Abstract
Objective
To evaluate the clinical outcomes of transvaginal ultrasound-guided radiofrequency ablation (RFA) combined with a levonorgestrel-releasing intrauterine system (LNG-IUS) for the treatment of symptomatic uterine adenomyosis.
Methods
Patients with symptomatic uterine adenomyosis treated with ultrasound-guided RFA in combined with an LNG-IUS from January 2013 to January 2016 and followed up for 3 years after treatment were selected. Assessment endpoints included the uterine volume reduction rate, dysmenorrheal score, symptom severity score and adverse events.
Results
Among the 72 patients, 64 completed the 3-year follow-up evaluations after treatment. No LNG-IUS expulsion was reported. Dysmenorrhea and symptom severity scores statistically significantly declined after the combined treatment of RFA and LNG-IUS was administered. The uterine volume significantly decreased, and the average reduction rate was 55%.
Conclusion
Ultrasound-guided RFA combined with an LNG-IUS might be a simple, safe and effective alternative for the treatment of symptomatic adenomyosis.
Adenomyosis is a common gynecological condition. The most common signs and symptoms include heavy menstrual bleeding, secondary dysmenorrhea and an enlarged uterus (which may produce pressure symptoms of the bladder or the bowel) [Citation1]. Currently, several minimally invasive treatments are used to treat adenomyosis in clinical practice. However, all of these treatment options have limitations, such as adverse events, insufficient efficacy or a high recurrence rate [Citation2]. Among the various options, hysterectomy remains the ‘gold standard’ treatment for adenomyosis, especially for severe adenomyosis [Citation3].
As a new conservative therapy, ultrasound-guided (US-guided) radiofrequency ablation (RFA) is a minimally invasive, safe, and effective treatment for symptomatic uterine adenomyosis [Citation4–6]. It can be performed as an outpatient procedure without general anesthesia and previous studies have reported that it can provide symptomatic relief and uterine volume reduction [Citation4–6]. However, our previous study also showed that approximately 20% of patients had recurrences after ablation [Citation4]. Therefore, measures are needed to effectively prevent recurrence in the long term after RFA for adenomyosis treatment.
The levonorgestrel-releasing intrauterine system (LNG-IUS) is an effective, reversible, and long-term treatment for adenomyosis [Citation7]. Although the expulsion rate is relatively high due to an enlarged uterus or heavy regular bleeding, numerous studies have shown that it reduces menstrual bleeding, dysmenorrhea and uterine volume and has an overall satisfaction rate of 72% [Citation8–10].
This study was performed to investigate the long-term efficacy and safety of transvaginal US-guided RFA combined with an LNG-IUS for the treatment of symptomatic uterine adenomyosis.
Materials and methods
Participants
This retrospective study was approved by the institutional review board of the Chinese PLA Rocket Force General Hospital, and all enrolled patients signed informed consent forms for the treatment and for any future use of the data collected.
The selection criteria for this study were as follows: (1) adenomyosis-related symptoms, e.g. dysmenorrhea, heavy menstrual flow or bulk pressure; (2) no desire for future childbirth; (3) declined to undergo surgery; (4) normal liver and kidney functions; (5) normal coagulation function and (6) normal ThinPrep cytology test results.
The exclusion criteria included pregnancy or lactation, suspected pelvic inflammatory disease, concomitant fibroids, breast cancer, suspected malignancy, ovarian cyst or other treatment for adenomyosis within the 3 months before the study.
US-guided RFA procedure
The radiofrequency generator used in this study (Ban Bian Tian Medical Apparatus and Instruments Company, Xi An City, China) was operated at 50 Hz with a transmitting power of 300 W. The radiofrequency electrode used (Ban Bian Tian Medical Apparatus and Instruments Company) was 35 cm long with a 1.5 cm exposed distal tip. The output energy was set at 30–50 W.A. The procedure was performed under intravenous conscious sedation. Patients were placed in the lithotomy position.
A radiofrequency electrode was introduced transvaginally under transabdominal ultrasound guidance. The electrode was penetrated through the endometrium and was inserted into the lesion. Once the hyperechogenic signal covered nearly 80–90% of the entire lesion on real-time ultrasound, the ablation was discontinued [Citation4]. Absence of vascularization was confirmed at the end by contrast-enhanced ultrasound. All patients received oral antibiotic prophylaxis for 3 days after the treatment.
LNG-IUS implantation
Ultrasound was performed before and after RFA to evaluate the uterus size. Patients with uterine lengths of less than 9 cm determined by ultrasound were implanted with the LNG-IUS (Mirena, BayerAg, Germany) [Citation11]. Positioning of the LNG-IUS was then determined by ultrasound after implantation.
