Abstract
Purpose
To compare the clinical outcomes of radiofrequency ablation (RFA) versus reoperation for benign thyroid nodules that developed after previous thyroid surgery.
Methods
A total of 53 patients with 53 benign nodules developed after previous thyroid surgery were evaluated in this retrospective study. Eighteen patients were treated by RFA (RFA group) and 35 patients underwent reoperation (reoperation group). The efficacy, safety, thyroid function, blood loss, hospitalization, total treatment time, and cost were compared between the two groups.
Results
In the RFA group, the mean volume decreased significantly from 12.78 ± 17.57 ml to 0.94 ± 1.01 ml (p = 0.043) with a volume reduction rate of 85.27 ± 14.35% and significant improvement in symptom and cosmetic scores (all p = 0.001). Therapeutic efficacy was achieved with a single session in all thyroid nodules. The total treatment time (6.12 ± 3.17 min vs. 110.26 ± 44.41 min, p < 0.001), blood loss (0 ml vs. 82.58 ± 105.55 ml, p < 0.001) and hospitalization(0 days vs. 9.66 ± 4.28 days, p < 0.001) were significantly lower in the RFA group than those in reoperation group, but the costs of treatment were similar(2262.12 ± 221.54 USD vs. 2638.04 ± 1062.90 USD, p = 0.081). The incidence of complications was significantly higher in the reoperation group than in the RFA group(31.43 vs. 0%, p < 0.001). Furthermorre, 65.17% of patients developed hypothyroidism after reoperation, whereas the thyroid function of the patients in the RFA group was unaffected.
Conclusion
For patients with benign thyroid nodules developed after previous thyroid surgery, RFA can be considered as a safe and effective alternative to reoperation with advantages of maintenance of intact thyroid function and low incidence of complications.
Introduction
Thyroid nodules are common in the general population [Citation1–3]. Although most asymptomatic benign nodules with modest growth should be only followed with nonsurgical intervention, some nodules that show progressive growth may require surgery [Citation3]. Thyroid surgery is the standard treatment [Citation4]. However, some patients develop another nodule in the thyroid tissue remnants approximately 10 years after the previous surgery [Citation5]. Those with symptomatic and cosmetic problems also require reoperation. Given the distorted anatomy of the thyroid and the postoperative adhesions after previous surgery, the incidence of complications in reoperation such as recurrent laryngeal nerves (RLN) injury and hypoparathyroidism were much higher than that in initial surgery [Citation6–8]. Moreover, the partial or total removal of the thyroid tissue remnants in reoperation results in a high incidence of hypothyroidism compared with that in the initial surgery. Thus, an alternative modality that is safer, has lower complications and allows gland retention is needed.
Radiofrequency ablation (RFA) and other thermal ablation techniques have been considered as promising strategies for the treatment of benign thyroid nodules [Citation9–11]. Significant volume reduction could be obtained after ablation [Citation12–21]. Comparative studies have suggested that RFA could be used as an alternative modality to surgery for the treatment of benign thyroid nodules with local symptoms or cosmetic problems [Citation22,Citation23]. However, to the best of our knowledge, no study has compared RFA with reoperation for the treatment of benign nodules that developed after previous thyroid surgery.
Therefore, the purpose of this study was to compare the clinical outcomes of RFA versus reoperation for benign thyroid nodules that developed after previous thyroid surgery.
Materials and methods
The Institutional Review Board of our institution approved this retrospective study (Approval number: S2019-211-01). All the patients were provided written information consent before RFA or reoperation.
