?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To compare the survival benefit, pain control and safety of low-power cumulative and traditional high-intensity focused ultrasound (HIFU) for metastatic pancreatic cancer.
Method
We retrospectively analyzed 55 patients with metastatic pancreatic cancer who received HIFU treatment between January 2008 and April 2014 in our department. 23 patients received low-power cumulative HIFU treatment (L group), 32 received the traditional HIFU treatment (T group). Performance status, cancer-related pain and serum biochemistry results were assessed before and after treatment. All patients were followed up until death. The survival rate and adverse events of the two groups were compared.
Results
The baseline characteristics of the two groups were generally well balanced (p > 0.05). The average KPS score after treatment was significantly improved in both groups compared with the baseline score. 36 patients exhibited tumor-related pain at baseline. The pain response rate was significantly higher in the L group (92.3%) than in the T group (52.2%) (p = 0.025). The median overall survival (OS) for the L group was 7.0 months, which was significantly longer than that of the T group (p = 0.000). The 3-month and 6-month survival rates were higher in the L group. The adverse events in both groups included abdominal pain, elevated C-reactive protein (CRP) and elevated amylase. The incidence was lower in the L group than in the T group.
Conclusion
Compared with traditional HIFU treatment, low-power cumulative HIFU treatment showed a significantly higher pain relief rate and survival benefit with a better safety profile in patients with metastatic pancreatic cancer.
Introduction
Pancreatic cancer is one of the most aggressive solid malignancies. In China, 86,000 new cases were diagnosed in 2012, which was the tenth highest incidence rate, while the mortality rate was the sixth highest [Citation1]. Most pancreatic cancer patients are diagnosed with advanced-stage disease, with a 5-year survival rate of only 3% [Citation2]. For patients in stage IV, palliation and lengthened survival are the primary goals. Current standard therapy is chemotherapy which showed limited survival advantage [Citation3–5]. An account of the specific anatomy location, the progression of primary disease usually cause severe symptoms, such as jaundice and abdominal pain, which not only affect the quality of life but also therapy options and survival time. It’s reported that not all stage IV patients die of metastatic disease. Approximately 30% of patients died due to the local disease development [Citation6].Therefore, to locally control of the primary tumor, to delay the emergence of tumor-associated complications, as well as to alleviate symptoms is important for stage IV pancreatic cancer patients. Effective and minimally invasive local therapies are urgently needed.
Radiotherapy is the most established local treatment method currently. However, for stage IV pancreatic cancer patients, the benefit is still controversial [Citation7–9] and the accumulated radiologic dose limits repeated applications. In recent years, there has been growing interest in the use of invasive ablation techniques for local disease control, such as radiofrequency ablation(RFA), microwave ablation (MWA), and irreversible electroporation (IRE) ablation [Citation10]. Several studies have evaluated the survival benefit of these ablative techniques in unresectable pancreatic cancer. The Median survival ranged from 5–20 months for RFA, 6–24 months for IRE which varied by different tumor stages (III–IV) and protocols [Citation11]. For WMA, only one study reported a longest survival of 22 month in 15 patients [Citation12]. RFA could produce a high temperature from 60° to 100° C [Citation13]. MWA needs shorter ablation times with less procedural pain compared to RFA [Citation14]. However, the complicated anatomical location of the pancreas limits the application of these invasive approaches, which are associated with an increased risk of injury to adjacent structures [Citation15,Citation16]. IRE, as a nonthermal ablation, could decreasing the risk of thermal damage to adjacent normal tissue. However, it is very expensive and not available in many centers. High-intensity focused ultrasound (HIFU) is a new noninvasive modality for tumor ablation. It generates ultrasound (US) waves and focuses them on the lesion precisely, producing thermal and cavitational effects that induce coagulation necrosis [Citation17]. It has been approved by the China Food and Drug Administration (CFDA) for the treatment of metastatic pancreatic cancer. Many clinical studies have shown the advantage of HIFU in pain reduction, performance status improvement, survival time extension and the great safety in advanced pancreatic patients, which confirmed it as a promising modality for palliative therapy [Citation18–23].
