Abstract
Objective
To develop a thermochromic tissue-mimicking phantom (TTMP) with an embedded 3D-printed bone mimic of the lumbar spine to evaluate MRgFUS ablation of the facet joint and medial branch nerve.
Materials and methods
Multiple 3D-printed materials were selected and characterized by measurements of speed of sound and linear acoustic attenuation coefficient using a through-transmission technique. A 3D model of the lumbar spine was segmented from a de-identified CT scan, and 3D printed. The 3D-printed spine was embedded within a TTMP with thermochromic ink color change setpoint at 60 °C. Multiple high energy sonications were targeted to the facet joints and medial branch nerve anatomical location using an ExAblate MRgFUS system connected to a 3T MR scanner. The phantom was dissected to assess sonication targets and the surrounding structures for color change as compared to the expected region of ablation on MR-thermometry.
Results
The measured sound attenuation coefficient and speed of sound of gypsum was 240 Np/m-MHz and 2471 m/s, which is the closest to published values for cortical bone. Following sonication, dissection of the TTMP revealed good concordance between the regions of color change within the phantom and expected areas of ablation on MR-thermometry. No heat deposition was observed in critical areas, including the spinal canal and nerve roots from either color change or MRI.
Conclusion
Ablated regions in the TTMP correlated well with expected ablations based on MR-thermometry. These findings demonstrate the utility of an anatomic spine phantom in evaluating MRgFUS sonication for facet joint and medial branch nerve ablations.
Introduction
Chronic low back pain (LBP) is defined as back pain of greater than six months duration [Citation1]. Chronic LBP continues to grow as a global problem, especially as the population continues to age [Citation2]. Clinicians struggle with defining the causes of chronic LBP and directing treatment to manage the specific etiologies of pain [Citation1,Citation3].
Lumbar facet joint pain accounts for about 10–15% of patients experiencing chronic LBP. Risk factors for lumbar facet joint pain include aging, spondylolisthesis, and degenerative disk disorders [Citation4]. Lumbar facet joint pain typically has an insidious onset. Intraarticular or medial branch block tests and diagnostic imaging, including computerized tomography and magnetic resonance imaging, are the current most reliable diagnostic approach for lumbar facet joint pain [Citation4].
The treatment for LBP due to lumbar facet joint is challenging. Though nonsteroidal anti-inflammatory drugs and acetaminophen are first-line LBP medical therapies, no studies to date have elucidated the efficacy of pharmacotherapy or noninterventional treatment specifically for lumbar facet arthropathy [Citation4]. Currently, surgical treatments for back pain may be considered when patients have debilitating pain refractory to conservative therapy and the anatomic abnormalities reflecting the distribution of pain identified, but the results are conflicting [Citation5]. Specifically, for lumbar facet arthropathy, fusion surgery in combination with facet blocks has shown some efficacy, but not enough supporting evidence in long-term follow-up with lack of comparison groups [Citation4]. Thus, many patients with LBP will likely not benefit from these surgical approaches [Citation4,Citation5]. Noninvasive and minimally invasive treatments such as medications, intraarticular steroid injections, and radiofrequency denervation have been utilized with mixed results depending on presenting symptomatology [Citation4]. While back pain management is a frequent and debilitating problem, the lack of consensus in treatment management makes chronic LBP an important health problem in need of improved treatments.
In recent years, an innovative ablation technique known as MRI-guided focused ultrasound surgery (MRgFUS) has been developed for treatment of low back pain, based on earlier successes in treating patients with pain derived from other bone and joint diseases [Citation6]. Trials targeting lumbar facet joints have demonstrated that MRgFUS can be a safe and effective noninvasive method for treatment of facet joint pathology [Citation7,Citation8].
Previous studies have demonstrated that MRgFUS is comparable to radiofrequency ablation techniques, with additional benefits of its real-time temperature monitoring and noninvasive technique [Citation8]. While MRgFUS has been tested on cadaver upper lumbar spine and swine sacroiliac joint models, the technology still lacks preclinical and phantom data trials that would allow it to become a widespread treatment option for back pain [Citation9,Citation10].