Patients
From May 2015 to March 2019, a total of 78 patients (75 females and 3 males, mean age 54.55 ± 12.56 years, range 14–77 years) with benign thyroid nodules that developed after previous thyroid surgery were evaluated in this study. A nodule that developed after previous thyroid surgery was defined as a new lesion in the remnant tissue or as the enlargement of the remaining thyroid tissue after previous surgery [Citation1]. All included patients met the following criteria: (1) nodule should be confirmed as benign via two separated fine-needle aspiration(FNA) or core-needle biopsy(CNB); (2) no suspicious malignant features on US; (3) thyroid nodules with solid portion over 80%; (4) underwent initial thyroid surgery for a benign thyroid nodule; (5) nonfunctioning thyroid nodules that were confirmed by thyroid scintigraphy; (6) complaint of cosmetic or symptomatic problems or concerns of nodules growing rapidly or malignant transformation. The exclusion criteria were as follows: (1) follicular neoplasm or malignancy findings on US-guided FNA or CNB; (2) nodule with benign result in biopsy but was suspected of malignancy in US; (3) contra-lateral vocal cord paralysis; and (4) coagulation disorder or serious heart failure, respiratory failure, liver failure, or renal failure.
The initially identified 78 patients were divided into two groups according to the treatment: the reoperation group (N = 56) and the RFA group (N = 23). Nodules with solid portions less than 80% (N = 2) and follow-up time less than 6 months (N = 3) were excluded. The remaining 18 patients (18 females, mean age 49.11 ± 13.84 years, range 22–74 years) with 18 benign nodules were included in the RFA group. Among the 56 patients who underwent reoperation, 35 patients (33 females and 2 males, mean age 55.83 ± 12.88 years, range 14–76 years) with 35 benign nodules were selected as the reoperation group. Flowchart of patient enrollment is shown in .
All the patients underwent laboratory tests, including complete blood count, thyroid function tests and coagulation tests. The following Information was also collected for each patient: demographics data, nodule characteristics, treatment-related variables and complications after treatments. Before treatment, each nodule underwent US to evaluate its size, location, margin, shape, echogenicity, calcification and vascularity. The volume of the thyroid nodule was calculated using the following equations: V = πabc/6 (V is the volume, while a is the largest diameter, b and c are the other two perpendicular diameters).
Before RFA, the symptom score was self-measured by patients using a 10-cm visual analogue scale (grade 0–10) [Citation9]. The cosmetic score was assessed by a physician (1, no palpable mass; 2, no cosmetic problem but palpable mass; 3, a cosmetic problem on swallowing only; and 4, a readily detected cosmetic problem) [Citation9]. The cost of RFA included the laboratory tests, RFA treatment, local anesthesia and radiofrequency needle fees. The cost of reoperation included the preoperative examination, operation, hemostatic materials and other consumables, general anesthesia, hospital bed, nursing and post-operative medication fees.
RFA procedure
All RFA procedures were performed by an experienced US physician with more than 20 years of experience in thyroid US and interventional US. A bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18-gauge bipolar RF applicator with a 0.9 cm active tip (CelonProSurge micro100-T09, Olympus Surgical Technologies Europe) were used in this study.
Patients lay on an operating table in the supine position with the neck extended. Local anesthesia with 1% lidocaine was administered. If the distance between the tumor and critical cervical structures (trachea, cervical artery, jugular vein, esophagus and recurrent laryngeal nerve, RLN) was <5 mm, the hydrodissection technique was used. RFA was performed using moving-shot technique. CEUS was performed immediately after the RFA procedure to evaluate the ablation area. If any enhancement existed, a complementary ablation could be performed. During the procedure, special attention was given to the protection of critical cervical structures to prevent significant complications such as hematoma or nerve injury. Each patient was observed for 1–2 h in the hospital, and any complication that occurred during and immediately after ablation were carefully evaluated according to the clinical signs and symptoms.
Clinical follow-ups were performed at 3, 6, 12 months and every 12 months thereafter. The ablated nodule was evaluated by US. Symptom scores, cosmetic scores and complications after RFA were evaluated at each follow-up. The volume reduction was calculated as follows: volume reduction rate (VRR)= ([initial volume-final volume] × 100%)/initial volume. Therapeutic efficacy was defined as a volume reduction of >50% at last follow-up. Regrowth was defined as an increase in nodule volume by >50% compared with the previously recorded volume [Citation24,Citation25]. Additional ablation may be considered if the nodule showed marginal regrowth or if cosmetic or symptomatic problems were incompletely resolved [Citation9].