Traditional HIFU therapy usually adopts a high input power and short pulse duration protocol. In this way, the temperature of the target lesion would increase quickly but is unstable, and easily to cause adjacent tissue damage. Here, we introduce a new HIFU therapy protocol called low-power cumulative HIFU. It uses a low power input and long plus emission, would increase the efficacy of heat accumulation while decrease the damage to the adjacent normal tissue. This new HIFU therapy might improve the therapeutic efficacy while decreasing the occurrence of adverse events.
In this study, we retrospectively analyzed 55 stage IV pancreatic patients who underwent HIFU treatment in only our medical center. We aimed to evaluate the clinical benefit and safety of low-power cumulative HIFU compared to traditional HIFU.
Materials AND methods
Patients
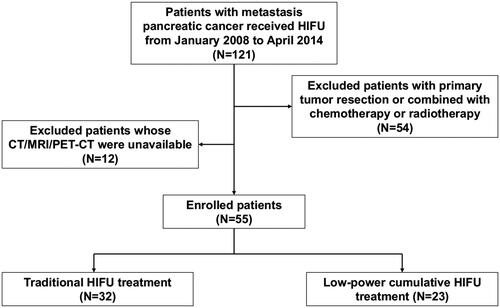
We retrospectively collected data from 55 metastatic pancreatic patients who refused chemotherapy or radiotherapy but accepted HIFU treatment as palliative therapy between January 2008 and April 2014 in the Department of Oncology Surgery, the Second Affiliated Hospital, Zhejiang University, School of Medicine. All patients fulfilled the following inclusion criteria: (1) 18 years or older; (2) histological/cytological diagnosis of pancreatic adenocarcinoma;(3) stage IV disease according to the 7th American Joint Committee on Cancer (AJCC) TNM classification; (4) tumor sufficiently visible on ultrasound;(5) Karnofsky performance status (KPS) of >50; and (6) estimated life expectancy ≥3 months. The main exclusion criteria were (1) noneligibility for general anesthesia; (2) tumor not sufficiently visible on ultrasound; and (3) extensive scarring along the acoustic path. Thirty-two patients accepted traditional HIFU treatment (defined as the T group) between 2008 and 2011, and 23 patients accepted low-power cumulative HIFU treatment (defined as the L group) between 2011 and 2014 ().
Figure 1. Flow diagram of the enrollment and data analysis of patients with metastatic pancreatic cancer treated with Low-power cumulative and traditional HIFU.
Informed consent of HIFU treatment and the possibility of future use of their medical data was obtained from each patient. The study was approved by the ethics committee of the Second Affiliated Hospital, Zhejiang University, School of Medicine.
Pretreatment procedure
Before HIFU therapy, the patient preparation included obtainment of medical history, physical examination, KPS evaluation, biochemical laboratory tests (complete blood count(CBC), C-reactive protein (CRP) level, serum chemistry panel, and amylase level), and imaging evaluation (computer tomography (CT)/magnetic resonance imaging (MRI)/Positron Emission Tomography-Computed Tomography (PET-CT)). The thickness of the abdominal wall, the largest and smallest distance between the target lesion and skin, the tumor size and the blood supply were evaluated. The frequency and duration of therapy were planned according to the tumor size and patient performance status aiming to cover the whole tumor.
The bowel preparation included a low-residue diet and the use of laxatives 3 days before treatment, no milk or other gas-producing food, and fasting for 12 h. The amount of gas in the gastrointestinal tract was evaluated by ultrasound, and a nasogastric tube was placed to remove gas if there was too much.
Device and treatment plan
The HIFU procedure was performed with an FEPBY02 HIFU system (Yuande Biomedical Engineering Co. Ltd, Beijing, China) as described by Hu et al. (2013). A vertical scanning mode was chosen with a slice thickness of 2 mm. The frequency of the ultrasonic transmitter was 1 MHz. Total acoustic output power and effective radiating area were measured by the manufacturer. When the input electric power was 200 W, the total acoustic power was measured as 100 W, and the effective radiating area (ERA) was 8.0 cm−2. The spatial peak time average intensity (Ispta) at 200 mm from the transducer was 800 W/cm2. The acoustic working frequency was found to be 1.0 MHz. The absorption coefficient of the tissue sample was assumed to be 0.6 dB cm−1 MHz−1 and there was a 14 cm water stand-off between the transducer and the the tissue surface, hence, the transmission loss of the propagation path up to the region of intensity was approximately: (20–14)1.0
0.6 = 3.6 dB. And the estimated in situ intensity (Ispta) and peak acoustic pressure were 348 W/cm2 and 3.2 MPa, respectively.
