Abstract
Background
Microwave ablation is effective for severe secondary hyperparathyroidism, but the difference in efficacy between microwave ablation and parathyroidectomy remains unclear. In this multicenter retrospective cohort study, we compared the long-term clinical efficacy of microwave ablation and parathyroidectomy for severe secondary hyperparathyroidism undergoing hemodialysis.
Materials and methods
The patients were divided into microwave ablation and parathyroidectomy groups. The primary endpoint was the proportion of patients with intact parathyroid hormone (iPTH) concentrations within the target range (100–600 pg/mL) during the efficacy assessment phase. The secondary endpoints were (i) differences in iPTH concentrations over time between the two groups, and (ii) decreases in iPTH concentrations over time in the two groups.
Results
Microwave ablation was performed in 47/92 patients and parathyroidectomy in 45/92. Primary endpoint: iPTH concentrations within the target range were achieved during the efficacy assessment phase in 26/47 patients (55.3%) and in 14/45 (31.1%) patients in the microwave ablation and parathyroidectomy groups, respectively (p = .02). Secondary endpoints: (i) Mean iPTH concentrations during the efficacy assessment phase were significantly higher in the microwave ablation versus parathyroidectomy groups (649 ± 519 pg/mL versus 136 ± 228 pg/mL, respectively; p < .01). (ii) Mean decrease in iPTH concentration from baseline was 725 ± 605 pg/mL versus 1369 ± 478 pg/mL in the MWA versus parathyroidectomy groups, respectively (p < .01).
Conclusions
Ultrasound-guided percutaneous microwave ablation provides higher iPTH target-achieving rates than parathyroidectomy in patients with severe secondary hyperparathyroidism undergoing hemodialysis.
Introduction
Chronic kidney disease, a worldwide public health problem, is increasing in incidence and prevalence. The condition gradually develops into end-stage renal disease, necessitating renal replacement therapy in the form of hemodialysis, peritoneal dialysis, or kidney transplantation. Secondary hyperparathyroidism is a common complication of chronic kidney disease and end-stage renal disease [Citation1,Citation2]. The condition is characterized by disturbances in mineral and bone metabolism, which are seen as serum parathyroid hormone, calcium, and phosphorus concentration changes. Secondary hyperparathyroidism contributes to soft tissue and vascular calcification, cardiovascular morbidity, and the risk of death [Citation2–4].
Drugs, including active vitamin D, are the main form of therapy for mild-to-moderate secondary hyperparathyroidism. Parathyroidectomy (PTX) is an effective therapy for severe secondary hyperparathyroidism [Citation5–7]. However, some patients, especially those with cardiopulmonary insufficiency, cannot tolerate this surgery. Thus, microwave ablation (MWA), a minimally invasive procedure, has been used to treat severe secondary hyperparathyroidism [Citation8,Citation9], especially in patients who cannot tolerate parathyroidectomy. MWA has advantages over PTX [Citation10], namely minimal invasiveness, ease of administration, and rapid recovery, and MWA has achieved good results in patients with severe secondary hyperparathyroidism [Citation11–13]. However, some studies have shown that MWA is less effective than PTX for severe secondary hyperparathyroidism [Citation14,Citation15]. Additionally, most studies have reported only the short-term results of MWA, generally those within 29 months [Citation11,Citation16,Citation17]. Thus, the long-term results of MWA for severe secondary hyperparathyroidism have not yet been conclusively established.
In the present multicenter retrospective cohort study, we compared the clinical outcomes of MWA and PTX for severe secondary hyperparathyroidism undergoing hemodialysis.
Materials and methods
Study design
This multicenter retrospective cohort study involved four hospitals. The study cohort comprised all patients with secondary hyperparathyroidism and end-stage renal disease who underwent MWA or PTX between 1 January 2010 and 31 March 2019. The participants were divided into two groups according to the initial therapy as MWA and PTX groups.
This study was approved by the Bioethics Committee of our institution, Beijing Friendship Hospital, Capital Medical University (Code: 2020-P2-023-01). Ethical approval exempts informed consent.