Tissue mimicking thermochromic phantoms (TTMP) have been previously used to test MRgFUS devices and assess peak temperatures in vitro [Citation11]. Thermochromic phantoms can be composed of polyacrylamide gel and thermochromic ink, which allows for irreversible color change at set temperature thresholds [Citation12]. Another study previously demonstrated the successful creation of a thermochromic phantom by combining polyvinyl alcohol crygogel with thermochromic ink [Citation13]. In addition, swine spine and vertebrae experiments were conducted to assess the localization and efficacy of MRgFUS for spine lesions using MR thermometry [Citation14]. These phantom models have created an alternative for preclinical testing that may expedite use of MRgFUS devices in clinical settings, which may give patients with chronic LBP more options in treatment management [Citation11].
Recently, several groups have developed the MRI compatible 3-dimensional model phantoms for MRgFUS treatment. Menikou et al. [Citation15] demonstrated that breast/rib phantom can be utilized as a very useful tool for evaluating ultrasonic protocols. Cao et al. [Citation16] showed the rib cage model is a valuable and useful tool that can provide realistic human anatomic structures and properties for evaluating the effects of the rib cage on ultrasound propagation. Beaulieu et al. [Citation17] demonstrated the use of 3D printed anatomical models to replicate bone ultrasonographic properties in water.
The current study built on the work of these prior studies to determine an optimal medium to create the spinal model, with acoustic properties closest to a human skull, followed by testing of the MRgFUS device across a range of parameters. This study aims to develop a novel thermochromic tissue-mimicking phantom with an integrated 3D-printed lumbar spine model suitable for evaluating the reliability in treatment planning and assessment of MRgFUS ablation of the facet joint and medial branch nerve.
Materials and methods
3D Lumbar spine model creation process
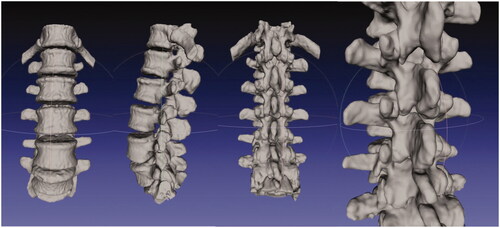
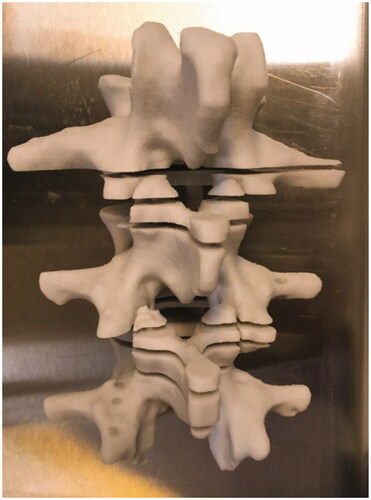
Multiple 3D-printed material test samples were made using polyamide (Materialise, Belgium), TangoBlack, VeroWhite, ABS plastic (Stratasys, Israel), and gypsum (3D Systems, CA, USA) of variable thickness from 1 to 10 mm. Sound speed and linear sound attenuation coefficients were then measured for each material using a well-validated through-transmission technique based upon multiple thicknesses [Citation18]. A 3D model of a normal lumbar spine was created after manually segmentation from an existing CT scan, displayed here in MeshLab. Each vertebrae was individually segmented and then reconstructed to form the lumbar spine in order to preserve spatial separation in the facet joints (). The final lumbar spine model was 3D-printed using gypsum, which demonstrated acoustic properties most similar to human cortical bone among the materials tested. The spine model had axial cuts placed at 1 cm intervals across the region of the facet joints prior to printing to allow for minimal disruption of surrounding friable gel when slicing ().
Thermochromic tissue-mimicking phantom formulation
A TTMP was made based on recently published formulations to mimic the acoustic and thermal properties of human soft tissue [Citation19]. Briefly, the phantom was composed of an aqueous solution of 40% (w/v) acrylamide/bis-acrylamide (VMR International, Secaucus, NJ), bovine serum albumin (3% w/v; Sigma Aldrich, Milwaukee, WI), silica beads (1.1% w/v; Sigma Aldrich, Milwaukee, WI) for ultrasound scattering, and thermochromic ink (MB Magenta NH 60 °C concentrate, 5% v/v; LCR Hallcrest, LLC, Glenview, IL). The thermochromic ink achieves color change from yellow to magenta as a function of temperature, with a gradual change starting from 40 °C to maximum intensity at 64 °C. Thus, the TTMP allows for a range of thermal evaluation from hyperthermic to ablative in the setting of an MRgFUS treatment.