Reoperation
Reoperation was conducted under general anesthesia by surgeons with more than 20 years of experience in thyroid surgery. The decisions regarding the extent of reoperation were determined by several factors, including the nodule location, volume, the remnant normal thyroid tissue remnants from the previous surgery and patients’ preferences. The types of reoperation for the patients were as follows: 12 patients underwent completion total thyroidectomy, 5 patients underwent subtotal thyroidectomy, 3 patients underwent lobectomy, and 15 patients underwent nodule resection only. A total of 17 and 18 patients who underwent total/subtotal thyroidectomy and lobectomy/nodule resection were categorized into the T subgroup and L subgroup, respectively.
Statistical analysis
Statistical analysis was performed using the SPSS Statistics software (Version 25.0). Continuous data were expressed as mean ± SD (range). The Mann-Whitney U test was used to compare the continuous data between the groups. The Chi-square test or Fisher’s exact test was used to compare the qualitative data between the groups. Wilcoxon signed rank tests were used to compare the mean volume, symptom and cosmetic scores before RFA and at each follow-up point after RFA. A difference with p < 0.05 was considered as statistically significant.
Results
Patient characteristics
shows the clinical characteristics of patients in the two groups. There were no significant differences between the two groups in terms of pretreatment demographic and nodule characteristics, including age, sex, interval time between initial surgery and treatment from this study, nodular volume, volume in each subgroup, mean diameter, location, echogenicity, composition, calcification, vascularity, thyroid function, cosmetic score, and symptom score.
Table 1. Clinical characteristics of patients undergoing reoperation and RFA.
Treatment outcomes
In RFA procedure, power of 3 W was used in 2 nodules; 5–6 W was used in 7 nodules; 7–8 W was used in 7 nodules and 9 W was used in 2 nodules. The mean energy was 2397.78 ± 1394.10 J (range 510–4930 J).
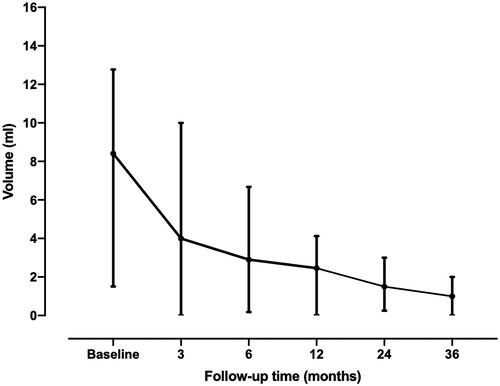
shows the volume and VRR at each follow-up period after RFA. The mean follow-up time was 26.20 ± 16.31 months (range, 8–63 months). The mean volume of the nodule decreased significantly from 12.78 ± 17.57 ml to 0.94 ± 1.01 ml with a mean VRR of 85.27 ± 14.35% (). Therapeutic efficacy was achieved with a single session in all thyroid nodules and no nodule regrowth was found. A total of two ablated nodules (11.11%) disappeared during the follow-up. At the last follow-up, the symptom scores significantly decreased from 2.89 ± 2.39 to 0.78 ± 1.17(p = 0.001), and the cosmetic scores significantly decreased from 2.39 ± 1.20 to 1.39 ± 0.61(p = 0.001). shows a representative case before and after RFA.
Figure 3. US images of a solid thyroid nodule developed after previous thyroid surgery. (A) The longitudinal US showed a benign nodule located in the left lobe. (B) At 3 months after RFA, the volume reduction rate was 67.71%. (C) At 12 months after RFA, the volume reduction rate was 72.99%.
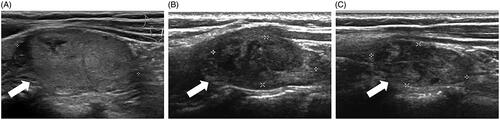
Table 2. Changes of volume and VRR at each follow-up in RFA group.