Outcome measures
Ultrasound was performed immediately before RFA (baseline), 3 and 6 months after RFA, prior to LNG-IUS implantation and at 3 months, 6 months and every year thereafter the implantation (). The uterine volume was evaluated by ultrasound and calculated using the following formula: 0.5233 × superoinferior diameter × anteroposterior diameter × transversal diameter.
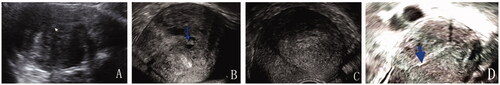
Figure 1. (A) Transabdominal sonographic image before treatment. The enlarged uterus (uterine length = 11 cm) with the adenomyosis affected the posterior uterine wall (arrow = endometrium); (B) Transvaginal sonographic image 3 months after the ablation (arrow = the secondary cystic changes); (C) Transvaginal sonographic image 6 months after the ablation. The uterine size was appropriate for the implantation of the LNG-IUS and the LNG-IUS was implanted. (D) Transvaginal sonographic image 3 years after the procedure (arrow = the bottom of the LNG-IUS). The uterus returned to its normal size.
Venous blood was extracted from all patients before and after ablation. Hemoglobin levels were assessed only after the procedure and at procedure 3 and 6 months post procedure. A hemoglobin level < 110 g/L was considered to reflect anemia.
All patients underwent a thorough clinical evaluation that included dysmenorrhea symptom severity(visual analog scale, VAS) and symptom severity score(SSS) questionnaires including eight items: heavy bleeding during the menstrual periods, passing blood clots during the menstrual periods, fluctuations in the length of the monthly cycle compared to the previous cycles, fluctuations in the duration of the monthly cycle compared to the previous cycles, feeling tightness or pressure in the pelvic area, frequent urination during the daytime or nighttime, and experiencing fatigue [Citation12]. The VAS ranged from 0 to 10. The SSS score ranged from 0 to 100.
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 26.0; SPSS Inc., Chicago, IL). Means were compared by t-tests, and proportions were compared by chi-square or Fisher’s exact tests. Paired t-tests were used to analyze changes in each score from baseline. A value of p < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 73 women with symptomatic uterine adenomyosis were treated by US-guided RFA combined with the LNG-IUS from January 2013 to January 2016.Nine patients with incomplete data were excluded from this study. The mean age was 39.2 ± 4.9 years, the mean BMI was 22.5 ± 2.9 kg/m2 and the mean preoperative CA125 level was 81.24 ± 46.56 U/ml ().
Of 64 individuals, 51 suffered from dysmenorrhea. Forty-two individuals had heavy menstrual bleeding, 15 patients suffered from anemia, 6 patients had a history of LNG-IUS expulsion, and 18 patients had severe adenomyosis according to the following clinical criteria: an enlarged uterus (> 12 weeks gestation); heavy menstrual bleeding and/or dysmenorrhea; and ultrasound revealed that adenomyosis affected the outer 2/3 of the myometrium [Citation13–14].
The mean uterine volume before ablation was 307.0 ± 183.1 cm3, with uterine anteposition, retroposition and mesoposition in 33, 25, and 6 women, respectively. Adenomyosis affected the posterior uterine wall in 43 patients (66.2%), anterior uterine wall in 25 patients (39.0%) and fundal uterine wall in 8 patients (12.5%) (). Ten patients had an enlarged uterus (>12 weeks gestation) at baseline, and the uterine length ranged from 10.5 cm to 12.2 cm.
Technical feasibility and efficacy
The mean ablation time was 31.5 ± 7.2 min (range 10–46 min). The mean number of punctures ranged from 1 to 4.
Thirty-eight patients underwent intraoperative LNG-IUS implantation immediately after ablation. Of the remaining 26 patients, 8 patients received implantation 1 month after ablation, 6 patients received implantation 3 months after ablation, and 8 patients received implantation 6 months after ablation. No LNG-IUS expulsion was reported at the study endpoint, including 6 patients who had a history of LNG-IUS expulsion before the study.
Safety
There were no intraoperative complications. Adverse reactions reported during follow-up included vaginal discharge (64 cases, 100%), amenorrhea (23 cases, 36.5%), lower abdominal pain (17cases, 26.5%), abnormal uterine bleeding (13 cases or 20.3%, which disappeared within 3–6 months), and weight gain >2 kg (11 cases, 17.1%). In addition, 15 patients complained of vaginal discharge lasting over 2 weeks and they were given oral antibiotic prophylaxis for 3 days to prevent infection. The longest vaginal discharge lasted 31 days. The remaining 49 patients presented with vaginal discharge lasting 1–14 days. All adverse events were classified as grade A or B according to the unified standardized Society of Interventional Radiology (SIR) grading system. Side effects of LNG-IUS implantation were well tolerated in this study.