Patient cohort
The inclusion and exclusion criteria were as follows. Inclusion criteria were (a) age: 18–80 years, (b) hemodialysis thrice weekly for ≥6 months, (c) hyperparathyroidism secondary to end-stage renal disease, (d) intact parathyroid hormone (iPTH) concentration >800 pg/mL before initial MWA or PTX, and (e) follow-up duration of >12 months. In accordance with the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines [Citation18], severe hyperparathyroidism was defined as persistent serum iPTH concentrations of >800 pg/mL. Exclusion criteria were (a) history of MWA or PTX before January 2010, (b) history of thyroidectomy before or during the study period, and (c) lost iPTH data for more than 20% of the follow-up time points.
Intervention
Participants were treated initially with MWA or PTX and then with the treatment specified by the clinical practice guidelines. Participants could be retreated with MWA or PTX if necessary.
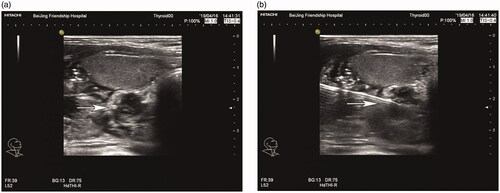
MWA, a form of thermal ablation, can generate very high temperatures very quickly, and the high temperatures can destroy targeted cells. The MWA procedure has been reported previously [Citation9]. Briefly, the microwave therapy apparatus was a KY2000 ablation system (Nanjing Kangyou Applied Research Institute, Nanjing, China) operating at a frequency of 2450 MHz and equipped with a 2-mm outside diameter antenna with a 5-mm tip. The patient’s neck was sterilized and local anesthesia using 2% lidocaine hydrochloride was applied. Next, 5 ml of 2% lidocaine hydrochloride was diluted in 15 ml of normal saline and injected into the area around the parathyroid nodule to develop a heat insulation layer. Thereafter, an ablation needle was inserted into the parathyroid tissue for ablation under ultrasound guidance (). Ablation was then performed with MWA power set between 25 and 35 Watts according to the target nodule size.
Figure 1. Parathyroid nodule presenting as a hypoechoic signal before MWA (a); the ablation needle was inserted into the parathyroid nodule under ultrasound guidance (b). MWA: microwave ablation.
PTX was performed under general anesthesia in operating rooms and comprised one of the following three surgical procedures: total PTX, total PTX with auto-transplantation, and subtotal PTX [Citation19].
Clinical data collection and follow-up
Baseline data for patients’ age, sex, dialysis vintage, iPTH concentrations, and treatment procedure were collected. Serum iPTH, calcium, and phosphorus concentrations were collected at the following time points: baseline, Month 1 (±2 weeks), Month 3 (±2 weeks), Month 6 (±1 month), and then at 6-month intervals (±1 month) until Month 60 or death, kidney transplantation, or transfer to another facility.
Endpoints
The primary endpoint was the proportion of patients with iPTH concentrations of 100–600 pg/mL during the efficacy assessment phase (Month 6 to Month 60 or death, kidney transplantation, or transfer to another facility). The target range of iPTH concentration was in accordance with the Kidney Disease Improving Global Outcomes Guideline [Citation20], which specify maintaining iPTH concentrations within a range of approximately two to nine times the upper limit of normal for the assay. The upper limits of normal for iPTH differed between the four participating hospitals, with two to nine times the upper limit ranging from approximately 100–600 pg/mL. The secondary endpoints were (a) differences in iPTH concentrations over time between the two groups and (b) decreases in iPTH concentrations over time in the two groups.
Other study variables
Changes in serum calcium and phosphorus concentrations over time were also analyzed in this study.
Adverse events
(i) Low serum iPTH concentration and hypocalcemia were assessed in this study. Low serum iPTH was defined as mean iPTH concentration <100 pg/mL during the efficacy assessment phase. Hypocalcemia was defined as serum calcium >2.1 mmol/L at baseline and decreasing to <2.1 mmol/L at any time point within the first 6 months after the initial MWA or PTX.