Comparison
shows the treatment-related variables, complications, and hypothyroidism in the two groups. The total treatment time (6.12 ± 3.17 min vs. 110.26 ± 44.41 min, p < 0.001), blood loss (0 ml vs. 82.58 ± 105.55 ml, p < 0.001), and duration of hospitalization (0 days vs. 9.66 ± 4.28 days, p < 0.001) were significantly lower in the RFA group than those in the reoperation group (all p < 0.001). Although the treatment cost was higher in the reoperation group, the difference was not significant (2262.12 ± 221.54 USD vs. 2638.04 ± 1062.90 USD, p = 0.081).
Table 3. Treatment-related variables, complications and Hypothyroidism of the Reoperation group versus RFA group.
In the subgroups of reoperation, the total treatment time in the T subgroup was significant longer than that in the L subgroup(127.06 ± 46.12 vs. 94.39 ± 37.33, p = 0.029). No significant differences were found in the blood loss, hospitalization and cost in the two subgroups (all p > 0.05).
The incidence rates of complications in the reoperation group and RFA group were 31.43% and 0%, respectively (p < 0.001). In the reoperation group, 24 patients (68.57%) had local pain after treatment, which resolved spontaneously before leaving the hospital. Two patients (5.71%) had transient hypoparathyroidism and recovered within one week. A total of seven patients had transient voice or swallowing change. Three of them only had voice change (3.57%) and refused laryngoscopy. They recovered spontaneously within one month. The other four patients (11.42%) had transient RLN injury diagnosed by laryngoscopy. They were treated with dexamethasone, and the injury resolved within three to six months. Meanwhile, two patients had permanent RLN injury by laryngoscopy (5.71%). No significant differences were found regarding complications in the two subgroups. In the RFA group, all patients tolerated the RFA procedure. Only seven patients (38.89%) experienced local pain after RFA treatment, which resolved spontaneously within two to three days.
The final pathology in the reoperation group showed that 8 thyroid adenomas, 6 follicular adenomas and 21 nodular goiters. After reoperation, 26 patients had hypothyroidism and needed thyroid hormone supplementation. However, three patients already had hypothyroidism and were on levothyroxine before reoperation. Therefore, a total of 23 (65.71%) patients had hypothyroidism because of the reoperation, whereas thyroid function was not affected in patients after RFA (p < 0.001). The incidence of hypothyroidism in the T subgroup was significantly higher than that in the L subgroups(94.12 vs. 38.89%, p < 0.001).
Discussion
This study compared the clinical outcomes of RFA versus reoperation for patients with benign thyroid nodules that developed after previous thyroid surgery. This study showed a high percentage of volume reduction in nodules treated with RFA. The therapeutic efficacy rate was 100%, and all nodule-related symptom and cosmetic problems were clinically improved. Moreover, thyroid function was well-maintained after RFA, with no patient developing hypothyroidism. However, reoperation was associated with a higher incidence rate of complications, and 65.71% of the patients who underwent reoperation developed hypothyroidism and needed thyroid hormone supplementation. The costs of treatment were similar, but the total treatment time, blood loss, and duration of hospitalization were significantly lower in the RFA group than those in reoperation group.
For the last few years, RFA has been recommended as an effective treatment for benign thyroid nodules with significant volume reduction and clinical improvement of local symptoms or cosmetic problems [Citation9–11]. In a recent meta-analysis, the VRR for benign thyroid nodules at 6,12 and 24 months after RFA was 68, 75 and 87, respectively [Citation16]. Ha et al. [Citation26] also reported that the mean volume of benign nodules in patients with previous lobectomy significantly decreased from 9.7 ml to 2.8 ml after RFA, with a mean VRR of 87.2%. Similar results were obtained in this study despite the larger initial volume (12.78 ml). These results indicated that the efficacy of RFA for medium-sized benign nodules was not be affected by the postoperative adhesions and distorted anatomy from previous thyroid surgery.
