, 5 in group 2 and 4 in group 3), necessitating only conservative treatment (short manual compression) and another short vaso-vagal reaction (patient n°34). All patients were discharged in the afternoon following the procedure, with a prescription of paracetamol if needed and were contacted by phone on the evening of the day following the procedure to check for the absence of delayed complications. No time correlation was detected between these complications and a learning curve in the 90 patients.
Discussion
Several studies have demonstrated that US-guided RFA is a safe and clinically effective procedure for the treatment of BTN [Citation1–8], with a 62–85% VRR at one-year follow-up after a single RFA session. None of these has, however, addressed specifically the issue of the learning curve. However, a large heterogeneity in results has been shown in the literature [Citation24,Citation25], and the experience of the operator could be one of the underestimated explanatory factors. In this report, the first 90th RFA procedures in treating BTN of a single operator were studied in terms of clinical efficacy, technique efficacy, volume reduction ratio and ablation ratio. The main results of our study is that no learning curve was demonstrated for clinical efficacy, but on the contrary clearly existed regarding TE, VRR and AR. However, after 60 patients improvement only persisted in the latter and for small to medium nodules.
Clinical efficacy
Clinical efficacy on pressure symptoms and esthetic complaints did not differ between the three groups and therefore was very rapidly acquired. It has been shown [Citation26] that a 50% volume reduction is most often sufficient to improve patients’ symptoms. In our study, a volume reduction > 50% was already present in 63% of group 1, which may explain the absence of clear improvement with experience regarding clinical parameters.
Technical efficacy, volume reduction, persistent viable volume
This report demonstrated a learning curve pattern for all parameters linked to volume reduction. TE, VRR and AR all improved between groups 1 and 2 and were stable between groups 2 and 3, indicating a rather quick learning curve. This is comparable to what was shown by Dobnig et al., who suggested that the plateau of the learning curve was reached quickly, between the 16th and the 30th patient [Citation12]. Jawad et al. reported a mean 67% volume reduction at 6 months in their initial experience for 31 nodules, to be compared to 54% and 65% in groups 1 and 2 of this series, respectively, also rather close [Citation4]. Aysan et al. reported at 6-month follow-up a reduction percentage of nodule volume of 78% and 84% for the first 10 cases and last 90 cases, respectively [Citation5]. It is not stated whether this difference was significant, but the volume of the treated nodules clearly was very different between their two groups (7 ml and 18 ml, respectively). Of note, before their prospective study, 48 cases of BTN had already been treated with RFA. In the study by Sung et al. [Citation27], it was shown that RFA of autonomous functioning nodules performed by six radiologists who were trained in thyroid RFA during a program organized by the Korean Society of Thyroid Radiology (KSThR) lead to a final volume reduction of 81.7%. As a multicenter study including five institutions, it validated the concept that RFA performed by well-trained radiologists could provide reproducible results. In their study, Jung et al. stated that radiologists considered as experienced in the field and complying with the training programs of the KSThR should have performed at least 50 procedures [Citation6]. Deandrea et al. also published a randomized controlled trial reporting the efficacy of RFA in non-autonomous TN and comparing the results of two centers [Citation1]. The Korean center (the ‘more experienced group’ in this field) had an experience of about 3000 cases of thyroid RFA; the Italian center (the ‘less experienced group’ in this field) had a significant experience in interventional US-guided therapies (both PEI and RFA by other devices) and had previously treated 50 cases of BTN with the moving-shot technique, after an initial instruction given by a Korean radiologist. No significant difference in volume reduction was seen at 6-month evaluation between the two groups (Korean group: 77% vs. Italian group: 66%).
All in all, our report and all experiences derived from this literature study are consistent with the hypothesis that the volume reduction ratio is stable after a learning curve of 50–60 procedures. Up to now, there were until our study, and to our knowledge, no data regarding the ablation ratio learning curve. We have shown that the AR also improves between G2 and G1. To determine, if an improvement could still exist after 60 treated patients, nodules were further subdivided into large ones (>30 ml) and small and medium sized (≤30 ml).
In nodules ≤30mL, technical success and VRR still did not differ between groups 2 and 3. However, a very significant difference was observed for AR, with a value of 13.7%, 57.1% and 94.4%, in groups 1, 2 and 3 respectively. This means that volume reduction is quite rapidly mastered, but that more complete ablation with the moving shot technique takes more time to acquire. This indicator of AR, first described by Sim et al. [Citation15,Citation16], is all the more important that it has been shown and confirmed to be a risk marker for regrowth [Citation17–19]. Therefore, the strategy of minimally invasive treatment should attempt to complete ablation of the entire nodule. The amount of the undertreated portion is dependent on the operator’s proficiency, and a considerable under-treated remnant can be a source of recurrence.
Regarding large (>30 mL) nodules, an improvement was observed between groups 3 and 1 in AR and it could be speculated that these nodules require even a larger experience than 90 cases to learn how to ablate them more completely. The significant difference of TE and VRR between small to medium and large nodules in the whole series and in the three consecutively treated subgroups is in favor of this hypothesis. These results were also confirmed by our multivariable analysis, which confirmed that nodular baseline volume was an independent predictive factor of VRR and AR. This has practical implications, as nodules measuring more than 30 mL should be avoided by beginning practitioners in the field, who may rather start with 10–20 mL nodules.
Thus, VRR and AR, could be some of the criteria used by a practitioner for self-assessment.