(ii) All-cause death during the study period.
Statistical analysis
All data were analyzed according to the intention-to-treat principle. All continuous variables with a normal distribution are reported as the means and standard deviations. Variables with a non-normal distribution are reported as medians and interquartile ranges. Categorical variables are reported as frequencies.
Independent t-tests were used to compare variables with a normal distribution between the two groups, namely age, dialysis vintage, and baseline iPTH concentrations. The Mann–Whitney U test was used when the distribution was skewed, which was the case for the number of parathyroid nodules, diameter of parathyroid nodules, and follow-up time. The proportion of iPTH concentrations within the 100–600 pg/mL range was compared between the two groups using Fisher’s exact test. Because there were repeated measurement data during the efficacy assessment phase, a mixed linear model was used to compare serum iPTH, calcium, and phosphorus concentrations between the groups during the efficacy assessment phase.
Cumulative incidence curves for all-cause death were plotted using Kaplan–Meier survival curves and were compared using log-rank tests. Cox regression models with the time (in months) after initial MWA or PTX as the time scale were used to calculate hazard ratios with 95% confidence intervals for all-cause death. Multivariable Cox models were adjusted for age, dialysis vintage, diabetes, and serum calcium, phosphorus, and iPTH concentrations.
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS version 26.0; IBM Corp., Armonk, NY).
Results
Enrollment
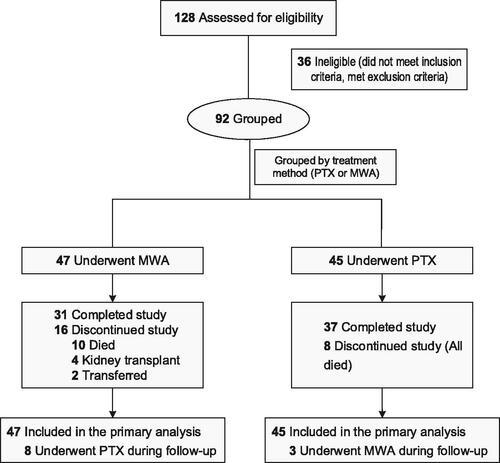
A total of 92 patients treated from January 2010 to March 2019 and followed-up until Month 60 were enrolled in this study; 47 in the MWA group and 45 in the PTX group. A flow chart of the study is shown in .
Baseline characteristics
Patients’ baseline characteristics and clinical data are summarized in . Most studied variables were well balanced between the two groups. However, the dialysis vintage was shorter in the MWA group than in the PTX group, and the baseline iPTH concentration was slightly lower in the MWA group than in the PTX group.
Table 1. Patients’ baseline characteristics.
Fewer parathyroid nodules were detected in the MWA group than in the PTX group; however, the sizes of the parathyroid nodules were comparable between the two groups. Regarding the surgical procedures, 53.3% of the patients in the PTX group underwent total PTX, while the remaining underwent total PTX with auto-transplantation, or subtotal PTX ().
Table 2. Characteristics of the parathyroid nodules and treatment received.
Endpoints
Primary endpoints: iPTH concentrations were within the target range (100–600 pg/mL) during the efficacy assessment phase in 26/47 (55.3%) patients in the MWA group and in 14/45 (31.1%) patients in the PTX group (p = .02). Most patients in the MWA group who did not achieve the target iPTH range had high iPTH concentrations (≥ 600 pg/mL), whereas most patients in the PTX group whose values were not in the target range had low iPTH concentrations (< 100 pg/mL, ).
Table 3. Proportion of patients achieving and not achieving the target range iPTH concentration by treatment group.