MRgFUS evaluation in TTMP
An ExAblate MRgFUS system (ExAblate 2000, Insightec, Haifa, Israel) connected to a 3 T MR scanner (SIGNA, GE Healthcare, Waukesha, WI, USA) was used. TTMP was positioned on the polyacrylamide gel pad over FUS transducer bath. Imaging was performed using a body transmit/receive coil. Two sets of experiments were performed: first, a total of 8 sonications were applied to test the acoustic power and energy required for local color change of the TTMP (). Each sonication point was separated by 2 cm: six sonications were performed with acoustic powers of 30, 60, and 90 W each at 30 and 60 s and two additional sonications of 100 W at 60 s and 200 W at 30 s were performed to assess change at maximal thermal dosage (). Second, the sonications were then designed to target the facet joints and medial branch nerve in the models with sonication energies ranging from 1800 to 2700 J. The ablation zone was approximately 10 mm in diameter. Temperature was monitored with a standard proton resonant frequency shift technique and the maximum temperature per sonication was recorded.
Figure 3. Insightec MRgFUS console display demonstrating a sonication of 5400 J (90 W, 60 s). Right bottom graph demonstrates a maximum temperature of 63 °C produced under these parameters. Green circles in the left panel indicate the locations of all 8 ablation targets, with the white circle indicating the target of the current sonication.
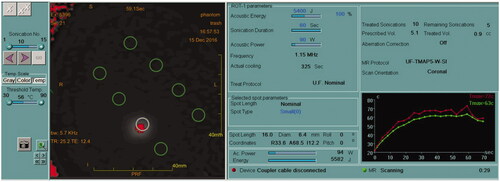
Figure 4. Thermochromic tissue-mimicking phantom with eight sonication sites after dissection is displayed. Maximum color change was noted for the three sonications with the highest total and no color change resulted from the two 30 W sonications. The remaining intermediate energy sonication sites demonstrated a submaximal color change.

Assessment
Following completion of the sonication trials, the gel phantom was dissected and the various targets were qualitatively assessed for presence or absence of color change (). The targeted regions on the phantom were compared qualitatively with expected regions of ablation of MR-thermometry for evaluating localization accuracy. In addition, the spinal canal and neural foramina were carefully examined for unexpected collateral heat deposition.
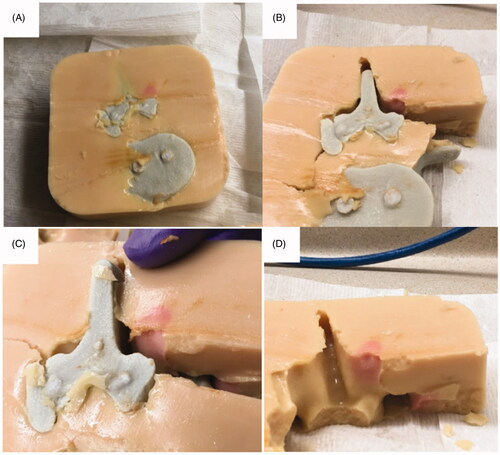
Figure 5. Ablation results of 3D printed tissue-mimicking thermochromic phantom of the lumbar spine. (A) 3D printed spinal segment was embedded in the thermochromic phantom. (B) Dissected cross-section shows red dot indicates ablated area. (C) Separation of the 3D spine model from thermochromic phantom demonstrates depth and color change in desired ablated area. (D) Close up image of dissected phantom demonstrates no evidence of heat deposition in non-targeted areas, including spinal canal and expected areas of the nerve roots.
Results
3D-Printing material analysis
For TangoBlack, VeroWhite, ABS plastic, and gypsum, the calculated linear sound attenuation coefficients were 131.6, 44.7, 134.1, and 271.4 Np/m-MHz and the sound speed were 1974.3, 2435.8, 1982.7, and 2122.4 m/s respectively (). The attenuation of the polyamide material was above the detection range of the system. Of the calculated material coefficients, the value of gypsum was most consistent to published values for the human skull, which has a reported sound attenuation coefficient of 240 Np/m-MHz and an average speed of sound of 2471 m/s [Citation20]. Thus, gypsum was used to 3D-print the lumbar spine model integrated within the TTMP ().
Table 1. Acoustic properties of various 3D-printed test materials.