For the low-power cumulative HIFU treatment group (L group), the HIFU parameters were as follows: ultrasonic power 100–300 W (input power varied depending on the depth of the tumor); unit transmission time or pulse length (T1)/intermission time or pulse repetition period (T2) 990 ms/10 ms; 40 transmissions per therapeutic point with a distance of 2 mm between adjacent therapeutic points; treatment of each unit (five therapeutic points) for 200 s with an interval of 2 min between each unit; spacing of 5 mm between adjacent treatment slices; total emission lasting < 90 min; and therapeutic duration < 2 h.
For the traditional HIFU treatment group (T group), the HIFU parameters were as follows: ultrasonic power 400–1,000 W (input power varied depending on the depth of the tumor); T1/T2 150–200 ms/150–200 ms; 50–80 total applications at each therapeutic point; spacing of 5 mm between adjacent treatment slices; total emission lasting < 30 min; and therapeutic duration < 1 h.
During the therapeutic process, the real-time ultrasound monitor was carried out. We would evaluate the target region every 200 s for low-power cumulative HIFU treatment and 120 s for traditional HIFU treatment, which depends on the time of each therapeutic unit, increase the power in a stepwise manner by 10–20%. As a palliative care, we aimed to control the power under the intensity that could cause tumor ablation. The contrast-enhanced ultrasound would be carried out every 10 min. If the imagine showed a strong echo area or no enhancement area following the injection of contrast agent, which are the signs of ablation, we would downregulate the power by 10–20%. The whole process would be monitored by an experienced operator who closely observed echogenic changes of targeted and non-targeted areas.
All HIFU treatments were performed under monitored sedative anesthesia. The times and interval of HIFU treatment was depended on the intensity of pain, the patients’ performance status and wiliness. The interval should be no less than 3 weeks.
Observation and measurement
The patients’ Karnofsky performance status scores were evaluated at baseline and 14 days after treatment. Vital signs and skin burn were evaluated within 6 h after treatment. CBC, CRP, serum chemistry, and amylase values were obtained 24 h after HIFU treatment. The abdominal pain status was evaluated at baseline and 3 h, 1 day and 3 days after treatment with a numerical rating score (NRS; 0–10: 0 ‘no pain at all’, 10 ‘most intense pain imaginable’, 1–3: ‘mild pain’, 4–6: ‘moderate pain’, 7–10: ‘sever pain’). An NRS reduction to ≤ 1 was defined as complete pain response. Partial pain reduction was related to an NRS reduction by ≥ 2 [Citation20,Citation24].
Treatment evaluation
All patients received CT or MRI, 1–3 months after last HIFU treatment. Because HIFU treatment was only performed at primary pancreatic tumors but not metastatic lesions, we just evaluated the treatment response of primary lesions. Evaluation of efficacy was based on Disease status was Response Evaluation Criteria in Solid Tumors (RECIST). Adverse events and complications, such as subcutaneous nodules, tumor rupture, and gastrointestinal perforation, were assessed one week after treatment. The follow-up was carried out every 3 months after the first HIFU treatment and until patient withdrawal or death. The last follow-up date was December 12, 2014, on which day all the patients had died.
Statistical analysis
All statistical analyses were conducted using SPSS 22.0(IBM SPSS, Armonk, NY, USA), and data are expressed as the mean ± SD. Continuous variables were compared with Student’s t-test. Categorical variables were compared using chi-squared analysis or Fisher’s exact test, and exact 95% CIs were computed. Overall survival (OS) time was measured from the date of first treatment to the date of death. OS estimates were analyzed using the Kaplan–Meier method with the log-rank test. A Cox proportional hazards model was used to estimate the hazard ratio and its 95% confidence interval. The association of HIFU treatments with adverse events was assessed using chi-squared analysis. Two-sided P values were computed; p < 0.05 was considered statistically significant.