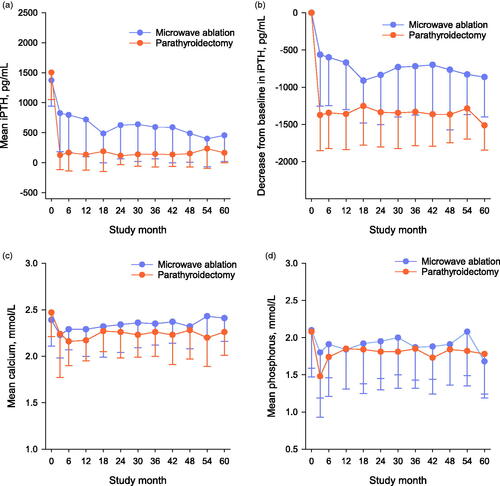
Secondary endpoints: (1) Mean iPTH concentrations during the efficacy assessment phase were significantly higher in the MWA group than in the PTX group (649 ± 519 pg/mL versus 136 ± 228 pg/mL, respectively; p < .01; ). (2) The decreases from baseline over time in mean iPTH concentration during the efficacy assessment phase were significantly smaller in the MWA group than in the PTX group, with mean decreases in iPTH concentration from baseline of 725 ± 605 and 1369 ± 478 pg/mL in the MWA and PTX groups, respectively (p < .01; ).
Other studied variables
Baseline serum calcium and phosphorus concentrations were both comparable between the two groups. Serum concentrations of both calcium and phosphorus decreased after the initial MWA or PTX; however, the concentrations of both parameters increased within 6 months and fluctuated during follow-up in both groups ().
Adverse events
(1) The proportions of patients with low serum iPTH concentrations and hypocalcemia were much higher in the PTX group than in the MWA group (). Of the 30 patients with low serum iPTH concentrations in the PTX group, 60% (18/30) underwent total PTX, 30% underwent (9/30) total PTX with auto-transplantation, and 10% (3/30) underwent subtotal PTX.
Table 4. Adverse events by treatment group.
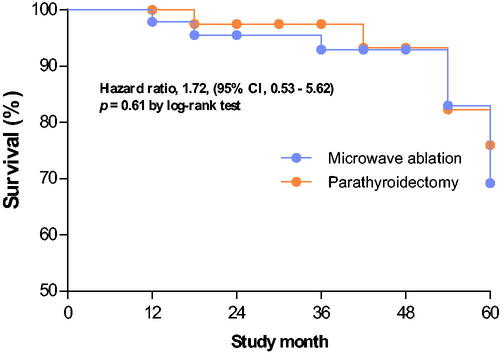
(2) All-cause death: Fifteen patients died during the study. The cumulative incidence of all-cause death was comparable between the two groups (p = .61 by the log-rank test; ). The multivariable-adjusted hazard ratio for all-cause death was also comparable between the MWA and PTX groups (p = .37; hazard ratio: 1.72, 95% confidence interval [CI]: 0.53–5.62).
Figure 4. Kaplan–Meier survival curves for patients with severe hyperparathyroidism who underwent microwave ablation or parathyroidectomy. There was no significant difference in the cumulative incidence of all-cause death between the microwave ablation and parathyroidectomy groups (p = .61, log-rank test).
Discussion
In this study, in our cohort of patients with severe secondary hyperparathyroidism undergoing hemodialysis, MWA more frequently achieved iPTH concentrations within the target range compared with PTX (57.4% versus 31.1%, respectively), and MWA rarely resulted in low serum parathyroid hormone concentrations. This finding is consistent with those of a previous single-center retrospective cohort study [Citation16] of 33 patients who underwent MWA and 48 who underwent parathyroidectomy. In the study, the recommended goal for iPTH concentration was achieved significantly more often in the MWA group than in the PTX group at 24 months (81.82% versus 52.63%, respectively), and persistently low iPTH concentrations were found more often in the PTX group (64.6%) than in the MWA group (0%) [Citation16]. Our study confirmed these results. However, our efficacy outcome was the average of multiple iPTH measurements during the efficacy assessment phase, whereas in the above-cited study, the efficacy outcome was the iPTH concentration at a single timepoint. We consider an average of iPTH concentrations over time preferable because the effect of iPTH on prognosis is long-term, not short-term. In another retrospective study [Citation14], 18/30 patients underwent MWA, and 12/30 underwent PTX. The authors reported that the recommended goal for iPTH concentration was achieved less frequently in the MWA group than in the PTX group at Month 29 (22.2% versus 50.0%, respectively). This result is inconsistent with ours; the following are possible explanations for this discrepancy. First, our efficacy outcome was the average of multiple iPTH measurements, whereas in the above-cited study, the efficacy outcome was the iPTH concentration at a single timepoint. Second, the sample size in the previous study was small (30 patients) and the longest follow-up time was 29 months. In the present study, the sample size was larger (92 patients), and the follow-up time was longer. These differences in sample size and follow-up time may have led to bias. Third, in the present study, eight non-responsive patients in the MWA group underwent PTX during the efficacy evaluation period, which may have affected the response rate.