MRgFUS procedure
Sonication of the TTMP produced successful ablative temperatures for the highest energy sonications only for the following combinations: 90 W for 60 s, 100 W for 60 s, and 200 W for 30 s. These energies resulted in localized intense magenta color change in the TTMP. At 30 W, the two sonication combinations did not result in a sufficient temperature for sufficient heating or ablation, reaching a maximum of 35 °C and 38 °C for 30 s and 60 s respectively. As expected, since the phantom was at room temperature, these temperatures did not result in color change in the TTMP. The remaining sonication energies demonstrated increased heating but sub-ablative maximum temperatures (between 40 and 60 °C) and sub-maximal color change intensity in the TTMP (). The areas of color change in TTMP models were consistent with the areas of temperature change displayed using the temperature mapping of MRI (between 65 and 100 °C). In addition, no heat deposition was observed in critical areas including the spinal canal and nerve roots ().
Discussion
As a noninvasive treatment, MRgFUS has been widely used for a variety of benign and malignant conditions throughout the body and is a promising technique in treatment of chronic LBP caused by facet joint arthropathy [Citation21–23]. In clinical practice, high spatial targeting accuracy of heating and assessing temperature elevation is important in mitigating harm to the surrounding structures. This capability combined with real-time MR-imaging and temperature monitoring allows for targeted ablation without the adverse effects associated with percutaneous procedures [Citation8]. While MRgFUS shows promise as a technique, the lack of preclinical data and trials is a barrier to more widespread adoption of this technology. Previous in vivo and in vitro experiments have been performed to evaluate MRgFUS as a treatment for facet joint arthropathy [Citation14]. This study demonstrated that a thermochromic phantom with an embedded anatomic 3D-printed spine model can serve as a testing tool in evaluating MRgFUS of facet joint arthropathy.
TTMPs have previously been used in experiments related to test MRgFUS hardware [Citation11,Citation14]. The ability to mimic tissue properties and subsequently detect color change as a function of temperature exposure makes the phantom adaptable to a range of anatomical structures and conditions. Prior work has demonstrated that accurate estimations of achieved temperatures in the phantom are possible by simple visualization through an experiment comparing TTMP color vs temperature calibration measured by calibrated optical temperature probes [Citation12]. In this study, we made a TTMP model based on the recently published formulations to mimic the acoustic properties of human soft tissue [Citation19]. The irreversible color changes in the phantom, which indicated the maximum temperatures achieved in the treated region, occurred immediately upon heating during MRgFUS and remained stable post-ablation, thus providing a record of the localization and temperature distributions. Successful ablative temperatures for the highest energy sonication of the TTMP were only seen at 90 W for 60 s, 100 W for 60 s, and 200 W for 30 s (). Two sonications performed at 30 W did not achieve sufficient temperature elevation for hyperthermia or ablation. Assessment of heat deposition showed no damage to non-targeted areas, specifically the spinal canal and nerve roots. These results demonstrated this phantom can be used to perform pre-clinical experiments of MRgFUS to develop novel treatment delivery strategies.
Currently, ex vivo studies are often used to evaluate the ablation effect and test the equipment function before MRgFUS treatment, but have several important limitations [Citation24,Citation25]. Tissues used in ex vivo studies may be heterogeneous, which will affect the penetration of ultrasound, thus affecting the evaluation of ablation effect [Li]. At the same time, in order to achieve adequate ablation effect, the tissues must be fresh and acquired immediately prior to the procedure, and these have limited shelf life. Furthermore, air bubbles and dissolved gas in ex vivo tissues will affect the penetration of ultrasound, so strict degassing must be carried out before the experiment, which can lead to heterogenous results [Li]. In contrast, the 3D-printed TTMP described in this work overcomes the above shortcomings and may be helpful for ongoing studies of new indications.
Our results demonstrated that the ablated regions in a tissue mimicking phantom correlated well to expected ablations based on MR-thermometry ( and ), highlighting the accuracy of this model to ablation of human tissue. Assessment of heat deposition showed no damage to non-targeted areas, specifically the spinal canal and nerve roots. Thus, this 3D replica of a segment of human spine and surrounding tissue can serve as a model for future testing of MRgFUS targeting lumbar facet joint arthropathy.
This study has several limitations. First, the reference linear sound attenuation coefficient and the sound speed coefficient of skull and spinal bone properties may differ. In addition, the TTMP composition is homogenous, making it hard to completely replicate the complexity of real human tissue. Another limitation is that one factor contributing to difficulty achieving ablative temperatures may be attributed to the phantom baseline temperature of 21 °C, which is lower than expected human body temperature. More energy would thus be required to create optimal hyperthermia for ablation than if the experiment was performed in vivo. This study is also limited because we did not perform quantified assessment for color change volume. Future studies can be conducted to gather quantitative data in measuring the ablated volumes produced with different technical parameters to further evaluate optimal treatment parameters.