Potential explicative factors for the efficacy learning curve
Time trend correlations have shown a significant increase in delivered energy per volume with experience. It also has been demonstrated previously that there is a strong correlation between the delivered energy per mL and TE and VRR [Citation28]. By multivariable analysis, energy delivered per volume was confirmed as an independent factor of VRR and AR. Therefore, part of the learning curve was related to learn how to increase the deposited energy, by sufficiently waiting for bubbles to appear or later for a steep increase of impedance, before treating the next area. Solely did AR improve between groups 3 and 2 whereas delivered energy per volume, ablation duration and power output were not different between these two groups. It is very likely that mastering RFA to treat the margins of the nodule and its deep parts is more difficult and has its own learning curve.
Tolerability and safety
No patients required hospitalization or experienced permanent adverse sequelae. Therefore, there were no major complications according to the classification by Mauri et al. [Citation21]. The total number of mild complications (transient vocal cord palsy, nodular rupture, vaso-vagal reaction forcing to interrupt the procedure) was 3.3%. This is in the upper range of what has been previously published for experienced practitioners [Citation29]. In the study by Aysan et al. there was no difference in complication rates between the 10 first and last patients among a cohort of 100 patients [Citation5]. Interestingly, the three complications of this series occurred in patients number 41, 73 and 79 and not in the early treated patients. This may be due that, with the increasing experience, the operator begins to push his/her limits beyond what was attainable by that time. There were no obvious common features between the three nodules in terms of location, volume or delivered energy. Analyzing these complications confirms that special care should be taken to respect the region close to the laryngeal inferior nerve and the entry point of the electrode in the nodule, which should not be heated. Regarding side effects, there were only minor hematomas with no obvious relation with experience and another short vaso-vagal reaction.
Limitations
This report was a retrospective study. It is based on the experience of a single radiologist. Thus, it could be of value to compare it with other operator’s learning curve, with different backgrounds, to determine if there really is a common learning pattern. However, comparison with the literature shows similarities. Regarding immediate and delayed appreciation of ablated tissue volume, use of CEUS might increase the detection of incomplete treatment [Citation17–19]. However, it has been shown a considerable similarity in terms of findings of power Doppler US and CEUS imaging for thyroid nodule [Citation30], probably even better when microvascular imaging is used. CEUS was not used in this study as it has not gained State approval for use in thyroid imaging.
As a conclusion, this study has shown that there is a learning curve in RFA for the treatment of BTN, which involves the accumulation of experience in delivering the energy and completeness of treated volume. RFA is clinically effective since the beginning of learning, especially if starting with small to medium-sized nodules. After 60 nodules optimal technical efficacy (VRR > 50%) is reached. Increasing experience furthermore allows to increase the ablation ratio, hoping to reduce the recurrence rate and the need for a second RFA session. Last, evaluation of residual viable tissue during treatment may be a way for further optimization of the RFA session. CEUS and or microvascularity assessment might increase the detection of incomplete treatment but more studies are needed. With adequate experience, RFA can be used to ablate BTN with low morbidity and a high complete ablation rate. Practitioners could use the volume reduction ratio and ablated volume ratio to assess their progression in the RFA learning curve.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available but can be provided by the corresponding author on reasonable request.
References
- Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25(8):890–896.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Jawad S, Morley S, Otero S, et al. Ultrasound-guided radiofrequency ablation (RFA) of benign symptomatic thyroid nodules – initial UK experience. BJR. 2019;92(1098):20190026.
- Aysan E, Idiz UO, Akbulut H, et al. Single-session radiofrequency ablation on benign thyroid nodules: a prospective single center study: radiofrequency ablation on thyroid. Langenbecks Arch Surg. 2016;401(3):357–363.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Ben Hamou A, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules? Int J Hyperthermia. 2019;36(1):666–676.
- Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;104(9):3751–3756.
- Kim J-H, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015;18(4):423–430.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association Clinical Practice Guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Dobnig H, Amrein K. Monopolar radiofrequency ablation of thyroid nodules: a prospective Austrian single center study. Thyroid. 2018;28(4):472–480.
- Kassite I, Bejan-Angoulvant T, Lardy H, et al. A systematic review of the learning curve in robotic surgery: range and heterogeneity. Surg Endosc. 2019;33(2):353–365.
- Hatlie MJ. Climbing ‘the learning curve’. New technologies, emerging obligations. JAMA. 1993;270(11):1364–1365.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33(8):905–910.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Yan L, Luo Y, Zhang M, et al. Vital volume increase versus clinical evaluation as the indication of additional radiofrequency ablation for benign thyroid nodule: a single center retrospective study. Int J Hyperthermia. 2020;37(1):777–785.
- Yan L, Luo Y, Xie F, et al. Residual vital ratio: predicting regrowth after radiofrequency ablation for benign thyroid nodules. Int J Hyperthermia. 2020;37(1):1139–1148.
- Jiao Z, Luo Y, Song Q, et al. Roles of contrast-enhanced ultrasonography in identifying volume change of benign thyroid nodule and optical time of secondary radiofrequency ablation. BMC Med Imaging. 2020;20(1):79.
- Masson E. La recherche clinique en radiologie interventionnelle depuis la mise en place de la loi Jardé [Internet]. EM-Consulte. [cited 2020. Nov 11]. Available from: https://www.em-consulte.com/article/1245370/la-recherche-clinique-en-radiologie-interventionne.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019 May;29(5):611–618.
- Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):1–919.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653–3659.
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015;25(1):112–117.
- Deandrea M, Trimboli P, Mormile A, et al. Determining an energy threshold for optimal volume reduction of benign thyroid nodules treated by radiofrequency ablation. Eur Radiol. 2020. DOI:https://doi.org/10.1007/s00330-020-07532-y
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Caresio C, Caballo M, Deandrea M, et al. Quantitative analysis of thyroid tumors vascularity: a comparison between 3-D contrast-enhanced ultrasound and 3-D Power Doppler on benign and malignant thyroid nodules. Med Phys. 2018;45(7):3173–3184.