Both MWA and PTX have been used to treat patients with severe secondary hyperparathyroidism [Citation15,Citation21]. PTX is commonly considered to reduce mortality in patients with secondary hyperparathyroidism undergoing hemodialysis [Citation21–26]. However, whether MWA is associated with long-term survival benefit in such patients has rarely been investigated.
In the present study, we found a comparable cumulative incidence of all-cause death in the MWA and PTX groups; however, iPTH concentrations were significantly lower after MWA than after PTX. We consider the major reasons for this are as follows: First, in the present study, MWA more frequently achieved iPTH concentrations within the target range compared with PTX. The target ranges for iPTH differ between published guidelines [Citation20,Citation27], with the maximums ranging between 60 pg/mL and 600 pg/mL. iPTH concentrations less than 600 pg/mL may not be associated with increased mortality; only very high iPTH concentrations are reportedly associated with an increase in mortality [Citation28,Citation29]. In this study, although mean iPTH concentrations were significantly higher after MWA than after PTX (649 ± 519 pg/mL versus 136 ± 228 pg/mL, respectively; p < .01), few patients still had very high iPTH concentrations after MWA. Second, MWA rarely resulted in low serum iPTH concentrations, whereas PTX was more likely to result in low serum iPTH concentrations. Some researchers have suggested that very low iPTH (<50 pg/mL to 65 pg/mL) can be associated with increased mortality [Citation30,Citation31].
This study has several limitations. First, information for some adverse events, such as hoarseness, and muscle spasms, was not available in this retrospective study. The only adverse events we assessed were low serum iPTH concentrations and hypocalcemia, which were data available from the hospital records. Second, the small size of the study sample may have skewed the results; larger studies are needed to assess efficacy and safety. Third, this was a retrospective study; thus, treatment was not randomly allocated. Some patients with higher numbers of parathyroid nodules may have been inclined to undergo treatment by PTX. This may have caused selection bias, which may have affected our findings.
In conclusion, MWA more frequently achieves sustained serum iPTH concentrations within the range of 100–600 pg/mL compared with PTX in patients with severe secondary hyperparathyroidism undergoing hemodialysis, and rarely results in low serum parathyroid hormone concentrations. Ultrasound-guided percutaneous microwave ablation is a promising and minimally invasive means of treating severe secondary hyperparathyroidism.
Acknowledgments
The authors thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing a draft of this manuscript.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Dang Z, Tang C, Li G, et al. Mineral and bone disorder in hemodialysis patients in the Tibetan Plateau: a multicenter cross-sectional study. Ren Fail. 2019;41:636–643.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. CJASN. 2015;10:98–109.
- Seo KW, Park JS. Myocardial calcification due to uncontrolled hyperparathyroidism. J Korean Med Sci. 2018;33:e162.
- Wan J, Li W, Zhong Y. Parathyroidectomy decreases serum intact parathyroid hormone and calcium levels and prolongs overall survival in elderly hemodialysis patients with severe secondary hyperparathyroidism. J Clin Lab Anal. 2019;33:e22696.
- Ivarsson KM, Akaberi S, Isaksson E, et al. Cardiovascular and cerebrovascular events after parathyroidectomy in patients on renal replacement therapy. World J Surg. 2019;43:1981–1988.