Results
Patient clinical and pathologic baseline characteristics
In 55 patients, 23 patients (15 males and 8 females) accepted the low-power cumulative HIFU treatment (L group) with an average age of 60.9 ± 10.5 years. 32 patients (15 males and 8 females) were included in the traditional HIFU treatment group (T group), with an average age of 64.8 ± 12.9 years. Tumors were located in the pancreatic head in most of the patients (14 of 23 in the L group and 17 of 32 in the T group). Patients’ performance status was comparable between the two groups, with no significant difference in the percentage of patients with KPS scores ≤60. Thirty-six patients (13 in the L group and 23 in the T group) exhibited tumor-related pain symptoms. The average NRS score was 2.8 ± 1.4 in the L group and 2.7 ± 1.3 in the T group, with no significant difference. The average frequency of HIFU treatment was higher in the T group than in the L group (p = 0.008). Other baseline characteristics of the two groups were generally well balanced (p > 0.05) ().
Table 1. Baseline clinical characteristics of all treated patients.
Performance status improvement and pain response
All patients were involved in the performance status evaluation with the Karnofsky performance status (KPS) score. The average KPS scores at baseline in the L group and T group were 66.1 ± 9.4 and 68.1 ± 12.0, respectively. The average score 14 days after treatment was significantly improved in both groups compared with that at baseline (79.6 ± 9.3 after 14 days in the L group, p = 0.000; and 75.3 ± 9.5 after 14 days in the T group, p = 0.000). However, the improvement rates between the two groups were similar (p = 0.666) ().
Table 2. Performance status improvement and pain evaluation in patients.
At baseline, 36 patients (13 in the L group and 23 in the T group) exhibited tumor-related pain symptoms, and 7 of them accepted opioid medication. All 36 patients were involved in the pain response evaluation. No patients had NRS ‘severe pain’. In L group, the number of patients in NRS ‘mild pain’ was 7, in ‘moderate pain’ was 6. In T group, the number was 17 and 9, respectively. Three days after the last HIFU treatment, none of the patients reported pain in the two groups. For the 7 patients who received opioid medication, 1 patient in the L group received a dose reduction, and the other 6 maintained the same dosage. The change of NRS assessment score was listed in . In the L group, 11 patients achieved complete pain reduction in L group, 1 patients achieved partial pain reduction, 1 patients achieved no pain reduction. There were 11 complete pain reduction, 8 partial pain reduction, 4 no pain reduction in the T group. The efficacy of pain reduction is superior in L group with a significant statistical difference (p = 0.044) ().
Therapeutic effects and survival analysis
During the HIFU treatment, none of the patient showed non-perfused region in the tumor according to the ultrasound monitoring. 54 patients were included in the response evaluation based on CT, except one in T group who died 27 days after HIFU treatment. In the L group, 1 patient achieved a partial response (PR), 20 stable disease (SD), 2 progressed disease (PD), giving an overall response rate (ORR) of 4.3%, disease control rate (DCR) of 91.3%. In the T group, none patients achieved PR, 25 and 6 patients had SD and PD, giving an ORR of 0%, DCR of 80.6% ().
Table 3. Primary tumor response before and after HIFU treatment.
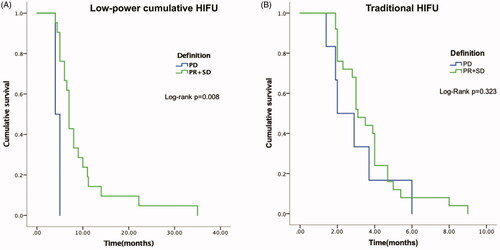
All 55 patients had died by the last follow-up date. The median overall survival (mOS) was 7.0 months (95% CI, 6.1–7.3 months) in patients receiving low-power cumulative HIFU treatment and 3.0 months (95% CI, 2.7–3.2 months) in patients receiving traditional HIFU treatment. There was a statistically significant difference between the two groups (p = 0.000) in OS. The stratified hazard ratio for death from any cause with low-power cumulative HIFU treatment versus traditional HIFU treatment was 0.459 (95% CI: 0.327–0.645, p = 0.000). In the low-power cumulative HIFU group, the 3-month and 6-month survival rates were 100% and 65.9%, respectively. However, the 3-month and 6-month survival rates were 59.4% and 9.4%, respectively, in the traditional HIFU group (). With regard to the correlation between tumor mass shrinkage and survival, patients who achieved disease control (SD + PR) had significantly longer mOS than whom got PD in L group (7.0 vs 4.0 months, p = 0.008, ). While in T group, the mOS of patients with disease SD + PD and PD were similar (3.1 vs. 2.0 months, p = 0.323, ).