Treatment effectiveness
The uterine volume was significantly reduced after RFA.Compared with the mean baseline uterine volume, the volume reduction rate was 48.9% at 3 months, and 57% at 12 months after the ablation and then the volume slightly decreased (). The average reduction rate was 55%.
In the 15 patients combined with anemia before treatment, their hemoglobin levels were significantly increased at 3 and 6 months after ablation (p < 0.05), and reached approximately normal levels.
The dysmenorrhea score decreased at each follow-up visit compared with baseline (7.2 ± 1.9). The lowest score was 2.2 ± 0.9, which was achieved at 12 months after ablation, followed by a slight increase to 2.3 ± 1.0 at 3 years. Similarly, the SSS score also showed statistically significant improvement, with scores decreasing from the baseline level of (36.4 ± 13.6) to the lowest level of (17.5 ± 4.3) at the 3-year follow-up (p < 0.05) ().
Table 1. Baseline information; means ± SD.
Table 2. Changes of post-ablation uterine volume, VAS score, SSS score and Hb levels.
One patient out of 64 patients showed no improvement and then underwent total hysterectomy. The remaining 63 patients had no relapse after the treatment.
Discussion
Adenomyosis or its variance, localized adenomyosis (adenomyoma) is caused by the invasion of ectopic endometrial glands and stroma in the myometrium [Citation15]. Hysterectomy is a definite solution, but most women do not want to lose their uterus. The conservative treatment of uterine adenomyosis is a clinical challenge. There are many conservative treatment options. However, there is still no consensus on the optimal strategy by which to manage symptomatic adenomyosis [Citation16]. Image-guided tumor ablation techniques (radiofrequency, microwave and high-intensity focused ultrasound) have been introduced for the treatment of adenomyosis in the recent years. The safety and effectiveness of this relatively new strategy for adenomyosis is under evaluation [Citation4–6,Citation17–20].
The present study showed that the combined RFA and LNG-IUS protocol effectively relieved the severity of the symptoms in women with adenomyosis, as demonstrated not only by the patient pain scores and menorrhagia scales but also by the uterine size.
Both the VAS and SSS scores significantly decreased after the combined treatment and were maintained at a very low level during the 3-year follow-up. It is difficult to compare the present results with the previous studies in which RFA was solely applied because the severity of the adenomyosis and follow-up period are different [Citation4–6]. The results in our study also showed that the mean uterine volume significantly decreased by 57% at 1 year after procedure.In the previous studies, the 1-year uterine volume reduction rate of single RFA was 41 − 47% [Citation4–5]. Based on the above comparisons, we speculate that combined treatment may provide a superior clinical effect compared to RFA treatment on its own.
None of the patients had relapse after the treatment during the 3- year follow-up period, in contrast to our previous study, in which approximately 20% of patients had recurrence after RFA without LNG-IUS implantation at the 1-year follow-up [Citation4].
Adenomyosis typically involves an irregular and massive myometrium [Citation15]. Complete ablation of the adenomyotic tissue is difficult because the boundary between the adenomyotic lesion and the normal tissue is unclear under ultrasound guidance during the application of thermal energy. The mean ablation rate reported ranged from 50% to 76% [Citation4–6,Citation17–20]. It is plausible that residual lesions may continue to grow and relapse [Citation21–22]. The LNG-IUS is suitable for maintenance therapy because it directly delivers 20 mg/days of an levonorgestrel into the uterine cavity for longer than 5 years. Levonorgestrel can downregulate endometrial estrogen and progesterone receptors by constantly releasing progesterone into the uterine cavity [Citation23]. This leads to a loss of sensitivity of the endometrium to circulatory estradiol, generating a strong proliferative antagonism in the endometrium [Citation24]. Lin et al. reported that adjuvant application of LNG-IUS after adenomyomectomy in the treatment of adenomyosis can effectively reduce postoperative recurrence [Citation25]. Our findings suggest that the LNG-IUS could maintain the inhibitory state of the uterus after RFA and effectively control adenomyosis thus reducing postablation recurrence.
There were no major complications, such as perforation or thermal injuries of the pelvic organs, infection and sepsis, in the present group of patients. Our study showed that RFA combined with intraoperative LNG-IUS implantation was technically feasible and successfully performed in 38 patients. Gao reported one case of LNG-IUS perforation from incision of the uterus after an “H” type incision of adenomyotic lesions combined with an LNG-IUS [Citation26]. No perforation occurred in the present study. The remaining 26 patients with enlarged uterus achieved an appropriate uterine size for the implantation of the LNG-IUS, 3–6 months after the RFA. No expulsion was reported, including in 6 patients who had a history of LNG-IUS expulsion before the study. Park et al. reported up to a 37.5% rate of LNG-IUS expulsion rate in the treatment of symptomatic adenomyosis [Citation27]. Even LNG-IUS placement after GnRH agonist therapy in adenomyosis patients still results in a 14.3% expulsion rate [Citation28]. RFA can effectively reduce the uterine volume. The uterine volume decreased quickly after ablation not only due to lesion involution but also from the ablation-induced tissue contraction as a result of dehydration [Citation29]. In this study, RFA decreased the uterine volume by 48% at 3 months post-ablation and 57% at 12 months after ablation. In addition, it significantly reduced menstrual flow. Thus, most patients achieved an appropriate uterine size with implantation of the LNG-IUS after RFA.