- Plas W, Dulfer RR, Koh EY, et al., on behalf of the Dutch Hyperparathyroidism Study Group (DHSG). Safety and efficacy of subtotal or total parathyroidectomy for patients with secondary or tertiary hyperparathyroidism in four academic centers in the Netherlands. Langenbecks Arch Surg. 2018;403:999–1005.
- Filho WA, van der Plas WY, Brescia M, et al. Quality of life after surgery in secondary hyperparathyroidism, comparing subtotal parathyroidectomy with total parathyroidectomy with immediate parathyroid autograft: prospective randomized trial. Surgery. 2018;164:978–985.
- Zhang J, Qiu M, Sheng J, et al. Ultrasound-guided percutaneous thermal ablation for benign parathyroid nodules. Acad J Second Mil Med Univ. 2013;33:362–370.
- Wang G, Liu S, Liu X, et al. Microwave ablation: an effective treatment for mild-to-moderate secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2017;33:1–52.
- Wei Y, Peng LL, Zhao ZL, Li Y, et al. Complications encountered in the treatment of primary and secondary hyperparathyroidism with microwave ablation - a retrospective study. Int J Hyperthermia. 2019;36:1264–1271.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282:576–584.
- Zhao J, Qian L, Zu Y, et al. Efficacy of ablation therapy for secondary hyperparathyroidism by ultrasound guided percutaneous thermoablation. Ultrasound Med Biol. 2016;42:1058–1065.
- Li X, Wei Y, Shao H, et al. Efficacy and safety of microwave ablation for ectopic secondary hyperparathyroidism: a feasibility study. Int J Hyperthermia. 2019;36:647–653.
- Diao Z, Liu X, Qian L, et al. Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2016;32:614–622.
- Gong L, Tang W, Lu J, et al. Thermal ablation versus parathyroidectomy for secondary hyperparathyroidism: a meta-analysis. Int J Surg. 2019;70:13–18.
- Jiang B, Wang X, Yao Z, et al. Microwave ablation vs. parathyroidectomy for secondary hyperparathyroidism in maintenance hemodialysis patients. Hemodial Int. 2019;23:247–253.
- Diao Z, Wang L, Li D, et al. Efficacy of microwave ablation for severe secondary hyperparathyroidism in subjects undergoing hemodialysis. Ren Fail. 2017;39:140–145.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201.
- Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2018;13:952–961.
- KDIGO CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
- Ho LC, Hung SY, Wang HH, et al., Tainan RENal Disease Study (TRENDS) group. Parathyroidectomy associates with reduced mortality in Taiwanese dialysis patients with hyperparathyroidism: evidence for the controversy of current guidelines. Sci Rep. 2016;6:19150.
- Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30:2027–2033.
- Komaba H, Taniguchi M, Wada A, et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;88:350–359.
- Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010–2016.
- Chen L, Wang K, Yu S, et al. Long-term mortality after parathyroidectomy among chronic kidney disease patients with secondary hyperparathyroidism: a systematic review and meta-analysis. Ren Fail. 2016;38:1050–1058.
- Apetrii M, Goldsmith D, Nistor I, et al. Impact of surgical parathyroidectomy on chronic kidney disease-mineral and bone disorder (CKD-MBD) – a systematic review and meta-analysis. PLoS One. 2017;12:e0187025.
- Fukagawa M, Yokoyama K, Koiwa F, et al., Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288.
- Floege J, Kim J, Ireland E, et al., on behalf of the ARO Investigators. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955.
- Fernández-Martín JL, Martínez-Camblor P, Dionisi MP, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30:1542–1551.
- Lee SA, Lee MJ, Ryu GW, et al. Low serum intact parathyroid hormone level is an independent risk factor for overall mortality and major adverse cardiac and cerebrovascular events in incident dialysis patients. Osteoporos Int. 2016;27:2717–2726.
- Jean G, Lataillade D, Genet L, et al. Association between very low PTH levels and poor survival rates in haemodialysis patients: results from the French ARNOS cohort. Nephron Clin Pract. 2011;118:c211–6.