Figure 2. Kaplan–Meier overall survival (OS) curves for treatment. In the low-power cumulative HIFU treatment group, the median OS was 4.0 months longer than that of the traditional HIFU group, with a statistically significant difference (p = 0.000).
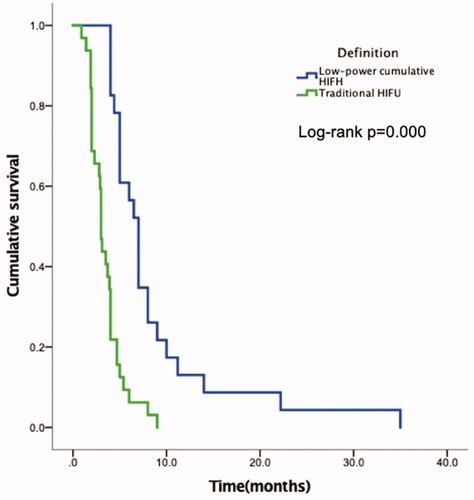
Figure 3. (A) Kaplan–Meier overall survival curves for patient in the low-power cumulative HIFU treatment group according to the tumor response. (B) Kaplan–Meier overall survival curves for patient in the traditional HIFU treatment group according to the tumor response.
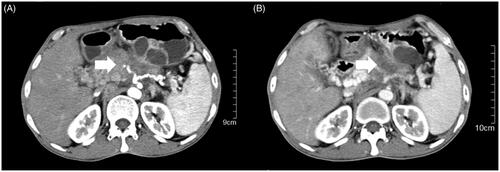
One patient in the low-power cumulative HIFU group was diagnosed with pancreatic cancer with peritoneal lymph node metastasis. His primary tumor mass shrank by 11% after 3 HIFU treatments without adverse events (). In addition, the back pain was significantly relieved after the first treatment, with a decrease in the NRS from 3 to 0. Ultimately, the patient had a high quality of life with a survival time of 8 months.
Adverse events
Biochemical laboratory tests were carried out before and after the treatment. Common adverse events, such as fever, abdominal pain, and skin burns, were observed after therapy. Abdominal pain was defined as newly emerging or aggravated pain 3 h after a single HIFU treatment. In both groups, the most common adverse events were transient abdominal pain and elevated CRP. The incidence of abdominal pain was slightly lower in the low-power cumulative HIFU group than in the traditional HIFU group (30.4% in the L group vs. 34.4% in the H group). However, all the patients reported pain reduction 24 h after treatment. In the traditional HIFU group, one patient experienced skin burn, and one had elevated amylase levels. The incidence of all observed adverse events was lower in the low-power cumulative HIFU group than in the traditional HIFU group; however, the difference was not statistically significant ().
Table 4. Summary of adverse events after HIFU treatment.
Discussion
Although most patients with stage IV pancreatic cancer die because of metastatic disease, some of them die from local disease progression. Local therapy, by relieving or postponing the symptoms such as jaundice and back pain, which negatively affect patients’ performance status and quality of life, have great significance. HIFU is a noninvasive technique for thermal ablation without the percutaneous placement of probes into the tumor [Citation16]. It focuses ultrasound beams on the target lesion precisely but not in the intervening tissue [Citation18], which causes tissue damage by two known mechanisms, heat and cavitation effects [Citation25]. The thermal energy would induce the tumor necrosis, eliminate the nerve compression, which might be the mechanisms of pain reduction [Citation26]. In addition, unlike radiotherapy, HIFU could be performed repeatedly without an accumulated thermal energy dose limitation. It is considered a potent alternative palliative treatment for metastatic pancreatic cancer patients and has been approved by the China Food and Drug Administration (CFDA). A number of studies have shown that HIFU can relieve symptoms and prolong survival safely in advanced pancreatic patients [Citation20–23]. Wang and colleagues [Citation26] retrospectively analyzed 40 advanced pancreatic cancer patients treated with traditional HIFU. Pain relief was achieved in 87.5% of patients. In 27 patients with stage IV, the median overall survival was 6 months. A Germany retrospective study involved 29 stage IV pancreatic cancer patients [Citation27] showed 46.5 ± 19.6% tumor volume reduction 3 months after HIFU treatment and overall survival of 15.5 months. Zhou [Citation18] reviewed 241 papers about HIFU treatment for advanced pancreatic cancer (stage III and IV) involving 3,198 patients. All of these studies adopted traditional HIFU treatment. For 653 patients who were treated with HIFU alone, the pain relief rate and clinical benefit rate were 71.33% and 71.06%, respectively. The mean OS duration was 10 months. The most common side effects were superficial skin burns, edema, fever and gastrointestinal dysfunction.