Although reoperation is the standard treatment modality for symptomatic benign nodules that developed after previous thyroid surgery, it is associated with a high incidence of complications from 21.34 to 47.37%, because of the normal tissue plane distortion and scar formation from the previous surgery [Citation6,Citation7,Citation27,Citation28]. RLN injury is the most major complication, and its incidence after reoperation has been reported to be higher than that in the initial surgery [Citation29]. The current study found that, the incidence of transient and persistent RLN injury in the reoperation group was 11.42 and 5.71%, respectively. Although the incidence was higher than that in regular thyroid surgery, it was consistent with the findings of previous studies [Citation6,Citation28], which reported that the incidence rates of transient RLN injury and permanent RLN injury were 0–22% and 0–13% in the thyroid reoperation, respectively. It was not only associated with the distorted anatomy of the thyroid and postoperative adhesions from initial surgery but also with other situations, such as clamping, stretching, ischemia or the injury due to electric surgical knives during the reoperation procedure. By contrast, no patient experienced RLN injury in the RFA group. It was consistent with the findings of a recent meta-analysis that reported an incidence rate of 1.44% after RFA [Citation30]. Several reasons were related to the low incidence of RLN injury in RFA, including real-time US imaging, experienced US physicians and safe, dedicated ablative techniques such as the moving-shot technique and hydrodissection technique [Citation9,Citation31].
Hypoparathyroidism is another major complication of reoperation. Hypocalcemia due to hypoparathyroidism could lead to serious problems. Medas et al. [Citation6] reported markedly higher incidence rates of transient hypoparathyroidism (56.6 vs. 25.9%) and permanent hypoparathyroidism(10 vs. 2%) in the reoperation than those in the initial surgery. However, hypoparathyroidism after RFA has not been reported to date [Citation29]. In this study, hypoparathyroidism occurred in 5.71% of the patients in the reoperation group, but no patient in the RFA group developed hypoparathyroidism. Thus far, no reliable marker that is predictive of postoperative hypocalcemia has been identified [Citation32]. The most practical approaches include the identification the parathyroid glands and the careful protection of their vascular supply [Citation32]. However, owing to the varied location of the parathyroid gland and the distorted anatomy after the previous surgery, these strategies were not always possible [Citation33].
RFA could not only reduce the rate of complications, but also preserve the thyroid function. In this study, 65.71% of patients had hypothyroidism after reoperation, but the thyroid function of patients in the RFA group was well-maintained. During RFA, only the targeted nodule was ablated, and most normal thyroid tissues were spared, thus reducing the injury to normal thyroid tissue remnants. By contrast, because of the different volumes of the newly developed nodules and the different sizes of the normal thyroid tissue remnants left by the previous surgery, reoperation involved nodule resection or total thyroidectomy. It could lead to hypothyroidism, which is related to life-long thyroid hormone supplementation and adverse effects on the bones and the cardiovascular systems [Citation2,Citation4,Citation34].
As a minimally invasive technique, RFA only needed local anesthesia in the outpatient department with no hospitalization, less treatment time and no blood loss, thus leading to lower operative-related risks for patients with a history of surgery. To the best of our knowledge, this is the first study to compare RFA with reoperation for the treatment of benign nodules that developed after previous thyroid surgery. Reoperation is undoubtedly the definitive solution to a symptomatic thyroid nodule, which can not only totally remove the nodule but also allow for the final pathology [Citation35]. However, by comparison, RFA has many advantages over reoperation, including a less invasive procedure, fewer complications and well preservation of thyroid function. Therefore, RFA could be a promising treatment for patients with benign thyroid nodule that developed after a previous surgery.
This study has some limitations. First, it was a single-center retrospective study. Thus, the possibility of selection bias could not be eliminated. Future prospective studies or randomized controlled trials are needed. Second, the sample size was small and, particularly for patients with large nodules. Third, the surgical techniques used in the reoperation were different. Although the reoperation group was further divided into two subgroups on the basis of the reoperation type and although the treatment-related variables and complications were reported separately, bias could not be completely avoided. Moreover, because of the insurance policy, some surgery-related variables, such as hospitalization in Asian countries, may be different from those in Western countries. Fourth, the follow-up time was relatively short. Nodules treated with RFA tended to regrow slowly, often within at least two or three years after the RFA [Citation36]. Long-term outcome comparisons should be compared in the future. Fifth, although the nodules in the RFA group all underwent two separated FNAs or CNBs, a false negative result or presence of occult microcarcinoma could not be completely excluded.