Conclusions
This study created and assessed a 3D anatomic lumbar spine model using gypsum surrounded by a thermochromic tissue-mimicking phantom. At high ablation energies, color change depicted ablation regions which correlated well to MR-thermometry expected ablation regions. No near or far-field heat deposition was detected in non-target areas. Based on our results, we conclude that the 3D integrated spine phantom model can potentially serve as an alternative method in assessing facet joint and medial branch nerve ablation with MRgFUS in the treatment chronic LBP, creating an alternative to more expensive and time-consuming pre-clinical tests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am. 2014;98:777–789, xii.
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–2037.
- Alleva J, Hudgins T, Belous J, et al. Chronic low back pain. Dis Mon. 2016;62:330–333.
- Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007;106:591–614.
- Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79:1067–1074.
- Namba H, Kawasaki M, Izumi M, et al. Effects of MRgFUS treatment on musculoskeletal pain: comparison between bone metastasis and chronic knee/lumbar osteoarthritis. Pain Res Manag. 2019;2019:4867904.
- Krug R, Do L, Rieke V, et al. Evaluation of MRI protocols for the assessment of lumbar facet joints after MR-guided focused ultrasound treatment. J Ther Ultrasound. 2016;4:14.
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain-case series of an innovative new technique. Eur Radiol. 2012;22:2822–2835.
- Karakitsios I, Mihcin S, Saliev T, et al. Feasibility study of pre-clinical Thiel embalmed human cadaver for MR-guided focused ultrasound of the spine. Minim Invasive Ther Allied Technol. 2016;25:154–161.
- Kaye EA, Monette S, Srimathveeravalli G, et al. MRI-guided focused ultrasound ablation of lumbar medial branch nerve: feasibility and safety study in a swine model. Int J Hyperthermia. 2016;32:786–794.
- Eranki A, Mikhail AS, Negussie AH, et al. Tissue-mimicking thermochromic phantom for characterization of HIFU devices and applications. Int J Hyperthermia. 2019;36:518–529.
- Mikhail AS, Negussie AH, Graham C, et al. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med Phys. 2016;43:4304.
- Ambrogio S, Baêsso R de M, Gomis A, et al. A polyvinyl alcohol-based thermochromic material for ultrasound therapy phantoms. Ultrasound Med Biol. 2020;46:3135–3144.
- Harnof S, Zibly Z, Shay L, et al. Magnetic resonance-guided focused ultrasound treatment of facet joint pain: summary of preclinical phase. J Ther Ultrasound. 2014;2:9.
- Menikou G, Yiannakou M, Yiallouras C, et al. MRI-compatible breast/rib phantom for evaluating ultrasonic thermal exposures. Int J Med Robot. 2018;14.
- Cao R, Huang Z, Nabi G, et al. Patient-specific 3-dimensional model for high-intensity focused ultrasound treatment through the rib cage: a preliminary study. J Ultrasound Med. 2020;39:883–899.
- Beaulieu A, Linden AZ, Phillips J, et al. Various 3D printed materials mimic bone ultrasonographically: 3D printed models of the equine cervical articular process joints as a simulator for ultrasound guided intra-articular injections. PLoS One. 2019;14:e0220332.
- Yeshurun L, Azhari H. Non-invasive measurement of thermal diffusivity using high-intensity focused ultrasound and through-transmission ultrasonic imaging. Ultrasound Med Biol. 2016; 42:243–256.
- Negussie AH, Partanen A, Mikhail AS, et al. Thermochromic tissue-mimicking phantom for optimisation of thermal tumour ablation. Int J Hyperthermia. 2016;32:239–243.
- Pichardo S, Sin VW, Hynynen K. Multi-frequency characterization of the speed of sound and attenuation coefficient for longitudinal transmission of freshly excised human skulls. Phys Med Biol. 2011;56:219–250.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4:294–302.
- Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74:1412–1418.
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16:140–146.
- Bove T, Zawada T, Serup J, et al. High-frequency (20-MHz) high-intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217–228.
- Li T, Chen H, Khokhlova T, et al. Passive cavitation detection during pulsed HIFU exposures of ex vivo tissues and in vivo mouse pancreatic tumors. Ultrasound Med Biol. 2014;40:1523–1534.