Although the traditional HIFU technique has been approved for its considerable clinical benefits and acceptable adverse events, it still has limitations. To achieve maximal therapeutic efficacy, it is essential to deliver an appropriate thermal energy at the target lesion with less damage to the adjacent normal tissues. The ultimate thermal energy at the target area is determined not only by the input power, pulse duration but also the efficacy of thermal energy accumulation. The traditional HIFU protocol usually uses a high input power (400–1000 w) and short pulse duration (150–200 ms), which releases high energy over a short time. This model can elevate the temperature in the target tissue in as quickly as tens of seconds [Citation28], but cause the sharp temperature gradient between the target area and surrounding tissues, which makes a rapid heat flow out of the therapeutic region. Meanwhile, the interval between two pluses (150–200 ms) is long, which makes the temperature in the target tissue decrease quickly and hard to accumulate thermal energy. In this mode, it’s difficult to achieve the desired stable therapeutic temperature in the target lesion while maintaining a safe boundary for the surrounding normal tissues. Cheng et al observed the histological changes after HIFU treatment in an animal model and found that there were still active cancer cell islands in the necrotic region [Citation29]. In addition, third-degree adverse events, such as skin burn, duodenal perforation, pancreatic-duodenal fistula, and mesenteric arterial thrombosis, have been reported as serious complications [Citation22,Citation30]. Determining a way to improve the therapeutic efficacy while reducing the incidence of adverse events is a future goal.
Here, we introduce a new HIFU treatment called low-power cumulative HIFU, which has been used in clinical practice for years in our medical center. Compared to the traditional treatment, it uses lower input power (100–300 w) and prolonged pulse duration (990 ms), which produce a continuous emission in low-power conditions and non-ablative thermal therapy. In our previous phantom experiment, in this new setting, the temperature in the target area increased slowly and evenly, which reduce the temperature gradient between therapeutic points and maximize the effect of heat accumulation. As a result, although the input power is lower, the actual temperature and thermal energy in the same area might be higher than traditional model. All of these factors might increase the therapeutic benefit. In addition, the low input power decreases damage to adjacent normal tissue. In our previous study, which involved 71 local advanced pancreatic cancer patients without analgesic adjustment, the rate of no cancer-associated pain was significantly improved from 70.42% to 92.96% after HIFU treatment [Citation31]. In another study involving 59 advanced pancreatic cancer patients, the new HIFU treatment displayed good safety with no impact on the endocrine and exocrine functions of the pancreas [Citation32].
In this study, all the patients had stage IV pancreatic cancer but refused or were unsuitable for chemotherapy and radiotherapy (because of poor performance status or elderly age), only accepted HIFU treatment as sole therapy. The frequencies of HIFU treatment was depended on the intensity of pain, the patients’ performance status and wiliness. The treatment frequencies in low power cumulative group was fewer than that in traditional group, which might be patients who underwent the new HIFU treatment achieved a better performance status and life qualities. None of the patient showed non-perfused region in the tumor according to the ultrasound monitoring or CT scan post therapy. This might attribute to the lower input power in both groups we set as the aim of palliative therapy. However, the KPS score improved significantly after HIFU treatment in both groups. 91.3% and 80.6% patients got primary tumor controlled in low power HIFU group and traditional HIFU group, respectively. For the patients with cancer-related pain, the pain control was superior in the low-power cumulative HIFU treatment group and the difference was significant. The better pain reduction may be associated with the damage to the tumor infiltration caused by more effective tumor necrosis, as a kind of hyperthermia, under the new HIFU therapy. In addition, the higher efficacy of thermal energy accumulation offered more effective local disease control and translated into a survival benefit. In the low-power cumulative HIFU treatment group, the patients who achieved SD or PR showed superior overall survival than whom got PD. Although the frequencies of HIFU treatment was less than that of the traditional group, the mOS in low-power cumulative group was 4.0 months longer. Meanwhile, the rate of adverse events in the two groups was similar, with a lower tendency for adverse events in the low-power cumulative group. The gradual accumulation of thermal energy decreased the damage to the adjacent normal tissues.