In Conclusion, for patients with small to medium-size benign thyroid nodules that developed after previous thyroid surgery, RFA can be considered as a safe and effective alternative to reoperation with advantages of maintenance of intact thyroid function and low incidence of complications.
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Funding
Reference
- Zatelli MC, Lamartina L, Meringolo D, et al. Thyroid nodule recurrence following lobo-isthmectomy: incidence, patient's characteristics, and risk factors. J Endocrinol Invest. 2018;41(12):1469–1475.
- Gharib H, Papini E, Paschke R, et al. American association of clinical endocrinologists, associazione medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J. Endocrinol. Invest. 2010;16(Suppl 1):1–50.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a mayo clinic case series. Mayo Clin. Proc. 2018;93(8):1018–1025.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Phitayakorn R, McHenry CR. Follow-up after surgery for benign nodular thyroid disease: evidence-based approach. World J Surg. 2008;32(7):1374–1384.
- Medas F, Tuveri M, Canu GL, et al. Complications after reoperative thyroid surgery: retrospective evaluation of 152 consecutive cases. Updates Surg. 2019;71(4):705–710.
- Lefevre JH, Tresallet C, Leenhardt L, et al. Reoperative surgery for thyroid disease. Langenbecks Arch Surg. 2007;392(6):685–691.
- Terris DJ, Khichi S, Anderson SK, et al. Reoperative thyroidectomy for benign thyroid disease. Head Neck. 2010;32(3):285–289.
- Kim JH, Baek JH, Lim HK, et al.; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Guang Y, He W, Luo Y, et al. Patient satisfaction of radiofrequency ablation for symptomatic benign solid thyroid nodules: our experience for 2-year follow up. BMC Cancer. 2019;19(1):147.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Tang X, Cui D, Chi J, et al. Evaluation of the safety and efficacy of radiofrequency ablation for treating benign thyroid nodules. J Cancer. 2017;8(5):754–760.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J. Clin. Endocrinol. Metab. 2015;100(2):460–466.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19(3):219–225.
- Faggiano A, Ramundo V, Assanti AP, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J. Clin. Endocrinol. Metab. 2012;97(12):4439–4445.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16(4):361–367.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label. Thyroid. 2020;30(6):847–856.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321–1325.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33(8):905–910.
- Ha EJ, Baek JH, Lee JH, et al. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid. 2013;23(3):289–293.
- Pelizzo MR, Variolo M, Bernardi C, et al. Complications in thyroid resurgery: a single institutional experience on 233 patients from a whole series of 4,752 homogeneously treated patients. Endocrine. 2014;47(1):100–106.
- Hardman JC, Smith JA, Nankivell P, et al. Re-operative thyroid surgery: a 20-year prospective cohort study at a tertiary referral centre. Eur Arch Otorhinolaryngol. 2015;272(6):1503–1508.
- Sun W, Liu J, Zhang H, et al. A meta-analysis of intraoperative neuromonitoring of recurrent laryngeal nerve palsy during thyroid reoperations. Clin Endocrinol (Oxf)). 2017;87(5):572–580.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine. 2014;47(2):537–542.
- Ertaş B, Veyseller B, Karataş A, et al. Hypoparathyroidism in total thyroidectomy due to benign thyroid diseases. Clin Ther. 2018;40(5):762–767.
- Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123(3):372–381.
- Bernardi S, Dobrinja C, Carere A, et al. Patient satisfaction after thyroid RFA versus surgery for benign thyroid nodules: a telephone survey. Int J Hyperthermia. 2018;35(1):150–158.
- Sim JS, Baek JH. Long-term outcomes following thermal ablation of benign thyroid nodules as an alternative to surgery: the importance of controlling regrowth. Endocrinol Metab. 2019;34(2):117–123.