The limitations inherent to the current study include the small number of patients, the lack of a control group and its single-center nature. Another limitation to the study was the use of ultrasound imaging for the evaluation of the size of the uterus, which is known to be an observer-dependent technique [Citation30]. Magnetic resonance imaging was not used routinely to evaluate the volume changes of the uterus because the cost was high. Nevertheless, our study is the first report about the efficacy and safety of combination treatment of US-guided RFA and LNG-IUS for symptomatic uterine adenomyosis with a long-term follow-up.
Conclusion
Our findings suggest that combined transvaginal US-guided RFA combined with LNG-IUS implantation is simple, safe and efficacious in patients with systematic adenomyosis. These treatments relieved dysmenorrhea and menorrhagia, reduced the intrauterine device expulsion rate and allowed patients to avoid hysterectomy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101(1):237–241.e1.
- Alvi FA, Glaser LM, Chaudhari A, et al. New paradigms in the conservative surgical and interventional management of adenomyosis. Curr Opin Obstet Gynecol. 2017;29(4):240–248. Aug
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90(1069):20160119.
- Lin XL, Hai N, Zhang J, et al. Comparison between microwave ablation and radiofrequency ablation for treating symptomatic uterine adenomyosis. Int J Hyperthermia. 2020;37(1):151–156.
- Nam J-H. Pregnancy and symptomatic relief following ultrasound-guided transvaginal radiofrequency ablation in patients with adenomyosis. J Obstet Gynaecol Res. 2020;46(1):124–132.
- Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3- year follow-up study on the effificacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79(3):189–193.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrelreleasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373. e1–7.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76(3):195–199.
- Maheshwari A, Gurunath S, Fatima F, et al. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–392.
- Rose S, Chaudhari A, Peterson CM. Mirena (Levonorgestrel intrauterine system): a successful novel drug delivery option in contraception. Adv Drug Deliv Rev. 2009;61(10):808–812.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Sheth SS, Ray SS. Severe adenomyosis and CA125. J Obstet Gynaecol. 2014;34(1):79–81.
- Abbott JA. Adenomyosis and Abnormal Uterine Bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68–81.
- Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):511–521.
- Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018;51:119–137.
- Hai N, Zhang J, Xu R, et al. Percutaneous microwave ablation with artificial ascites for symptomatic uterine adenomyosis: Initial experience. Int J Hyperthermia. 2017;24:1–13.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124(3):207–211.
- Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol 2012;81:3624–3630.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27(27):677–681.
- Louis LS, Saso S, Chatterjee J, et al. Adenomyosis and infertility. Reprod Biomed Online. 2012;24(5):586.
- Li Q, Yuan M, Li N, et al. The efficacy of medical treatment for adenomyosis after adenomyomectomy. J Obstet Gynaecol Res. 2020;46(10):2092–2099. Oct
- Pontis A, D’Alterio MN, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. 2016;32(9):696–700.
- Fraser IS. Non-contraceptive health benefits of intrauterine hormonal systems. Contraception. 2010;82(5):396–403.
- Lin CJ, Hsu TF, Chang YH, et al. Postoperative maintenance levonorgestrel-releasing intrauterine system for symptomatic uterine adenomyoma. Taiwan J Obstet Gynecol. 2018;57(1):47–51. Feb
- Gao Y, Shan S, Zhao X, et al. Clinical efficacy of adenomyomectomy using H. type incision combined with Mirena in the treatment of adenomyosis. Medicine (Baltimore). 2019;98(11):e14579.
- Park DS, Kim ML, Song T, et al. Clinical experiences of the levonorgestrel-releasing intrauterine system in patients with large symptomatic adenomyosis. Taiwanese J Obstetr Gynecol. 2015;54(4):412–415.
- Zhang P, Song K, Li L, et al. Effificacy of combined levonorgestrel releasing intrauterine system with gonadotropin-releasing hormone analog for the treatment of adenomyosis. Med Princ Pract 2013;22:480e3.
- Wall MS, Deng X-H, Torzilli PA, et al. Thermal modification of col- lagen. J Shoulder Elbow Surg. 1999;8:339–344.
- Moshesh M, Peddada SD, Cooper T, et al. Intraobserver variability in fibroid size measurements: estimated effects on assessing fibroid growth. J Ultrasound Med. 2014;33(7):1217–1224.