There are some limitations in this study. First, this was a single-center retrospective study with a small number of patients. Second, we did not evaluate the tumor response rate under a regular interval, as most patients asked for palliative therapy and refused repeated CT/MRI scans. However, our results demonstrated that low-power cumulative HIFU treatment could provide a superior survival benefit while being safe for stage IV pancreatic cancer patients.
Conclusion
According to our study, compared to traditional HIFU treatment, low-power cumulative HIFU treatment showed a significant benefit in pain control and the survival rate with fewer side effects. Such a new approach might be suitable for the local treatment of stage IV pancreatic cancer patients. A large, multicenter, prospective study will be needed to confirm these results.
Disclosure of interest
No potential conflict of interest was reported by the author(s).
References
- Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28(1):1–11.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–1813.
- Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33(16):1770–1778.
- Loehrer PJ, Sr., Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112.
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–1599.
- Ierardi AM, Lucchina N, Bacuzzi A, et al. Percutaneous ablation therapies of inoperable pancreatic cancer: a systematic review. Ann Gastroenterol. 2015;28(4):431–439.
- Petrou A, Moris D, Paul Tabet P, et al. Ablation of the locally advanced pancreatic cancer: An introduction and brief summary of techniques. J Buon. 2016;21(3):650–658.
- Lygidakis NJ, Sharma SK, Papastratis P, et al. Microwave ablation in locally advanced pancreatic carcinoma–a new look. Hepatogastroenterology. 2007;54(77):1305–1310.
- Girelli R, Frigerio I, Giardino A, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398(1):63–69.
- Ierardi AM, Biondetti P, Coppola A, et al. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018;7(2):59–66.
- Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med. 2014;119(7):483–498.
- Rombouts SJ, Vogel JA, van Santvoort HC, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg. 2015;102(3):182–193.
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther. 2010;6(4):414–420.
- Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract. 2014;2014:205325
- Xiaoping L, Leizhen Z. Advances of high intensity focused ultrasound (HIFU) for pancreatic cancer. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. Int J Hyperthermia. 2013;29(7):678–682.
- Marinova M, Rauch M, Mücke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26(11):4047–4056.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4(3):294–302.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086.
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10(2):123–129.
- Strunk HM, Henseler J, Rauch M, et al. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. Rofo. 2016;188(7):662–670.
- Cranston D. A review of high intensity focused ultrasound in relation to the treatment of renal tumours and other malignancies. Ultrason Sonochem. 2015;27:654–658.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27(2):101–107.
- Marinova M, Huxold HC, Henseler J, et al. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic Cancer. Ultraschall Med. 2019;40(5):625–637.
- ter Haar G. Ultrasound focal beam surgery. Ultrasound Med Biol. 1995;21(9):1089–1100.
- Shu-Qun Zx-D C, Zhao-You T. Histological changes in rabbit liver tumor after treatment with high intensity focused ultrasound. Chinese Jounal of Experimental Surgery. 1996;13:135–136.
- Li JJ, Xu GL, Gu MF, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol. 2007;13(19):2747–2751.
- Shi Y, Ying X, Hu X, et al. Pain management of pancreatic cancer patients with high-intensity focused ultrasound therapy. Pakistan Journal of Pharmaceutical Sciences. 2017;30(1 Suppl):303–307.
- Shi Y, Ying X, Hu X, et al. Influence of high intensity focused ultrasound (HIFU) treatment to the pancreatic function in pancreatic cancer patients. Pak J Pharm Sci. 2015;28(3 Suppl.):1097–1100.